Introduction
Recent trends show an increase in the consumption of processed meat products in South Korea due to the rise in single-person households, camping culture, and diversification of home substitute products. From 2016 to 2018, there was an approximately 29% increase in the use of raw materials for sausages, bacon, seasoned meat, ground processed meat, and other meat products (Korea Agro-Fisheries & Food Trade Corporation, 2020). Important sausage products in the Korean Food Code refer to meats or processed meat products that are ground, seasoned, smoked, or heat-treated after being stuffed into casings and then refrigerated or frozen. These include fermented and mixed sausages (Ministry of Food and Drug Safety, 2023).
Fermented sausages undergo fermentation and maturation at low temperatures over one to two months without heating or pasteurization. During production, a starter is added to prevent contamination; concurrently, the growth of other contaminating microorganisms is inhibited (Kim et al., 2022; Klingberg et al., 2005). Commonly used fungal starters for fermented sausages include Lactobacillus plantarum, Lactobacillus sakei, Pediococcus pentosaceus, Staphylococcus xylosus (Agüero et al., 2020; Klingberg et al., 2005), Debaryomyces hansenii (Álvarez et al., 2022; Leroy et al., 2006), and Penicillium nalgiovense (Papagianni and Papamichael, 2007; Van Ba et al., 2016), which are added or sprayed onto the surface of sausages. Fermented sausages inoculated with fungi exhibit higher protein and fat breakdown activity than that exhibited by traditionally fermented sausages, resulting in the accumulation of free amino acids and fatty acids (volatile chemical precursors) and enhanced flavor (Bruna et al., 2001).
Temperature and humidity in the fermentation chamber must be maintained consistently for the starter fungi to thrive until the product is fully mature (Álvarez et al., 2022). Additionally, Cladosporium spp., such as Cladosporium cladosporioides, Cladosporium herbarum, and Cladosporium oxysporum, can adversely affect the quality of fermented sausages through the production of off-flavors, specific color formation due to spore-induced pigmentation, and the development of hairy mycelia (Alía et al., 2016; Lozano-Ojalvo et al., 2015). Because fermented meat products are exposed to open environments for extended periods during production, spoilage fungi pose a high risk of contamination. Therefore, technological developments are needed to ensure the microbiological safety of fermented sausages without heat treatment.
In small-scale farms, a common method to prevent microbial contamination in fermented sausages is to use lactic acid bacteria to lower the pH and inhibit the growth of harmful microorganisms (Kim et al., 2022). While research has explored technologies like high-pressure processing (Balamurugan et al., 2020; Dučić et al., 2023) and pulsed light (Rebezov et al., 2021) to control microorganisms in fermented sausages, their adoption on small-scale farms is limited by their high cost. These technologies can also cause changes in the sensory properties of meat, particularly its color and aroma (Rebezov et al., 2021). Recognizing the need for practical solutions in small-scale fermented meat production, bio-control agents derived from useful microorganisms and plant-based materials are considered the most appropriate biological control substances (Álvarez et al., 2022).
This study aimed to investigate the antifungal effects of essential oils extracted from various plants that are considered safe for human consumption to inhibit the growth of contaminating fungi in fermented sausages. Essential oils are hydrophobic liquids containing various volatile aromatic compounds that have demonstrated antibacterial and antifungal properties, which enhance the safety of food products against harmful microorganisms (Hou et al., 2022; Puškárová et al., 2017). For example, clove oil, rosemary, basil, thyme, and oregano were found to exhibit broad-spectrum antimicrobial effects against fungi and both gram-positive and gram-negative bacteria (García-Galdeano et al., 2020; Mutlu-Ingok et al., 2020; Puškárová et al., 2017; Yassin et al., 2020). Based on their findings, clove, marjoram, and black pepper essential oils were selected for further investigation of their mold reduction potential in this study. The selected essential oils were analyzed for their antifungal activity against mold in fermented sausages. Subsequently, we confirmed the antimicrobial effects of the fermented sausages using the most effective natural substance identified for mold reduction.
Materials and Methods
A sample (25 g) was taken from each fermented sausage and diluted with 225 mL of 0.1% peptone water. After digestion, serial dilutions of the sample suspensions were prepared in 0.1% peptone water and plated on potato dextrose agar (PDA; Difco, Sparks, MD, USA). This was performed according to the solid surface sampling method proposed by the Ministry of Food and Drug Safety of Korea.
For identification, each separate fungus was grown on PDA at 25°C for 7 d. Single colonies were streaked and incubated on PDA plates. After incubation, a small volume (10 μL) of fungal mycelia was captured with a loop and suspended in 900 μL of UltraPure Distilled Water (Invitrogen, New York, NY, USA). The suspension was boiled at 100°C for 2 min and stored at −70°C after cooling. The samples were sent to SolGent (Daejeon, Korea) for DNA sequencing. Internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA were amplified with two universal primers: ITS1 (5’-GTA GGT GAA CCT GCG G-3’) and ITS4 (5’-TCC GCT TAT TGA TAT GC-3’). The polymerase chain reaction was conducted under the following conditions: initial denaturation at 95°C for 15 min, 30 cycles of denaturation at 95°C for 20 s, annealing at 50°C for 40 s, and extension at 72°C for 1.5 min, with a final extension for 5 min at the same temperature. The purified polymerase chain reaction products were sequenced using an ABI PRISM 3730LX DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences were assembled to obtain a full-length sequence, which was identified using the National Center for Biotechnology Information GenBank database (http://www.ncbi.nlm.nih.gov).
This study selected three essential oils (clove, black pepper, and marjoram; doTERRA, Pleasant Grove, UT, USA) aimed at reducing molds derived from fermented sausages. To assess their antifungal activity, the essential oils were purchased and diluted in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) to prepare concentrations of 10%, 25%, 50%, and 75% (v/v). A 100% pure essential oil concentration was also tested, with pure dimethyl sulfoxide (DMSO, Sigma-Aldrich) used as the control. Fungal spore suspensions were prepared following the method described by Lee et al. (2022). Fungal colonies cultured at 25°C were individually suspended in a 0.03% Tween 80 solution (Sigma-Aldrich). Each spore concentration was adjusted to 106 conidia/mL using a hemocytometer (C-Chip disposable hemocytometer, INCYTO, Cheonan, Korea), and 300 μL was spread on PDA agar. Paper discs (8 mm diameter, Advantec, Toyo Roshi Kaisha, Tokyo, Japan) were placed on the inoculated agar, and 10 μL of the diluted essential oil was applied. The samples were then incubated at 25°C for 3–5 d, and the inhibition zone formed was measured in mm using a caliper to evaluate antifungal activity.
