Introduction
Today, with the development of human culture and economic resources, food has undergone qualitative improvement as well as quantitative increase, and consumers’ selection criteria have come to place as much emphasis on external appearance as on internal quality. Among external factors, color influences consumers’ perception of food preference and acceptability (Bittencourt et al., 2005). Colorants are a class of food additives used to impart or restore color to food. Annatto extract, a natural coloring agent that imparts yellow to red hues to food, is extracted from the hull of annatto (Bixa orellana L.) found mainly in Central and South America and East Africa (Scotter et al., 1994).
Bixin (methyl hydrogen 9′-cis-6,6′-diapocarotene-6,6′-dioate, C25H30O4) and norbixin (9′-cis-6,6’-diapocarotene-6,6’-dioate, C24H28O4) are the main pigments in annatto (Rahmalia and Naselia, 2021). Both compounds are apocarotenoids. Cis-bixin accounts for more than 80% of the total carotenoid content, while trans-bixin and norbixin account for <5% of the total pigment content (EFSA ANS Panel, 2016). Cis-bixin is soluble in most organic solvents and can be transformed by alkaline hydrolysis into the water-soluble analogue, cis-norbixin, in the form of potassium or sodium salts (FAO, 2006).
Annatto is permitted as a food colorant in a wide range of food products (Lancaster and Lawrence, 1996; Scotter, 1995; Scotter et al., 2002), including high-fat dairy products, such as butter and cheese, as well as cereals, snack foods, condiments, creamers, ice cream, flour, sugar confectionery, soft drinks, fish, teas, coffee, kimchi, vinegar, and processed spices (limited to products containing red pepper or red pepper powder; MFDS, 2023a; MFDS, 2023b). A market survey of the types of foods that use annatto among foods sold in large discount stores and the Internet in 2022 found that it is mainly used for coloring cheese, cheese sauce, meat processed products (ham), and processed milk drinks.
The acceptable daily intake (ADI) of annatto extracts is based on the amount of bixin and norbixin, not the total amount of pigment (JECFA, 1982). In 1974, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) established a provisional ADI of 1.25 mg/kg body weight (b.w.) for the sum of bixin and norbixin. However, in 2006, it was agreed to establish different ADI values for bixin- and norbixin-containing annatto extracts. Bixin was assigned a higher ADI than norbixin and its potassium and sodium salts (12 vs. 0.6 mg/kg b.w; EFSA FAF Panel, 2019). Meanwhile, in 2016, in response to a review of the toxicity data, the European Food Safety Authority Additives and Nutrient Sources (EFSA ANS) panel established ADI values for bixin and norbixin of 6 and 0.3 mg/kg b.w., respectively (EFSA ANS Panel, 2016).
For the determination of annatto, in particular, bixin and norbixin, several detection techniques, such as spectrophotometry, ultraviolet-visible spectroscopy (UV-VIS; Bareth et al., 2002), liquid chromatography-mass spectrometry (LC-MS; Scotter, 2009), nuclear magnetic resonance spectroscopy (Scotter, 2009), time-of-flight secondary ion mass spectrometry (TOF-SIMS; Bittencourt et al., 2005), and ultra-performance liquid chromatography (UPLC) UV/MS (van Scheppingen et al., 2012) were evaluated. However, this study aimed to simultaneously analyze bixin and norbixin using high-performance liquid chromatography-diode array detector (HPLC-DAD), a relatively simple and commonly available analytical instrument. In addition, DAD provides both sensitivity and specificity, as well as real-time qualitative (spectral) confirmation, enabling robust isomer identification and measurement (Scotter, 2009).
Therefore, this study verified the method by performing specificity, linearity, detection limit, quantitative limit, precision, accuracy, and inter-laboratory cross-validation. Furthermore, to demonstrate its feasibility, the method was applied to 122 annatto-containing products sold in Korea. In addition, in order to quantify the reliability of the results, this study performed mathematical processing and statistical methods to estimate the measurement uncertainty that occurs during bixin and norbixin analysis.
