Introduction
In recent years substantial emphases have been given to the improvement of meat quality and safety. Lipid oxidation is one of the major causes to deteriorate the meat quality via the production of off-flavors, odors, destruction of mostly polyunsaturated fatty acids (PUFAs), fat-soluble vitamins, and pigments (Morrissey et al., 1994). With regards to improve antioxidant activity and retard lipid oxidation in meat, different antioxidants are commonly added to pig diets. Owing to this issue, many studies have been conducted with different additives such as vitamins, minerals, and antioxidants which can improve sensory, antioxidant capacity, and nutritional characteristics of meat (Swigert et al., 2004). In particular, additives for the control of lipid oxidation and enhance the antioxidative stability in meat and meat products have become increasingly important. In the meat and meat products industry, lipid oxidation is a major deteriorative phenomenon that affects negatively color, flavor, and nutritional value (Asghar et al., 1988). And also, lipid oxidation is responsible for the formation of some toxic compounds in meat and meat products (Addis and Park, 1989). Owing to prevent lipid oxidation activities in meat and meat products, many synthetic and natural substances have been investigated as potential antioxidants. Nowadays, it has been recorded that due to consumer safety and toxicity the using trend of synthetic antioxidants decreases (Coronado et al., 2002). However, synthetic antioxidants have been known with toxicological and carcinogenic effects in some studies (Faine et al., 2006; Sarafian et al., 2002).
Therefore, the search for natural additives, especially from plant origin, has been increased over recent years (Ohlsson and Bengtsson, 2002). Interestingly, nowadays, however, there have been found a strong tendency to organic antioxidants from a natural source (plants and herbs) as an alternative to a synthetic antioxidant in the protection of animals and their products against lipid oxidation (Wenk, 2003). Compounds from natural plant sources such as fruits, grains, species, oilseeds, and vegetables have been investigated (Que et al., 2006). As a dietary antioxidant α-tocopherol (AT) received considerable attention in recent years (Lee et al., 1998) and is a highly effective antioxidant to enhance the shelf life from the animal origin (Jensen et al., 1998). Some plant fruits or extracts contain phenolic compounds which associate with anti-inflammatory, antioxidant, and antimicrobial activities in meat (Pereira et al., 2009). Of them, Bokbunja/Korean Black raspberry (Rubus coreanus Miquel) extracted is a plant source substance that contains anthocyanin, tannin, gallptannin, ellagic acid (EA), gallic acid, ferulic acid, and phenolics (Dietrich and Will, 1997; Jin et al., 2016). EA is a natural polyphenol antioxidant found in numerous fruits and vegetables including raspberries, strawberries, grapes, certain nuts, and other plant foods. Moreover, EA is a representative of a natural polyphenolic source compound that possesses several activities in form of pharmacological and biological aspects such as strong antioxidant, anti-mutagenic, anti-carcinogenic, anti-allergic, and anti-inflammatory (Bakkalbaşi et al., 2009; Hassoun et al., 2004). EA (2,3,7,8-tetrahydroxy chromeno [5,4,3-cde] chromene-5, 10-dione) is a phenolic constituent plant-derived naturally rich in raspberries exhibits a wide avenue of biological properties comprising antioxidant, antimutagenic, antiproliferative, and anticarcinogenic effects (Festa et al., 2001). In addition, EA can act as an effective 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging, superoxide anion radical scavenging, ABTS+ scavenging, hydrogen peroxide scavenging, ferric ions (Fe3+) reducing power, and ferrous ions (Fe2+) chelating activities (Kilic et al., 2014).
For meat quality registration, data obtained through basal diet testing is essential, but the literature contains no data on the effects of dietary supplementation with black raspberry (Rubus coreanus Miquel) fruit by-product (RCFB) on lipid oxidation, antioxidant capacity, and meat quality in finishing pigs. Therefore, the present study was conducted to evaluate the effect of dietary RCFB supplementation on the oxidative stability, antioxidant activity, and meat quality of LL muscle from Berkshire finishing pigs.