As the fermented sausages are produced and matured at relatively low temperatures and are ultimately distributed at refrigeration temperatures, temperature ranges of 4°C, 10°C, 15°C, and 20°C were selected for the temperature-resistance test. To assess whether the antifungal activity of the essential oils applied during sausage production was maintained throughout manufacturing and distribution, a temperature-resistance experiment was conducted. The 100% essential oils were stabilized by exposure to each temperature for 2 h to assess their inherent temperature resistance. Subsequently, the essential oils stored at different temperatures were applied to paper discs, inoculated onto agar with fungal spore suspensions, and cultured at 25°C for 3–5 d to measure the size of the inhibition zone in mm, following the same method as described in the antifungal activity of essential oils section above.
Dried cloves were purchased in South Korea (Barunmigak, Wonju, Korea) to extract their active components. The clove extract was prepared using the method described by Lee et al. (2013). The extraction was performed by adding 25 g of dried cloves to 500 mL of 75% ethanol in a storage bottle, and the extraction was maintained at 70°C for 1 h in a shaking water bath. The clove extract was then filtered through a filter paper (No. 1, Whatman, Maidstone, UK).
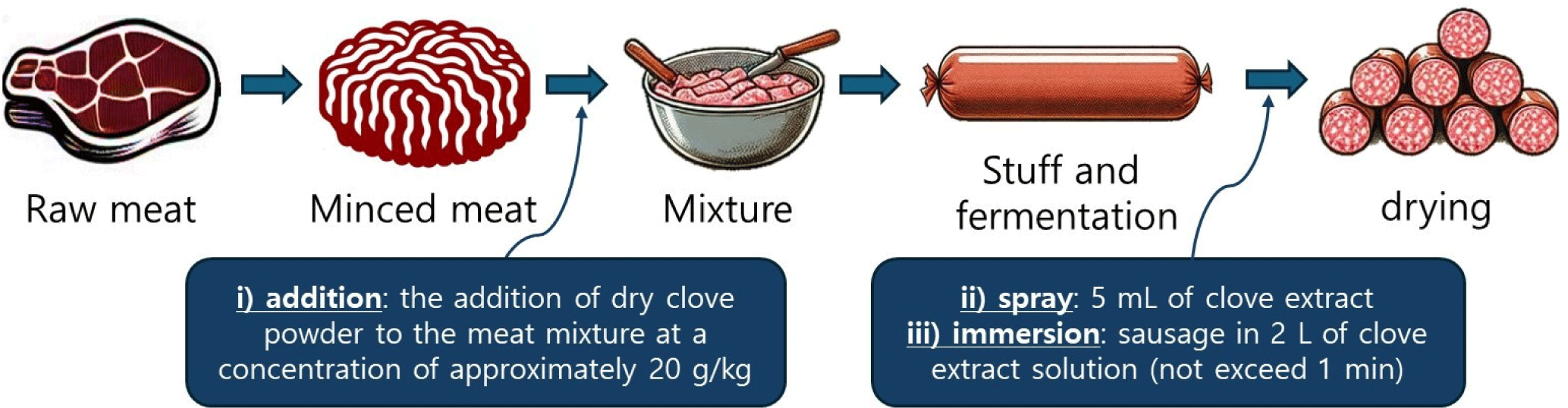
For this experiment, we produced sausages according to the local process and method described by Kim et al. (2022). The ingredients of each sample were compiled according to the producer’s instructions (Table 1). Following homogenization in a vacuum mixer, the mixture (including minced pork shoulder and pork fat, spices, salt, and herb mix) was inoculated with lactic acid bacteria (except for sample A). The treatment for estimating the efficacy of added clove powder involved the blending of ingredients during this mixing step. Subsequently, the fibrous casing was filled with the mixture to form a cylindrical shape. The casing surface was inoculated with a mold starter culture (P. nalgiovense) by spraying (only sample A). Sausages were hung with a string and fermented at 20°C–23°C with an RH of ~95%. Fermentation continued until a final pH of approximately 4.7–4.8 was attained (within approximately 48 h). Subsequently, the fermented sausages were dried until the weight decreased by approximately 55% at 7°C–9°C and an RH of 75%–80%. The samples were individually packaged in sterile bags, placed in a container with dry ice, and transferred to the laboratory within 12 h. The production process consisted of three methods (Fig. 1): i) addition: the addition of dry clove powder to the meat mixture at a concentration of approximately 20 g/kg; ii) spray: approximately 5 mL of clove extract, obtained using the method described above, was evenly sprayed onto the surface of the fermented sausages; iii) immersion: the fermented sausages were adequately immersed in 2 L of clove extract solution, ensuring that the immersion time did not exceed 1 min. Fermented sausages were prepared in two different regions of South Korea using the ingredients listed in Table 1 and following the approach of Kim et al. (2022).
A high-performance antimicrobial clove, identified for its superior properties in a previous experiment, was selected for incorporation into the fermented sausage production process via the addition, spraying, and immersion methods. Each fermented sausage was individually filled and manufactured, then placed in a fermentation room. Subsequently, one row from each rack was sampled for analysis, resulting in a total of three rows of fermented sausages being analyzed. Subsequent microbial analysis revealed that aerobic bacteria and fungi varied in each treatment group over the fermentation sausage production period (0, 1, 2, 3, 7, 14, 21, 28, and 35 d). We collected 10 g of each sample at each time point. The samples were diluted with 90 mL of saline (HUKO, Yokohama, Japan). After digestion, serial dilutions of the sample suspensions were prepared in 1 mL of saline (3M) and plated on PDA (Difco). We collected 1 mL of each microbial suspension and serially diluted it tenfold with 9 mL of a diluent saline solution (3M) to a total volume of 10 mL. Subsequently, the diluted suspensions were inoculated onto PDA plates. After incubating the plates at 25°C for 3–5 d, the number of colonies was counted and expressed as CFU/g.
The pH of fermented sausages was measured using a pH meter (Model Testo 205, Testo, Lenzkirch, Germany). The water activity (aw) of the fermented sausages was determined at 25°C with a Novasina AW SPRINT-TH 300 measuring instrument (Novasiva, Pfäffikon, Switzerland). All experiments were performed in triplicate.