Materials and Methods
To verify the applicability of the established test method, various types of cheese (sample numbers: 52), ham (20), ice cream (15), cheese-flavored snacks (15), processed milk (10), and cheese sauce (10) were purchased among the foods distributed in Korea. Monitoring was conducted by applying a pretreatment method suitable for the food type. All samples were frozen at –18°C after homogenization, and three repeated preparations were performed. The results were expressed as detection range, total average content, and positive average content.
The standards of bixin and norbixin (97.0% and 91.1% purity) were purchased from FUJIFILM Wako Pure Chemical Industries (Osaka, Japan). Acetic acid (Sigma-Aldrich, St. Louis, MO, USA) was applied in HPLC mobile phase. HPLC-grade water, methanol, and acetonitrile were used as solvents for extraction or mobile phase and were purchased from JT Baker (Phillipsburg, NJ, USA).
Each 10 mg of bixin and norbixin standards was precisely placed in a 100 mL volumetric flask and then dissolved in a 100% methanol solution to obtain 100 mg/L. The stock solution was refrigerated and applied in the experiment. Standard solutions were adjusted at concentrations of 0.2, 0.5, 2, 5, 10, and 25 mg/L by serially diluting the standard stock solution with 100% methanol.
Sample preparation was conducted in accordance with the previous publication method with some modifications, as described herein (Lee et al., 2021). In the case of a beverage, 3 g of the sample was accurately weighed in a 50 mL conical tube, 6 mL of methanol was added, and then vigorously stirred for 1 min using a vortex mixer. This solution was centrifuged at 1,970×g for 10 min with a centrifuge (FrontierTM 5000, OHAUS, Parsippany, NJ, USA), and the supernatant was filtered through a 0.45 μm syringe filter (Sartorius Minisart® RC, Sartorius, Gottingen, Germany). The supernatant filtrate was used as a HPLC test solution.
In the case of a solid sample, 2 g of the homogenized sample was accurately weighed in a 50 mL conical tube, and 10 mL of a solution of distilled water (pH 4.6): acetonitrile (1:10, v/v) was added. Samples containing fat components, such as meat products, were ultrasonically pulverized at 40°C or less for 5 min after adding 200 mg of ascorbyl palmitate. Afterward, 40 mL hexane was added to remove fat, and the mixture was vigorously stirred for 1 min using a vortex mixer. After centrifuging this solution at 1,970×g for 5 min, the hexane layer of supernatant was discarded, and the pH 4.6 distilled water acetonitrile solution layer was placed in a concentrating flask. The above process was repeated until no pigment appeared in the pH 4.6 distilled water acetonitrile solution layer. Then, after concentrating using a rotary evaporator, methanol was added to make 5 mL, which was filtered through a 0.45 μm syringe filter and used as a HPLC test solution.
Bixin and norbixin were analyzed using an Agilent Technologies 1200 series HPLC apparatus (Agilent, Santa Clara, CA, USA) equipped with a DAD. The analytes were separated at 495 nm on an Agilent XDB C18 column (5 μm, 4.6 mm×150 mm; Agilent) set at 35°C. The mobile phase composed of 2% aqueous acetic acid in water:methanol (15:85, v/v), and the flow rate and injection volume were 1.0 mL/min and 10 μL, respectively (MFDS and NIFDS, 2014).
In this study, specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy were calculated by referring to the ICH guideline (ICH, 2005). Based on this, the validity of the analysis method was verified. After preparing a standard solution by diluting the standard stock solution, a calibration curve was prepared from the area of the peak for each concentration collected by repeated analysis seven times using HPLC-DAD, and the linearity was evaluated by obtaining a coefficient of determination (r2). Accuracy, precision, LOD, and LOQ were then measured.
To calculate the LOD and LOQ, the three lowest concentrations of the calibration curve were selected and analyzed three times to create a calibration curve. The SD (σ) of the intercept values, the y-intercept of the calibration curve, and the average of the slope values (S) were used in the following Eqs. (1) and (2) to derive the LOD and LOQ:
Accuracy means the degree to which the measured concentration of the bixin and norbixin is adjacent to the true value, and this was evaluated through a recovery rate experiment. Precision represents the degree of closeness between each measurement value when a sample of the same concentration is repeatedly experimented and is expressed as a relative SD (RSD) for the repeated measurement results. For the accuracy test, bixin and norbixin standards were spiked at low, medium, and high concentrations (2, 5, and 10 mg/L) in cheese, processed milk, and ham samples that did not contain bixin and norbixin, respectively. For intra-day precision, three concentrations (2, 5, and 10 mg/L) were repeated six times within a day and for inter-day precision, the same centration were analyzed three times for three days.