Materials and Methods
In this experiment, RCFB was collected from a raspberry juice-making company (Gochang, Korea). The RCFB was dried in a vacuum hot dryer (60°C, 16 h) into final moisture of 4% and ground into fine powdered. The chemical composition of dried Bokbunja/Black raspberry fruit by-product was analyzed in triplicate for moisture (method 930.15 using drying oven), crude protein (method 954.01 using Kjeldahl apparatus), crude fat or ether extract (method 920.39 using Soxhlet apparatus), crude fiber content (method 978.10 using Soxhlet apparatus and furnace), and crude ash content (method 942.05 using furnace) by the methods of Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, those are manifested in Table 1. And also, nitrogen-free extract (NFE) was determined by using Equation 1:
To quantify the concentration of phenolic compounds from the RCFB powder, 20 mL of a 2% phosphoric acid (50% EtOH) solution was added to 0.1 g of the sample and then extracted for 2 h at room temperature. After that filtrated the extraction with Whatman No. 2 filter paper, and then the supernatant was taken with a syringe filter (0.45 μm) for quantification of active compounds by using HPLC (Agilent 1100 HPLC, Agilent, Santa Clara, CA, USA). The conditions of HPLC for phenolic compounds were equipped with column: Shinseido capcellpak C18 UG (5 μm, 4.6×250 mm), column temperature: 30°C, flow rate: 1.0 mL/min, injection rate: 10 mL, detector: DAD detector (280 nm), mobile phase: A: MeOH:H2O: phosphoric acid (20:79.9:0.1), B: 100% MeOH with gradient system begun with 95% of the mobile phase A and 5% of the mobile phase for B. And for EA quantification, 10 mL of a pretreatment solvent (EtOH:H2O:HCl=60:20:20) was added to 0.1 g of the sample, then hydrolyzed at 90°C for 1 h using a water bath equipped with a reflux extraction device. The hydrolysis solution was cooled at room temperature, dissolved in methanol (20 mL), filtered with a 0.45 μm syringe filter, and used for analysis in the same HPLC with the similar column, flow rate, and injection rate which was followed for catechin, epicatechin, and gallic acid determination. But others conditions were run with column temperature at 35°C, detector: DAD detector (370 nm), mobile phase: (A) 0.1% phosphoric acid in the water, (B) 100% MeOH with gradient system began with 70% of the mobile phase A and 30% of the mobile phase for B. The content of each compound is expressed as mg/100 g.
A total of 120 Berkshire pigs with an average body weight of 110 kg were used in this study. All pigs were randomly divided into two groups (male; castrated and female), 60 in each group, and were fed supplemented experimental diets with 0% and 0.3% RCFB for 60 d before slaughtered. This feeding trial was carried out at a Berkshire pig-producing private farm (Dasan Pig, Namwon, Korea). All animals were raised and handled in following the guidelines and instructions for the use and care of animals by Ministry for Agriculture, Forestry, and Fisheries, Korea.
Moisture contents of LL porcine muscle excised from pigs fed diets with RCFB supplementation with two different concentrations (0% and 0.3%) were determined by drying the samples (3 g) at 104°C following the procedure of (AOAC, 2000). The crude protein content was measured by the methods of (AOAC, 2000). Lipids were extracted from 5 g of muscle with chloroform/methanol (2:1), according to the method described by Folch and Lees (1951). Muscle pH values of LL porcine muscle were measured using a pH meter (Seven ExcellenceTM, Mettler Toledo, Greifensee, Switzerland). The water holding capacity of LL porcine muscle was measured by Uttaro et al. (1993) with minor modifications. In short, 5 g of minced meat samples were centrifuged at 4°C for 10 min with 1,500×g using a centrifuge machine (Combi 514-R, Hanil, Gimpo, Korea) and the weight of the samples was measured. The lightness (CIE L*), redness (CIE a*), and yellowness (CIE b*) of LL muscle samples were measured using a colorimeter (CR-410, Minolta, Japan). All values of color were taken in triplicate for each sample. Shear force values were measured using a Warner-Bratzler shear attachment on a texture analyzer (TA-XT2, Stable Micro System, Surrey, UK).
The fatty acids composition of porcine LL muscle was estimated by the method of O’Fallon et al. (2007), with minor modifications. The assay was performed using a Gas Chromatograph-Flame Ionization Detector (7890 series, Agilent) under the following conditions: injector split mode with a split ratio of 25:1, temperature 250°C. High purity air, H2, and He were used as carrier gases. The flow rate was maintained at 40 mL/min for H2 and 400 mL/min for air. An HP-88 column (60 m×250 μm×0.2 mm) was used for the analysis. The fatty acid composition is expressed as a percentage.