The malondialdehyde (MDA) content in the sausages was analyzed using the OxitecTM thiobarbituric acid reactive substance (TBARS) Assay kit (BIOMAX, Guri, Korea) according to the manufacturer’s protocol. We added 5 g of meat sample to 15 mL of distilled water and centrifuged the samples at 12,000×g for 10 min at 4°C. Subsequently, the obtained supernatants (200 μL) of samples were mixed with 200 μL of indicator solution and allowed to react for 45 min at 65°C. The absorbance was measured at 532 nm using a microplate reader (SpectraMAX PLUS, Molecular Devices, San Jose, CA, USA) and compared with the MDA standard curve to quantify the MDA content in the sausages.
The antifungal effects of the essential oil and fermented sausage quality properties were examined in triplicate, and treatment differences were evaluated. Values of p<0.05 were considered significant, and the data were grouped using Duncan’s multiple-range test. Additionally, temperature stability was analyzed statistically using a simple linear regression analysis to examine the correlation between temperature and the antimicrobial activity of each essential oil. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA).
Results and Discussion
Dry-fermented meat products are manufactured via controlled fermentation processes involving complex microbiomes, including many bacterial and fungal species with key roles. These production characteristics make fermented meat products a conducive environment for the growth of both beneficial and harmful microorganisms during the ripening stage. The proliferation of these organisms can be particularly facilitated by mold spores prevalent in production environments. Notably, Penicillium species can spread widely in production environments, and their development in or on the final products can impact consumers negatively.
Therefore, this study aims to inhibit the growth of dominant contaminating fungi in fermented sausages, selecting target fungi from samples collected in small-scale factories across Korea. The most frequently detected contaminant mold was selected as the target strain for reduction, and ITS region sequencing was conducted to analyze the genetic nucleotide sequences. Strains showing more than 99% similarity were isolated using GenBank database analysis. Four Penicillium mold species, Penicillium chrysogenum (GenBank No. MK140686.1), Penicilliumcommune (GenBank accession No. MW881072.1), Penicillium oxalicum (GenBank No. MT530069.1), Penicilliumsolitum (GenBank accession No. MK761050.1), as well as C. cladosporioides (GenBank accession No. MK111523.1), were selected as target microorganisms for this study. In particular, Penicillium constitutes over 50% of the total molds detected in fermented sausages and over 60% in fermented ham, with such high detection frequencies attributed to its ability to thrive at lower temperatures compared to other species (Lešić et al., 2021). Additionally, Cladosporium is among the most frequently detected genera during the production of fermented meat products, forming black spots that can compromise product quality and lead to economic losses (Alía et al., 2016). Therefore, we analyzed the antifungal abilities of the aforementioned essential oils against these five fungal strains, which are frequently detected in fermented sausages.
A previous study confirmed the excellent antifungal properties of essential oils, such as those from clove, rosemary, and basil, which were isolated from fermented sausages (Lee et al., 2022). This was achieved through a comparative analysis that included antioxidant and antifungal properties (Lee et al., 2022). However, prior to their practical application in sausage production, the antimicrobial efficacy of essential oils from marjoram and black pepper was tested. Additional validation included incorporating strains commonly detected in fermented meat products to ensure suitability for use in sausage production. Dried marjoram is usually used as a mixed herb in meat products (Mazza et al., 2023; Zhang et al., 2021); however, inhibiting mold contamination in long-term fermented meat products may pose challenges. The antifungal activities of clove, marjoram, and black pepper essential oils used to treat fermented sausages are shown in Fig. 2. A comparison of the antifungal activity of the essential oils against the five fungal species at different concentrations revealed significant concentration-dependent differences in all treatment groups (p<0.05). Among them, cloves exhibited the most pronounced inhibitory