Inter-laboratory reproducibility validation of accuracy and precision was performed by comparing the analytical results of the same sample using the same analytical method in three different laboratories (Lab A, Lab B, and Lab C). Cheese was used as a sample and recovery experiments were performed by adding 2, 5, and 10 mg/L of bixin and norbixin standards. Then, the accuracy and precision were calculated to confirm the recovery rate (%) and RSD%. The experiment was repeated three times.
Measurement uncertainty is defined in CODEX (CODEX Alimentarius Commission, 2008) as “a value related to a measurement result representing the distribution of values attributed to a measurand, which indicates the dispersion characteristics of a value reasonably estimated for a measurement result.” This study calculated measurement uncertainty based on the methods shown in the EURACHEM guide and GUM (guide to the expression of uncertainty in measurement). Measurement uncertainties related to standard solution dilution (uSSS), sample pretreatment (uSP), calibration curve preparation (uCal), and sample repeated measurements (uRP), which may occur during the analysis of bixin and norbixin, were estimated. These error factors were estimated and calculated with an expanded uncertainty (Uc) using a factor (k) of 2 at the 95% level (Ellison and Williams, 2012; JCGM, 2008).
Results and Discussion
The HPLC chromatograms of the blank, standard solution (bixin and norbixin, 10 mg/L), and samples are shown in Fig. 1. Specificity was verified by confirming that there were no substances interfering with the peak retention times of bixin and norbixin in the collected samples. The retention times of bixin and norbixin were 10.6 and 6.0 min, respectively. Both bixin and norbixin were analyzed seven times after diluting the standard solution to six concentrations between 0.2 and 25 mg/L to prepare a calibration curve. As a result of the measurement, the average coefficient of determination (r2) was 0.9999, representing good linearity, and the results are shown in Table 1. This result satisfied the minimum standard of 0.995 of the US Food and Drug Administration (FDA; US FDA, 2014). The LOD obtained through the calibration curve obtained by repeating the three low concentrations three times was 0.03 mg/L for bixin and 0.02 mg/L for norbixin, and the LOQ was 0.11 mg/L for bixin and 0.05 mg/L for norbixin. These results were similar or better to previous researches that calculated LOD and LOQ values of 0.05 0.6 and 0.16 0.7 mg/L for bixin and 0.03 0.6 and 0.10 0.7 mg/L for norbixin, respectively (Bareth et al., 2002; Chisté et al., 2011; Lee et al., 2021; Noppe et al., 2009).