The malonaldehyde (MDA) content of LL porcine muscle was quantified using the TBARS assay adopted with the procedure described by Ahn et al. (1998). Briefly, 5 g of porcine LL samples were homogenized by mixing 15 mL of distilled water and 50 μL of butylated hydroxytoluene (7.2% in ethanol, w/v). After performing the homogenization, 2 mL of homogenized samples were taken in a 15 mL test tube and 4 mL of thiobarbituric acid/trichloroacetic acid solution (20 mM TBA/15%, w/v) were added. After that, the mixture was thoroughly mixed with a vortex mixer. The mixture was then heated for 15 min in a hot-water bath at 90°C and subsequently cooled for 15 min with cool water. After that, the mixture was centrifuged at 5,300×g for 15 min and absorbance was measured at 531 nm by a Spectrophotometer (T 60 UV-visible, Oasis Scientific, Taylors, SC, USA). 1 mL of distilled water and 2 mL of TBA/TCA solution were mixed and used as blank. The amount of TBARS was expressed in mg of MDA per kg of the meat samples.
Antioxidant capacity of LL porcine muscle from Berkshire finishing pigs fed a diet containing 0% and 0.3% concentrations of RCFB supplementation was determined by applying the free radical scavenging assay, according to a method described by Blois (1958) with minor modifications, and is expressed as the DPPH radical scavenging activity (%). Briefly, 2 g of samples from each tested group were diluted with 18 mL of distilled water and then homogenized. After homogenization centrifuged the samples at 3,000×g for 10 min. Thereafter, 2 mL of DPPH (0.2 mM in methanol) solution was mixed with 0.4 mL of supernatant and 1.6 mL distilled water, and then absorbance was measured at 517 nm after storage 1 h at dark conditions. Ascorbic acid was performed as a control. The porcine samples were inspected on 0 and 7 d of refrigeration stored at 4°C.
GPX1 activity was determined by measuring the oxidation of NADPH (nicotinamide adenine dinucleotide phosphate) in the presence of GSH reductase from the supernatants of samples following the procedure described by Chen et al. (2000b) as adopted for meat analysis (Daun et al., 2001). To measure the glutathione peroxidase enzyme activity from blood, samples were taken from the jugular vein from pigs at slaughtering. As an anticoagulant, lithium heparin was used and samples of blood were stored at 4°C until analyzed. Briefly, recorded the oxidation of NADPH by reducing in absorbance at 340 nm. The assay mixture is enclosed with tert.butyl hydroperoxide (0.10 mmol/L), glutathione (0.63 mmol/L), NADPH (0.25 mmol/L), EDTA (5 mmol/L), and glutathione reductase (5 μg/mL) in the potassium phosphate buffer (50 mmol/L; pH 7.6). A mercaptosuccinate-containing blank was used and a serum control was included in every assay. Results are expressed as mg/mL of samples.
Data obtained were analyzed by multiple assay techniques, applying the Student-Newman-Keuls for significance test (p<0.05) using the general linear model of the SAS program (SAS, 2003). Significant differences were determined by applying the one-way ANOVA. Each treatment was performed in triplicate, and results are presented with the SEM value.
Results
Proximate composition of LL porcine muscle from Berkshire pigs fed with supplemented diets containing 0% and 0.3% RCFB is presented in Table 2. Results reveal that dietary 0% and 0.3% RCFB supplementation did not affect the proximate composition of meat excised from the male or female group. And also, the meat quality traits; pH, WHC, meat color, cooking loss, and shear force remain unaffected (p>0.05) by dietary RCFB supplementation with two different concentrations (0% and 0.3%) for both tested groups and presented in Table 3.
Lipid oxidation of porcine LL muscle deduced from TBARS from pigs fed with 0% and 0.3% RCFB dietary supplementation for two tested groups are presented in Table 4. On average during the entire storage of meat samples, the TBARS value of meat samples from 0.3% RCFB fed pigs was significantly lower than 0% RCFB fed or control pigs for male or female groups. The TBARS values from meat fed with 0.3% RCFB dietary supplementation were 0.06 and 0.11 mg MDA/kg for 0 and 7 d of storage respectively in the male group and values were significantly lower than those of meat samples from pigs diets with 0.3% RCFB or control (Table 4). And also, for female pigs TBARS values were 0.03 and 0.12 mg MDA/kg at 0 and 7 d of storage for the meat from 0.3% RCFB diets fed and were significantly lower than control pigs. Moreover, the result shows that TBARS values in meat obtained from pigs fed with 0% and 0.3% RCFB dietary supplementation for both tested groups were significantly increased with the d of storage.