effects on fungal growth. There was no difference between black pepper and the control group (8 mm) up to a concentration of 75%. However, at 100% concentration, they exhibited very slight effects (p<0.05), with a maximum inhibition zone of 8.93 mm. In contrast, clove exhibited the highest inhibitory effect among the tested oils. At a concentration of 10%, all essential oils demonstrated inhibitory zones of less than 10 mm against the five fungal species, except for clove essential oil. In particular, P. solitum showed high susceptibility to clove essential oil, demonstrating a remarkable inhibitory zone of 24.63 mm even at 10% concentration, presenting distinctive results compared to the other oils.
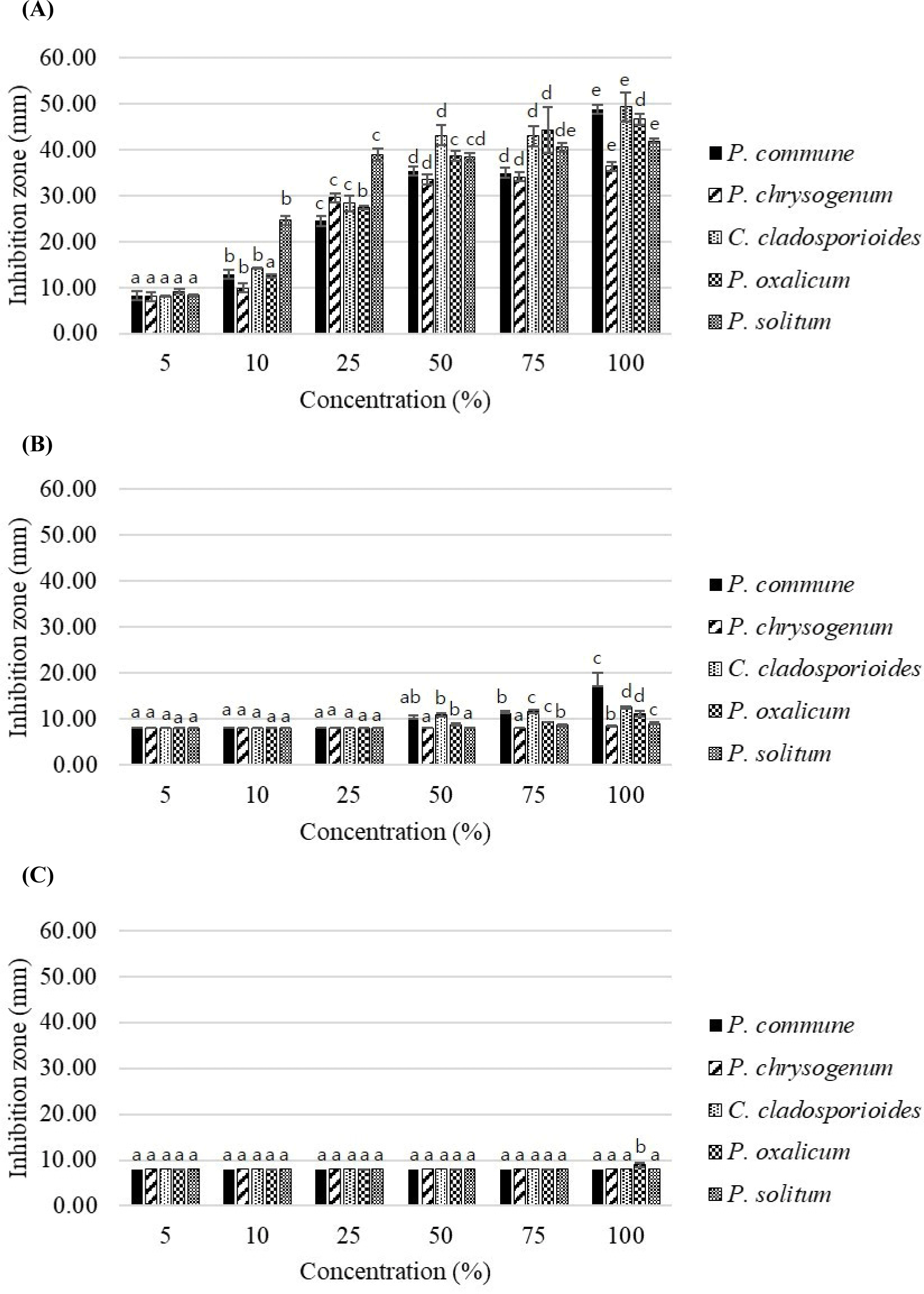
In the studies by Yang et al. (2021) and Yassin et al. (2020), the antifungal activity of essential oils was estimated by measuring and ranking the inhibition zone diameter (inner diameter of the 8 mm paper disk included) as follows: ≤36 mm, strong inhibition; 26–35 mm, moderate inhibition; 16–25 mm, mild inhibition; 10–15 mm, weak inhibition; <10 mm, no activity. Based on the above rankings, the 50% clove essential oil treatment induced a consistently strong inhibitory effect against all five fungal strains, averaging 37.80 mm. Furthermore, the 100% treatment displayed diameters exceeding 40 mm for all strains, except P. chrysogenum (36.39 mm), confirming excellent inhibitory efficacy across all tested strains compared to the other oils. On average, marjoram (11.70 mm) exhibited mild and moderate inhibitory effects at 100%. Studies have shown that essential oils extracted from plants that have been applied to foods such as sausages (Li et al., 2022; Soncu et al., 2020) and cheese (Li et al., 2022) control fungi and inhibit the growth of various harmful microorganisms responsible for foodborne illnesses. The oils used in the present study have been deemed safe for food applications by the U.S. FDA, as they are included in the Generally Recognized as Safe category of substances extracted from natural sources (Code of Federal Regulations, 2024; Jackson-Davis et al., 2023).
During the production process, fermented sausages undergo a prolonged fermentation and maturation stage in a curing chamber (10°C–15°C). Accordingly, the stability of the antifungal efficacy of 100% essential oils was analyzed at temperatures including refrigeration (4°C) and room temperature (20°C), as depicted in Fig. 3. Irrespective of temperature conditions, clove essential oil consistently exhibited inhibitory effects exceeding 36 mm on average. In another study, clove essential oil demonstrated significant inhibitory effects of over 40 mm against P. oxalicum, P. commune, and C. cladosporioides, irrespective of temperature conditions (Lee et al., 2022). Based on these findings, clove essential oil exhibits remarkably strong antifungal activity.
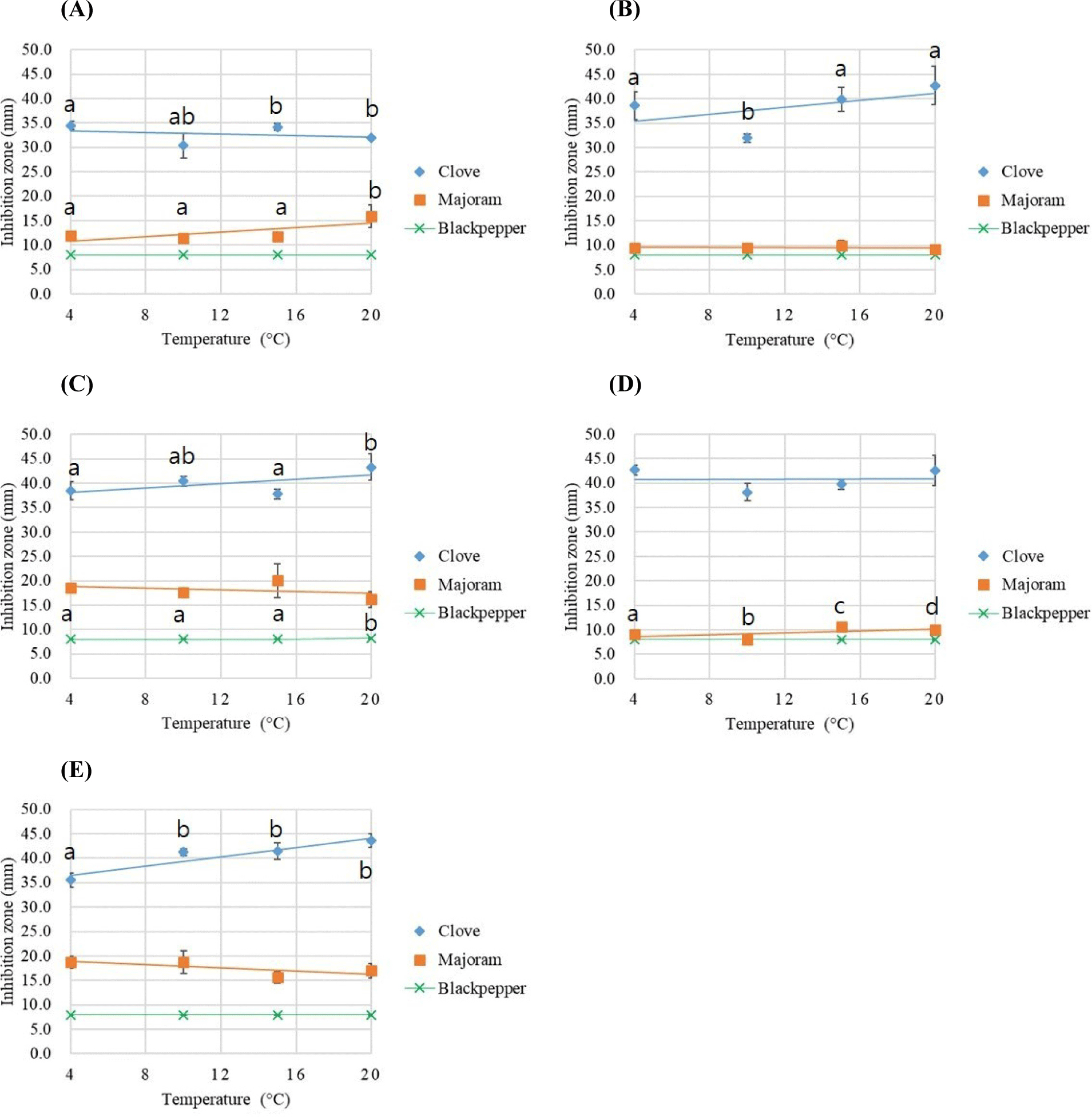
Similar trends were observed under identical temperature conditions. For marjoram, its efficacy against P. commune and P. oxalicum strains increased significantly with temperature (p<0.05). However, the overall average results for essential oils across strains did not show significant differences with temperature (p>0.05). Additionally, regression analysis revealed r-square values for each essential oil that ranged from 0.00 to 0.09, indicating little to no correlation. Therefore, the antifungal properties of the essential oils used in this study were minimally affected by temperature, indicating that their efficacy remains conserved during fermentation, refrigeration, and consumption, making them suitable for application. The results indicated a significant decrease in antioxidant activity and radical scavenging ability due to temperature variations; however, the storage period in that study was notably long, lasting at least one month (Dedebas et al., 2022). Another study confirmed that treating Litsea cubeba oil at high temperatures (30°C–100°C) effectively inhibited molds such as Cladosporium sp., Penicillium sp., and Aspergillus niger (Suhem et al., 2019). These findings suggest that high-temperature treatment can positively affect the inhibition of certain fungal strains. Overall, these studies demonstrate that temperature and storage duration significantly impact the antioxidant and antifungal properties of essential oils. Therefore, it is crucial to carefully consider temperature conditions and storage periods when using essential oils for preservation and antimicrobial treatments.