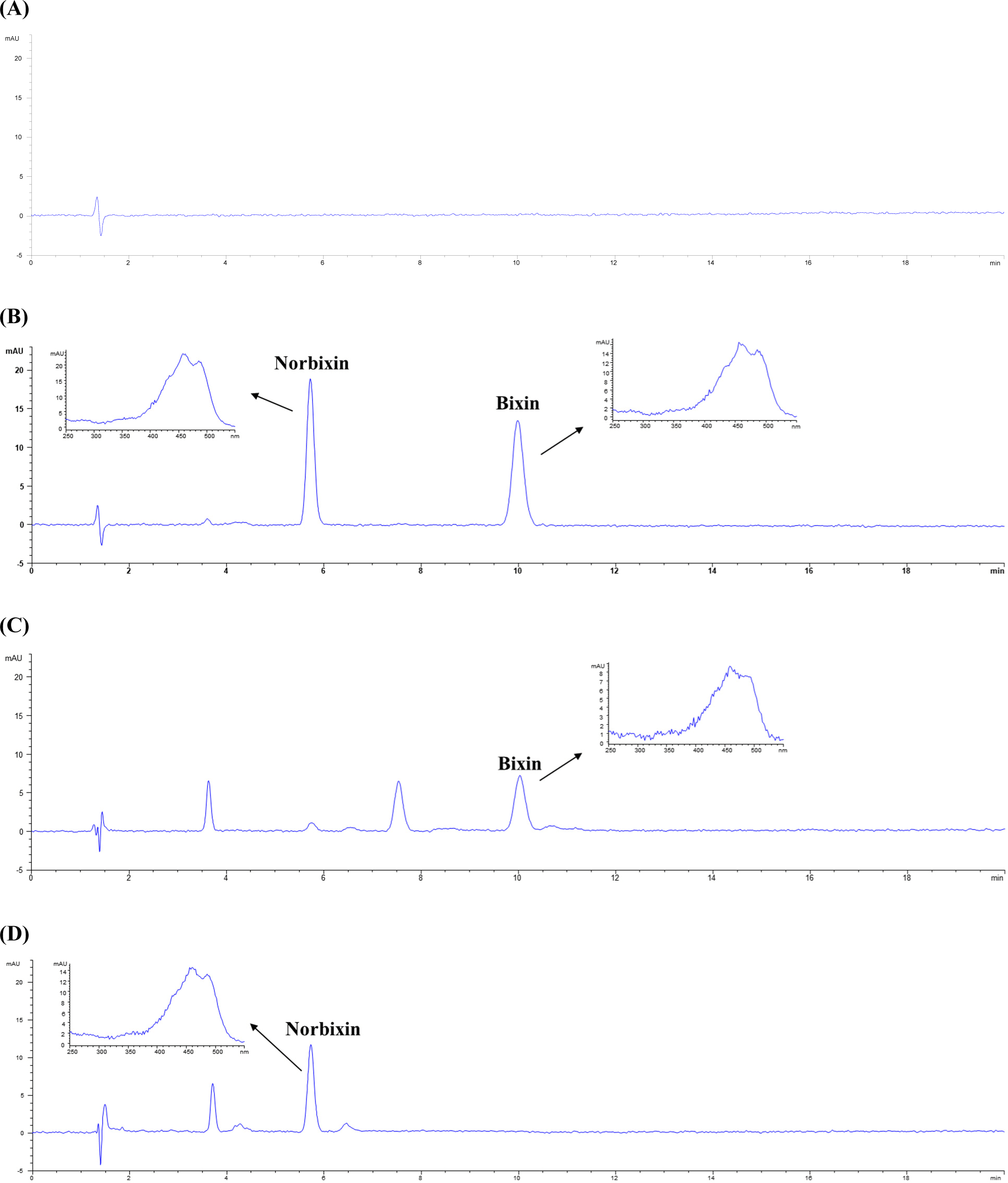
The intra-day and inter-day accuracy and precision of bixin and norbixin are shown in Tables 2 and 3. In order to evaluate these, the accuracy, as the recovery rate (%) test results obtained by spiking 2, 5, and 10 mg/L, was 87.99% 96.62% (intra-day) and 89.15% 96.99% (inter-day) for bixin, and 88.16% 105.07% (intra-day) and 91.23% 105.81% (inter-day) for norbixin. Intra-day RSD% for bixin and norbixin were 0.21% 1.53% and 0.30% 1.79%, respectively. Inter-day RSD% for bixin and norbixin were 0.52% 2.59%, and 0.75% 2.69%, respectively. These results were in accordance with the AOAC validation guidelines (AOAC, 2016).
Moreover, the Horwitz ratio (HorRat, r) values were 0.04 0.24 (intra-day) and 0.09 0.36 (inter-day) for bixin and 0.05 0.26 (intra-day) and 0.12 0.41 (inter-day) for norbixin (Horwitz and Albert, 2006). These results could be compared to previous publications. According to Bareth et al. (2002), the recovery rate of spiking 10 mg/L in cheese was 91.0% 99.7% with an RSD of 1.2% 2.4%. Additionally, in the conducted study of Scotter et al. (2002), the recovery rate of spiking 3 mg/L in cheese was 90% with an RSD of 2.6%, and the recovery rate of spiking 1.7 mg/L in yogurt was 92%, with an RSD of 4.8%.