The antioxidant capacity of porcine LL muscle from Berkshire pigs fed with dietary 0% and 0.3% RCFB supplementation based on its DPPH radical scavenging activity determined and is manifested in Table 4. The result shows that meat from 0.3% RCFB supplemented fed pigs evidenced with significantly higher DPPH radical scavenging activity compared to control or 0% RCFB supplemented fed pigs in the male group at 0 and 7 d of entire storage. And also, a similar trend was noted in the female group at 0 and 7 d of storage. It has been found that meat from the male group, more than 56.90%, and 53.75% DPPH radicals were scavenged in 0.3% RCFB supplemented fed pigs at 0 and 7 d of entire storage, respectively and were significantly higher than 0% RCFB fed pigs or control. Subsequently, meat samples from the female group, DPPH radical scavenging activities were 58.14% and 53.18% at 0 and 7 d of storage, respectively for meat fed with dietary supplementation with 0.3% RCFB in diets and were significantly higher than 0% RCFB fed pigs or control for both d of storage.
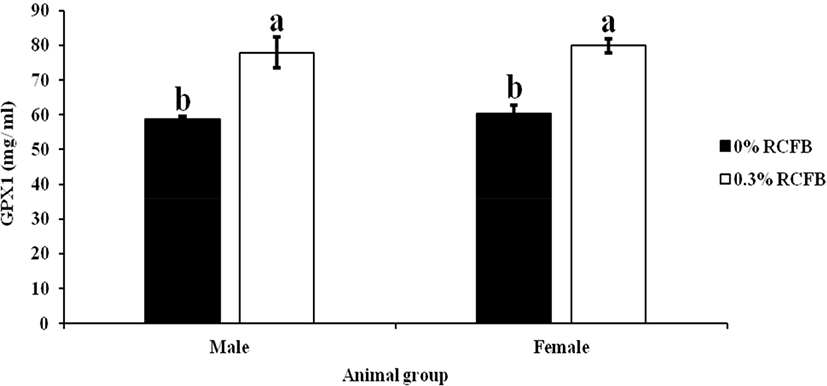
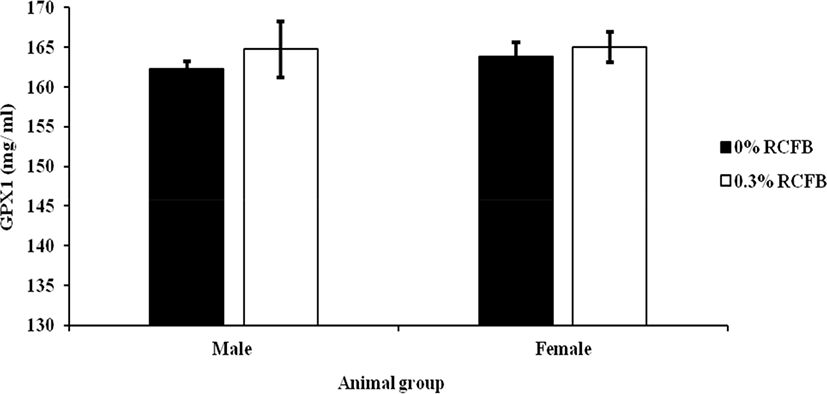
To investigate whether dietary RCFB supplementation in the diet was mediated by enhancing antioxidant enzymes or not, we measured GPX1 activities from blood plasma and LL porcine muscle for male or female pigs. GPX1 is an H2O2-scavenging enzyme activity for blood plasma and LL porcine muscle and results are presented in Fig. 1 and Fig. 2, respectively. Result reveals that the glutathione enzyme activity in blood plasma was significantly higher for meat from 0.3% RCFB fed pigs compared to control or 0% RCFB fed pigs for both tested groups. In addition, however, enzyme activity in muscle did not show any significant differences we observed (p>0.05) and are presented in Fig. 2.
By feeding dietary RCFB supplementation with two different concentrations (0% and 0.3%) of basal diet, the fatty acid composition of LL muscle from Berkshire finishing pigs was determined (Table 5). The result shows that none of the concentrations of RCFB supplementation in diets affects the fatty acid composition of meat from Berkshire finishing pigs (p>0.05) in the male or female group. Owing to sex, it was found that unsaturated fatty acids and PUFAs were significantly higher in meat from female pigs compared to male pigs. Moreover, saturated fatty acids in the meat from male pigs were significantly higher compared to female pigs. Furthermore, a lower ω-6/ω-3 ratio was observed in the meat obtained from male pigs compared to female pigs (p<0.05).