In this study, using the aforementioned methods, clove demonstrated the highest antifungal activity at the laboratory scale. Moreover, its antifungal effect remained stable across various temperatures, making it the optimal application method. Clove essential oil is highly effective at inhibiting fungal growth owing to its phenolic compounds, primarily eugenol, which can damage or reduce the expression of cell walls and membranes (Rodriguez-Garcia et al., 2016). They often inhibit the synthesis of DNA and proteins, leading to the deterioration of cell walls, cell lysis, and the disruption of membrane integrity (Rodriguez-Garcia et al., 2016). Based on the characteristics of clove and the results obtained from previous experiments, it was applied in an actual fermented sausage production process. Following the main procedures detailed in Table 1, we produced fermented sausages and treated them with clove extract spray, immersion, or the addition of clove powder. Subsequently, microbial and quality changes were analyzed over time to identify the most effective application.
In both sausages produced (samples A and B, Table 1), there was a significant increase in microbial contamination around day 7, followed by a decrease, and this trend was maintained until the completion of production by day 35. For aerobic bacteria, the growth reached up to 6–7 Log CFU/g before decreasing to an average level of 3 Log CFU/g by the end of production. In the case of sausage A (Figs. 4A and 5A), the effects of the different treatments were significant (p<0.05). Particularly, sausages treated with fungi starter spray on the surface showed a reduction of 1.3 Log CFU/g for fungi and 1.8 Log CFU/g for aerobic bacteria compared to the control group, indicating the production of a safe final product. However, in the addition treatment group, the sausage texture became harder and the color was significantly darker than that of the control, diminishing its aesthetic value as a product. Consequently, although there was a reduced effectiveness of approximately 0.6 Log CFU/g in aerobic bacteria with immersion treatment. However, it is impractical for workers to individually immerse each fermented sausage during production. Additionally, the amount used in the spray treatment is 1/400 (5 mL/2 L), making it significantly more economical. Consequently, from the perspective of fermented sausage manufacturers, spray treatment is advantageous for practical industrial applications.
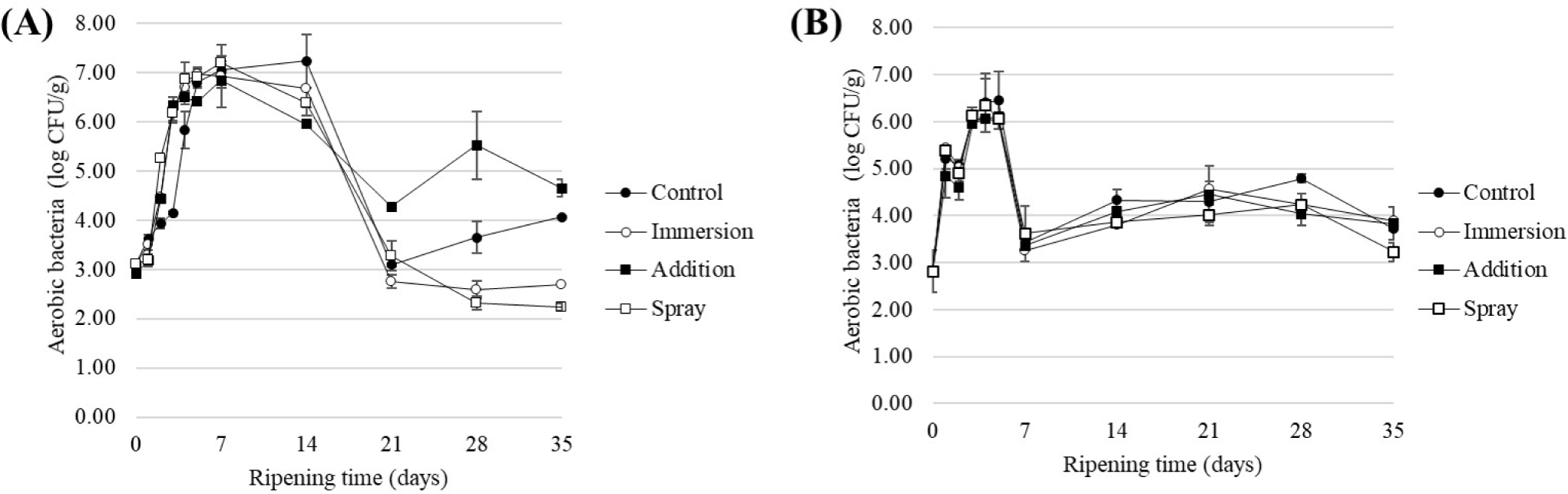
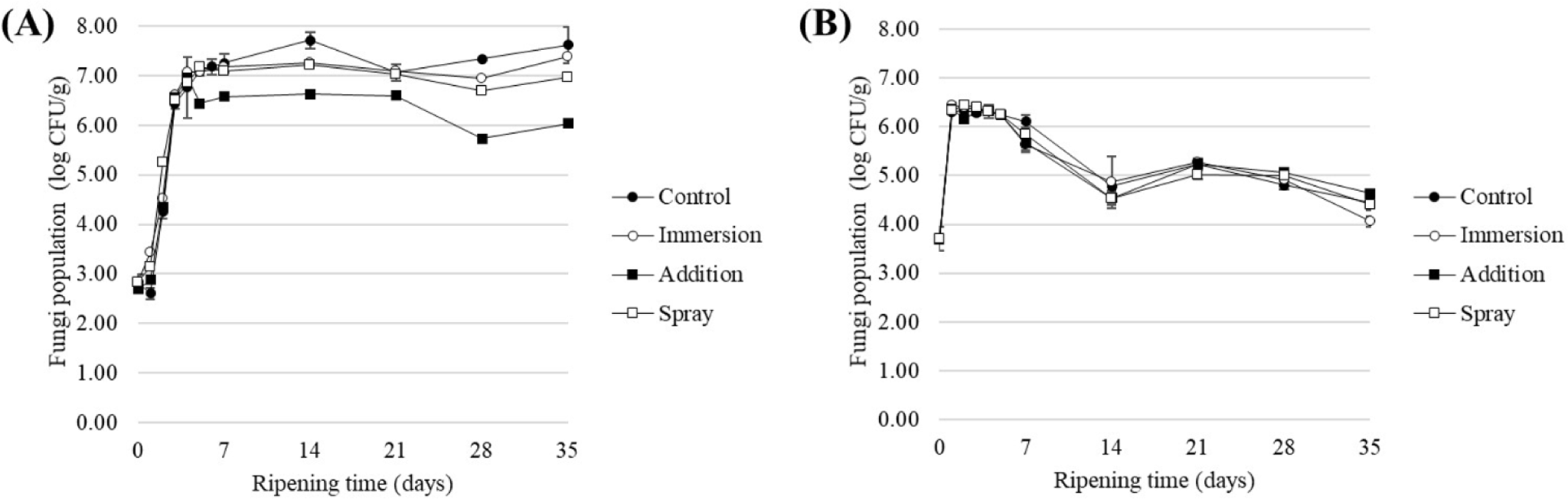
Sausage B also exhibited significant differences among the treatment groups, similar to A (Figs. 4B and 5B). Microbial levels initially rose to 6–7 Log CFU/g and then gradually decreased during ripening, with aerobic bacteria reaching 3–4 Log CFU/g and fungi decreasing to 4–5 Log CFU/g. On day 35, there were no significant differences among the treatment groups for aerobic bacteria (p>0.05), but significant differences were observed among the treatment groups for fungi, with a reduction of 60.19% compared to the control group with immersion treatment (p<0.05). Across both sausages, regardless of the production method, spraying clove extract on the surface after casing filling was effective in inhibiting aerobic bacteria in fermented sausages, whereas immersion was only effective in controlling fungi. Moreover, both types of sausages exhibited an initial increase in microbial populations, followed by a decrease in the final product. This trend, consistent