Thus, when comparing the test method established in this study with the test method of other studies, it was confirmed that the average recovery rate values were similar, and the LOD and LOQ values were similar or better. As a result, it was possible to prove that the established method is suitable for the determination of bixin and norbixin in various samples with the acceptable repeatability and reproducibility.
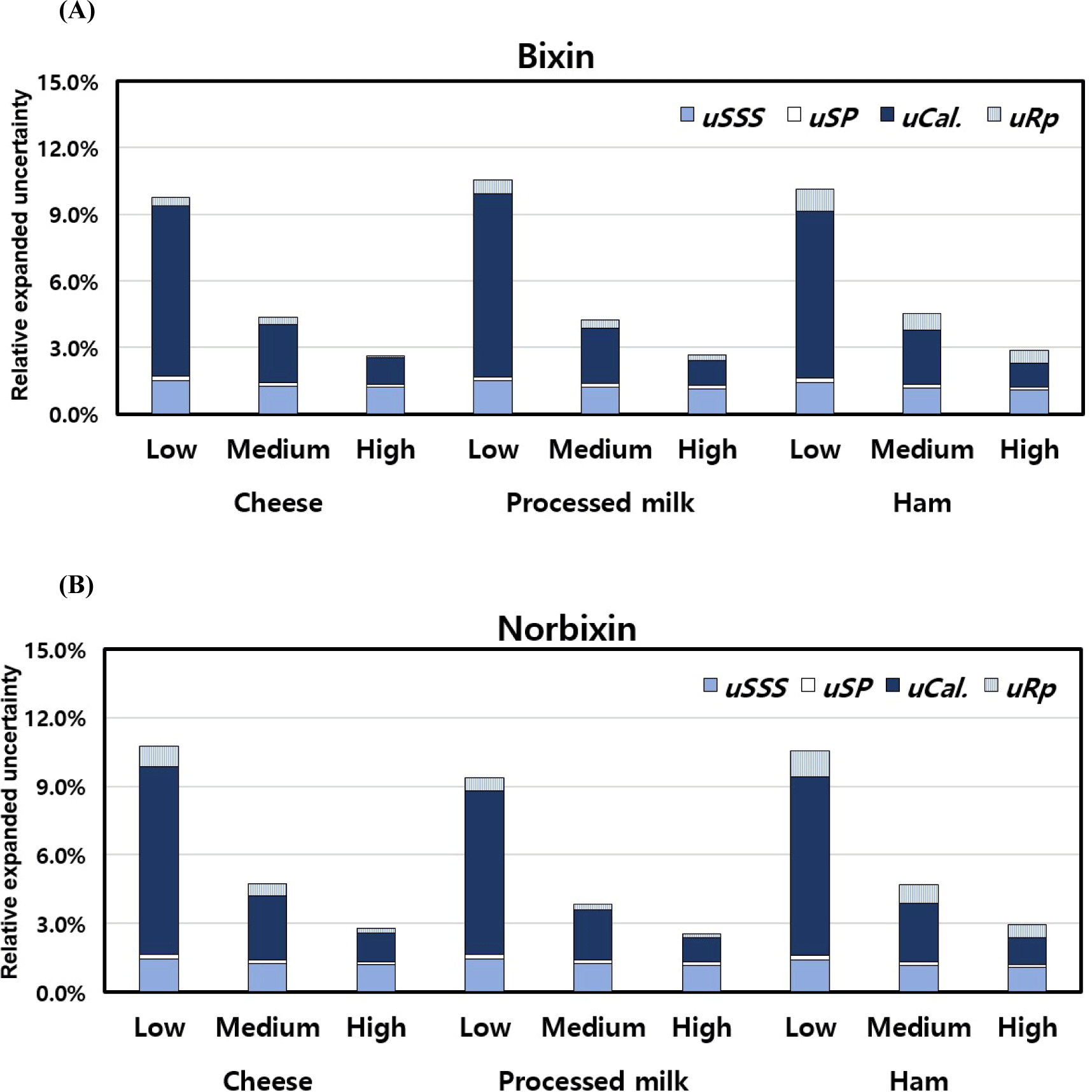
In this study, the measurement uncertainty of cheese, processed milk, and ham was evaluated by adding 2, 5, and 10 mg/L of bixin and norbixin standard products at low, medium, and high concentrations, respectively, prior to HPLC analysis. The estimation procedure was performed considering the uncertainty factors related to the analysis of bixin and norbixin, such as uSSS, uSP, uCal, and uRP. As shown in Tables 2 and 3, the Uc was 2.6% 10.7% for cheese, 2.5% 10.5% for processed milk, and 2.9% 10.6% for ham compared to the analytical results. The results were complied with the CODEX criteria (<22%; CODEX Alimentarius Commission, 2008). The contribution of each factor to the expanded uncertainty of the result is shown in Fig. 2. Even though there weren’t any noteworthy differences in uRP, uSP, and uSSS calculated for each spiking concentration of every sample, a marked escalation in the uCal uncertainty linked to the calibration curve’s preparation was noted as the concentrations of added bixin and norbixin diminished. Therefore, the researcher requires to enhance their proficiency skill for preparing the minimum concentration calibration curve for the sample analysis.