Discussion
Carcass composition is an imperative aspect of animal science relating to food production as the market value of carcass depends on the proximate composition of meat. The results of the present study indicate that dietary RCFB supplementation does not affect the proximate composition as well as meat quality traits also. In addition to the effect of sex, meat from male pigs had higher fat than female pigs and was a good accord previously reported by Barton-Gade (1987); Leach et al. (1996). And also, male pigs tended to have intense color and higher yellowness than female pigs (Barton-Gade, 1987). Gender did not affect the Warner-Bratzler shear force value which confirms previous observation reported by Hamilton et al. (2000). The cooking loss was higher in meat from male pigs which was inconsistent with the report described by Hamilton et al. (2000) in pigs. The possible explanation of lower cooking loss in female pigs might be due to lack of stimulating hormones responsible for collagen synthesis (particularly thermally stable collagen content). Another probable reason might be higher fat in meat from male pig and it also could be attributed to some related factors like cooking temperature, cooking time, internal muscle orientation, and collagen contents. The most important meat quality trait, pH is an important indicator of quality as it is allied to shelf life, color, and water holding capacity of meat. The ultimate pH of most pork with normal glycolysis ranges from 5.3 to 5.8 (Warriss, 1982). In our study, we observed the pH of meat within this range (5.45 to 5.64) which was a good accord with many studies. To our knowledge, there is no data available in the literature indicating a beneficial effect of dietary RCFB supplementation in pigs or other species still and this is the first report regarding the RCFB supplementation diet in pigs. However, polyphenols have been revealed to have anti-nutrient possessions due to their aptitude to association with different dietary components and interfere in their digestion stated by Butler and Rogler (1992). Therefore, further studies regard to dietary RCFB supplementation with different concentrations at different rearing stages should be warranted.
Oxidation in muscle lipids is the cause of the production of free radicals, which are implicated in the deterioration of meat color and flavor. And also, lipid oxidation is the oxidative deterioration of unsaturated fatty acid, defined as a free radical-mediated phenomenon involving a chain reaction mechanism. MDA resulted from lipid peroxidation is one of the amplest aldehydes generated during the secondary lipid oxidation and is most ordinarily used as an oxidation marker (Barriuso et al., 2013). The reduced oxidation has been recorded in meat samples procured pigs fed with added 0.3% RCFB supplemented diets for both tested groups. The presence of oxidized lipids in muscle tissue or food increased TBARS assessed from TBARS value (Ruban, 2009). The lower TBARS values in meat samples from pigs receiving the diets with 0.3% RCFB enriched in EA are probably the result of the presence of strong antioxidant properties (polyphenol compound). Previously, it is reported that polyphenols in plants act as an antioxidant by scavenging the free radical and play a vital role in the cellular antioxidant system, inhibiting oxidative reactions in unsaturated fatty acids in pigs (Havsteen, 2002). The results show that the supplemented diet with RCFB inhibited lipid oxidation in 0.3% RCFB supplemented fed pigs compared to 0% RCFB supplemented fed or control pigs for both tested groups. Thus, the aforementioned possible explanation might be due to phenolics compound in diet, EA that scavenged free radicals available in meat 0.3% RCFB fed pigs which are mediated or generated in the initiation phase, propagation phase, and or during the breakdown of the hydro-peroxidase (Kumar et al., 2015) in the meat of pigs.
DPPH radical scavenging activity is an assay to determine the antioxidant status of meat and meat products that can scavenge the free radicals involved in lipid peroxidation. Regardless of the sex, higher scavenging activity in pigs fed with 0.3% RCFB supplementation was due to supplementation of RCFB which contains ellagic phenolics acid which has high radical absorbance activity or has strong H. donating activity than can capable to inhibit the lipid peroxidation (Kumar et al., 2015). The potent radical scavenging capacity of 0.3% RCFB fed pigs due to strong phenolics compounds in diet exhibit EA. EA has four phenolic OH. groups with merged to benzofuran structure and previously reported as a strong DPPH radical scavenging activities in pigs (Han et al., 2006; Zafrilla et al., 2001). It is well reported that phenolics compounds from plant origin protect the UFA against oxidants and could energetic antioxidant response element (ARE) mediated gene expression (Chen et al., 2000a). Therefore, high amounts of reducing compound in pigs from 0.3% RCFB supplemented fed pigs for both tested groups compared to control feeding pigs could be liable for the regeneration of antioxidants present. Therefore, this is the first report with RCFB supplementation in pigs and we hypothesize that the supplementation of 0.3% RCFB into pig diets would result in a positive effect on the antioxidant capacity of the LL muscle of finishing Berkshire pigs.