with sausages produced using French, Spanish, and Italian methods, indicates that the decreasing strains during this phase are identified as spoilage bacteria, such as Pseudomonas and Enterobacteriaceae (Lebert et al., 2007). Shen et al. (2019) demonstrated that spraying methods are more efficient and suitable for large-scale producers in reducing Campylobacter jejuni on chicken than immersion. Shen et al. (2019) further indicated that immersion methods not only consume more water but also more money. For fermented sausage manufacturers, especially those operating on a small scale, individual immersion is impractical and resource-intensive due to the high consumption of ethanol and other resources, making it economically inefficient. This summary was incorporated into the discussion section.
During the drying period, the reduction of Escherichia coli in sausages after treatment with cinnamon oil was close to the results of the present study, with a decrease of 0.7 Log CFU/g compared to that in the control group (Ji et al., 2022). Compared to the single treatment with chitosan (>1.5 Log CFU/g), the additional effect of the composite treatment (about 2.0 Log CFU/g) was less than 1 Log CFU/g (Lekjing, 2016). However, even slight differences could be attributed to the presence of eugenol components in cloves. When comparing the antimicrobial effects of different essential oils in these aforementioned studies, similar trends were observed. However, their antimicrobial activity has not shown dramatic results at the laboratory scale. Barberis et al. (2018) mentioned that when applied directly to complex food matrices, active natural compounds may experience restricted availability because of their potential to bind to other hydrophobic compounds, such as certain proteins and lipids. Therefore, further research is required to investigate the synergistic effects between essential oils and other natural substances.
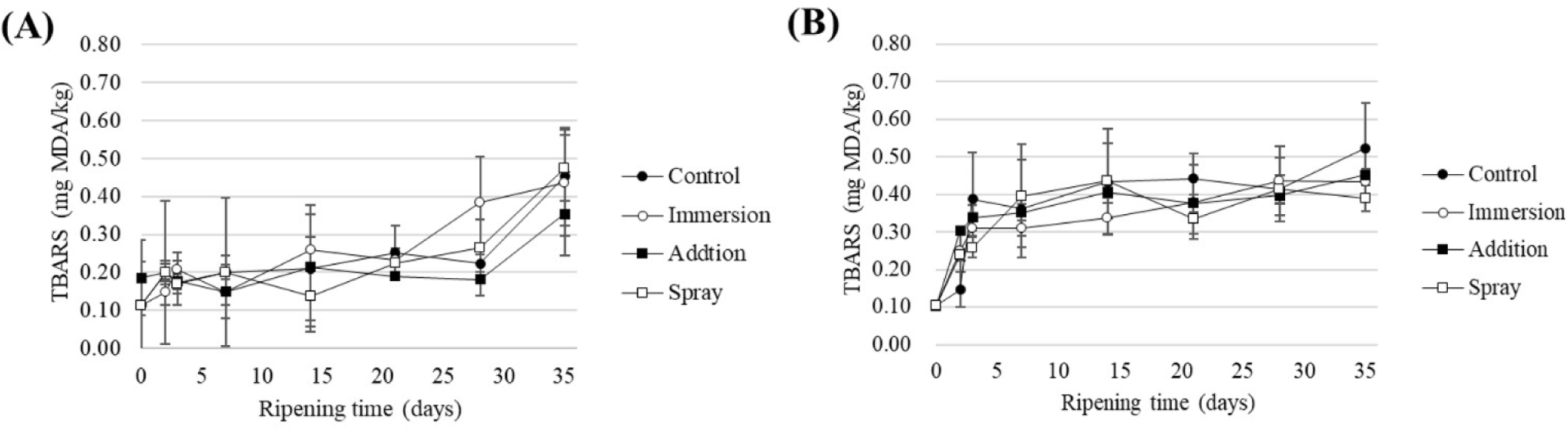
TBARS analysis was conducted to assess the lipid oxidation levels over the production period of fermented sausages, which are exposed to air for a long duration and have a high fat content. The TBARS values showed an increasing trend up to day 35, with no significant differences observed among all treatment groups in the final product (Fig. 6). Particularly, TBARS values in all treatment groups remained below 0.5 mg MDA/kg throughout the entire production period, similar to the untreated groups. The TBARS value of pork sausages reported by Wenjiao et al. (2014) ranged from 0.5–1.0 for about 35 d at 5°C–35°C. Variations in the initial MDA content of pork sausages may arise from differences in seasoning, fat composition, processing methods, and the specific pork muscle used in each sausage type (Wenjiao et al., 2014). Additionally, previous research by de Oliveira et al. (2012) suggested that TBARS values ranging from 0.3–1.0 are associated with the development of flavor in pork and beef, owing to lipid oxidation. These values may not always function as strict thresholds for off-flavors, as the utilization of essential oils, in addition to slowing down lipid oxidation, can also conceal initial off-flavor indicators (Jokanović et al., 2020). Moreover, Connell (1990) suggested that the limit of TBARS is 2.0 mg MDA/kg. In this study, since all treatment values were below 2.0 mg MDA/kg, it is anticipated that utilizing cloves in sausage production, following the methods presented in this study, will not have a significant impact.
The results of pH and water activity during the production period according to the clove treatment methods (control, immersion, addition, and spray) are presented in Figs. 7 and 8. The pH initially started at an average of 5.46 but decreased to 4.83 after 5 wk. This trend closely resembled the final pH of commercial fermented meat products, which are typically in the range of 4.8–4.9 (Klingberg et al., 2005). During fermentation, microbial proliferation increased to 6–7 Log CFU/g by the 7th day but reduced to 2–3 Log CFU/g during maturation, likely due to decreases in pH and water activity. Also, the addition of salt increases osmotic pressure, making it difficult for microorganisms to survive. The decreasing pH during fermentation creates an unfavorable environment for microorganisms (Lücke, 2000). Additionally, during aging, consistently low temperature and low water activity further restrict microbial growth (Lücke, 2000; Talon et al., 2007). Additionally, no significant difference was observed between the treatment groups prepared with clove extract and clove powder compared to the control (p>0.05). This result was consistent for both fermented sausage A, which used fungi as a starter, and fermented sausage B, which used lactic acid bacteria as a starter. Based on the pH analysis, all treatment groups performed statistically similar to the existing products, indicating that the utilization of clove during production has no impact on product quality.
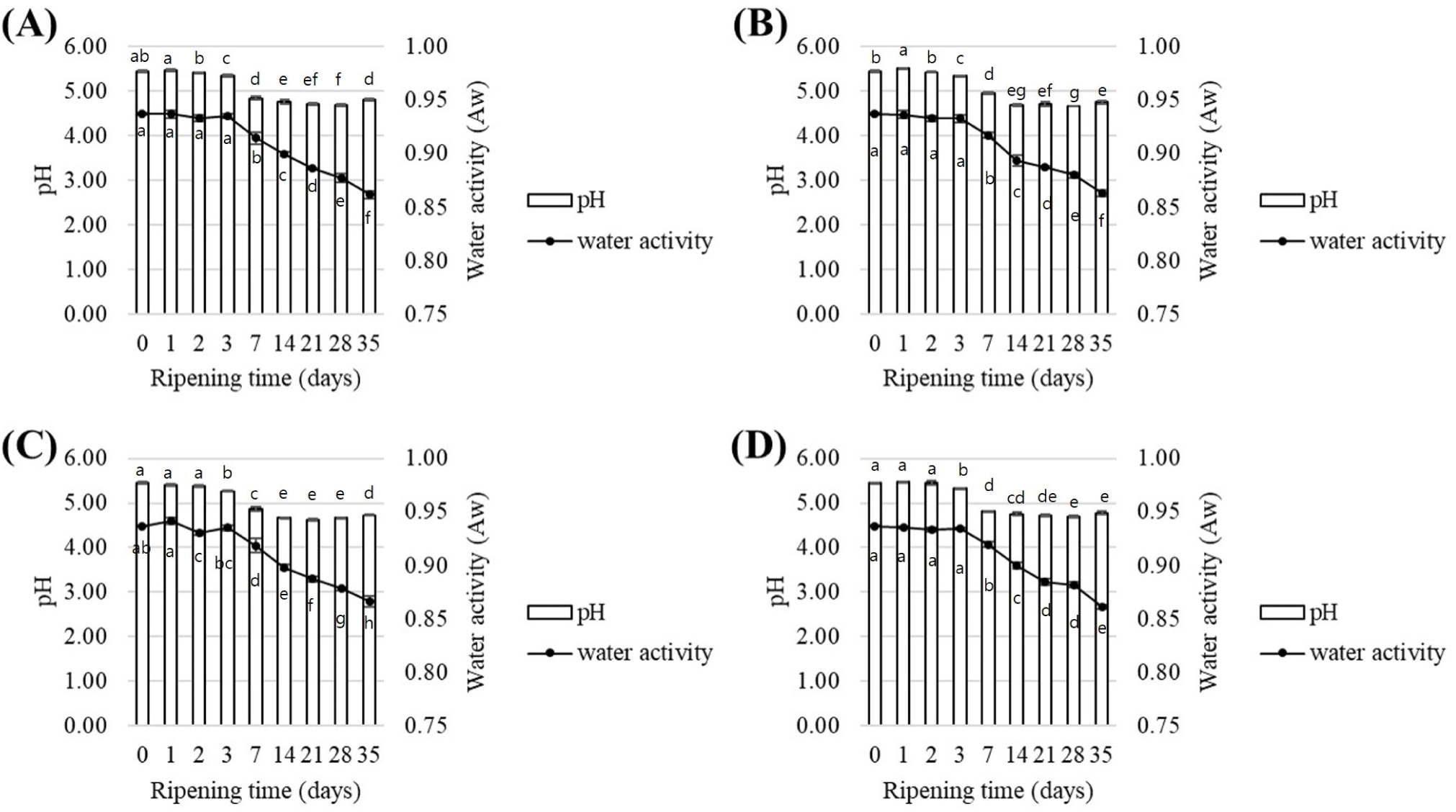
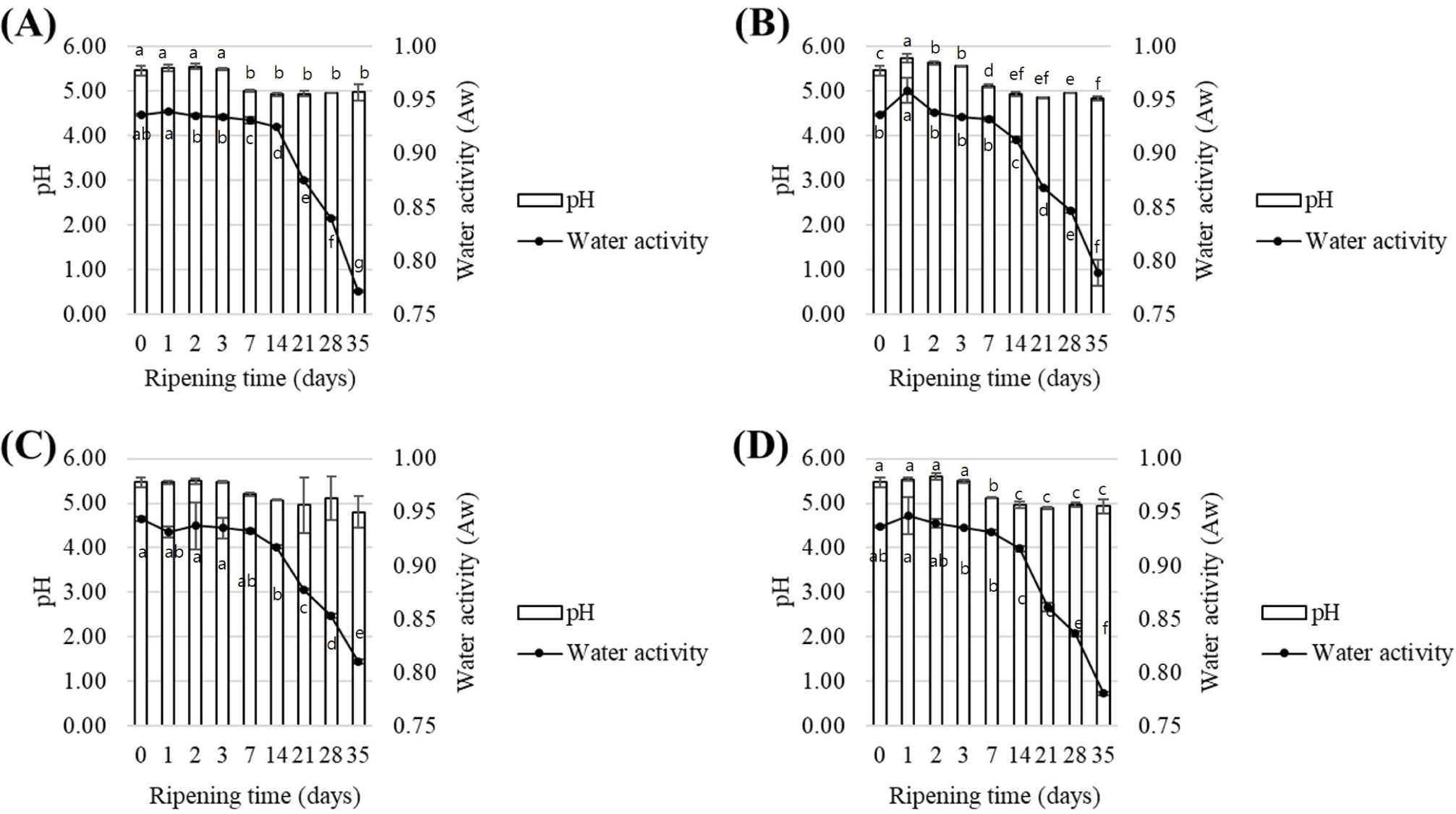
The average initial water activity of the fermented sausages produced using methods A and B was 0.94. However, after 5 wk, it decreased to 0.86 and 0.79, respectively. A decrease in water activity in the range of 0.80–0.84 has been found to inhibit the growth of Cladosporium spp. (Alía et al., 2016). Therefore, it was inferred that the reduction in water activity in the final product derived from this analysis could control the black spot spoilage on the surface during fermentation. Regardless of the clove treatment method, no significant difference in water activity was observed among sausages produced using the same production method (p>0.05), suggesting that clove use during production may not affect water activity, similar to the pH results.