Deteriorative oxidative reactions in the meat guide to the loss of both nutritional and food value. Endogenous antioxidative enzymes as superoxide dismutase, catalase, and glutathione peroxidase control the oxidation in muscle tissue. Of them, an antioxidant enzyme, GPX1 is the first line defense antioxidant in meat (Ray and Husain, 2002). Glutathione is a selenium-containing enzyme that catalyzes lipid reduction and hydrogen peroxide (Daun and Åkesson, 2004). The result shows that in blood plasma, the endogenous antioxidant enzyme activities were significantly higher in pigs fed with 0.3% RCFB supplementation than in fed control pigs for both groups. In the present study, 0.3% RCFB dietary feeds increased the GPX1 enzyme activity and the changes of the enzyme could be attributed to the presence of phenolic substances, rich in EA in the supplemented diets. Our findings are well consistent with the study reported by Rossi et al. (2013) who conducted with plant extract containing phenolic compounds in pigs’ diet. The compound we found EA from RCFB diets has strong antioxidant properties previously reported, which could defend organisms alongside oxidative stress. The finding of the present study was in accord with other studies, which have documented a significant relationship between phenolic content and antioxidant enzyme activity (Song et al., 2010; Yao et al., 2010). Therefore, a higher concentration of antioxidant enzyme activity in blood plasma, due to the addition of 0.3% RCFB dietary supplementation, may provide more efficient scavenging of free radicals in finishing pigs irrespective of sex. Many reports have been documented that phenolic compounds are significantly linked with exclusively soluble glutathione peroxidase enzyme activity in muscle tissue (Kumar et al., 2015) with their strong antioxidant activities. However, the result of enzyme activity in the muscle are inconsistent with previously reported data of EA as dietary supplementation in pig (Mishra and Vinayak, 2014) might be attributed due to oxidative stress of muscle that induced the glutathione depletion and or activation of some cofactors those reduced the glutathione, NADPH, and glucose 6-phosphate in muscle. Moreover, glutathione peroxidase enzyme activity partly depends on selenium concentration in the system and it may be hampered due to improper function of the liver also. Therefore, to elucidate the effect of dietary RCFB supplemented diet in muscle tissue precisely, further experiments are needed to be conducted with different doses of RCFB diets in different slaughtering phases of pigs.
The fatty acid composition plays an important role in human health and it is well reported that sex affects the specific enzymes and enzyme activities involved in long-chain PUFA metabolism (Zhang et al., 2007). Higher SFA content in male pigs was due to the higher content of fat in this study and might be attributed of hormonal differences on enzymatic system since lipid metabolism can be changed by manipulating the sex hormone status of the animal. In female pigs, our findings were agreed with De Smet et al. (2004) who concluded that high PUFA deliberation has found frequently been originated in total lipids or triacylglycerols. It is stated that the maximum recommended value of ω-6/ω-3 PUFA is 4.0 because it is a risk factor in cancers and coronary heart diseases, particularly the formation of blood clots formation to a heart attack (Enser et al., 1996). However, regardless of the diet fed, the concentration of linoleic acids was significantly higher in female pigs than male pigs resulted in a higher proportion of ω-6/ω-3 PUFA. Therefore, pigs fed with 0% and 0.3% RCFB dietary supplementation did not affect the fatty acid composition but partly differed by sex need to investigate with further studies.
Conclusion
According to the results obtained from the current study, we found that regardless of the sex, 0.3% RCFB supplemented diets were found to be an effective antioxidant in finishing pigs by enhancing the DPPH radical activity, decreasing the TBARS value in meat, and better antioxidant enzymatic activity (GPX1) in blood plasma procured from male or female Berkshire finishing pigs in LL muscles. Based on the data, EA (we determined) rich dietary supplementation, 0.3% RCFB can be used to prevent lipid oxidation as well as antioxidant capacity enhancement in the meat of pigs at the finishing phase. This is the first report showing that a high concentration of EA content in RCFB diets has been examined in this study as well as administrated through diet in pigs without a detrimental effect on meat quality traits. Further studies should be warranted to elucidate the effect of the different concentration levels of RCFB supplemented diets with maximum antioxidant potency in meat from different species of animals at different slaughtering ages which could open a new avenue for the meat industry with shelf life enhancement.