Color analysis revealed significant differences between the sausages treated with cloves and the control group. The color measurements of the sausage surface were recorded as 64.4 (CIE L*), 11.4 (CIE a*), and 12.2 (CIE b*), whereas those of the clove-added group were 53.4, 10.4, and 13.4 (data not shown). The visually dark appearance of clove-supplemented sausages, coupled with statistically significant differences in CIE L* (p<0.05), indicates that the color may not be suitable for production, turning dark brown instead of the desired reddish hue. Similar to the findings of Zhang et al. (2017), the addition of clove extract to sausages resulted in a significant decrease in CIE b* over time. Furthermore, CIE L* and CIE a* also decreased during ripening, consistent with the results reported by Rohlík et al. (2010). Additionally, the reduction in CIE L* and CIE b* after adding clove powder is likely due to its dark brown color, high polyphenol content, and oxidizing enzymes, which can cause enzymatic browning. This leads to an increase in the CIE a* value and a further decrease in the CIE L* and CIE b* values (Aljobair, 2022).
Conclusion
This study assessed the antifungal effects of essential oils from clove, marjoram, and black pepper on mold contamination in fermented sausages, particularly for small-scale farm applications. The findings revealed that clove essential oil, primarily due to its eugenol content, exhibited the strongest antifungal activity by disrupting fungal cell walls and membranes, thereby inhibiting growth. Our results also demonstrated that the application method significantly influences the antimicrobial efficacy of clove extract. Among the tested methods—addition, spraying, and immersion—spraying proved the most effective and economically feasible for small-scale producers. This method not only inhibited fungal growth but also controlled aerobic bacterial contamination without adversely affecting the pH, water activity, or lipid oxidation of the final product.
In conclusion, clove essential oil offers a promising natural alternative for enhancing the microbiological safety of fermented sausages in small-scale production. Adopting clove extract could reduce the reliance on chemical preservatives and heat treatments, aligning with consumer preferences for natural and minimally processed foods. Future studies should aim to refine these findings and explore the synergistic effects of combining clove essential oil with other natural preservatives to further improve the safety and quality of fermented meat products.