Recovery tests (accuracy) were conducted in three laboratories on cheese samples containing bixin and norbixin, and the results were compared. The results were expressed as recovery rate (%) ±SD (%), and the average and precision (%RSD) of each laboratory result are shown in Table 4. For bixin, recovery rate (%) ranged from 92.97%–95.47% in Lab A, 96.26%–97.99% in Lab B, and 102.52%–103.39% in Lab C. Regarding norbixin, it ranged from 93.79%–95.62% in Lab A, 94.00%–96.84% in Lab B, and 100.27%–101.53% in Lab C. In addition, RSD% was 2.85%–4.18% for bixin and 3.81%–4.51% for norbixin, which satisfied the reproducibility range, verifying the accuracy and precision of the proposed analysis method. All these results satisfied the AOAC guidelines (AOAC, 2016).
Although it is difficult to estimate the content of annatto extracts and water-soluble annatto specified in commercially available products, the content of the main pigments (bixin and norbixin) can be measured and calculated. A total of 122 products were selected and quantitatively analyzed as items with a high frequency of use among distributed foods specified to have an annatto extract and water-soluble annatto added. Bixin and norbixin were randomly detected in distributed foods labeled as containing “annatto extract,” whereas only norbixin, not bixin, was detected in distributed foods labeled as containing “water-soluble annatto.” Analysis was conducted by repeating the experiment three times for one sample, and various livestock products distributed in Korea, such as cheese, processed milk, and ham, were analyzed. The results are presented in Table 5.
As a result of the quantitative analysis conducted for the content of bixin and norbixin in a total of 122 foods from seven food categories distributed in Korea, bixin was detected in 30 samples with a rate of 25%, and norbixin was detected in 107 samples with a rate of 88%. In the case of bixin, ice cream showed the highest detection rate (73%), but cheese sauce showed the highest detection rate (7.54 mg/kg) in positive average content. Norbixin was detected in all types of natural cheese, ham, and processed milk (100%) among the collected samples, showing the highest detection rate, and the highest average content of detection was 2.77 mg/kg in natural cheese.
These results were similar to the previous publications. For example, in a research by Bareth et al. (2002), monitoring for cheese types showed “not detected” (ND, <0.15 mg/kg) for bixin and ND 11.89 mg/kg for norbixin. In addition, in a report of Scotter et al. (2002), monitoring for flavored processed cheese showed ND (<0.01 mg/kg) 0.4 mg/kg for bixin and 0.1 9.1 mg/kg for norbixin; in edible ices, ND for bixin and 0.5 8.3 mg/kg for norbixin; and in cheese-flavored snacks, 3.2 3.4 mg/kg for bixin and 0.5 1.1 mg/kg for norbixin.
Therefore, it was confirmed that the proposed analytical method is suitable for the quantification of bixin and norbixin in various foods. The results also provide risk assessment data for bixin and norbixin as food additives in food products from animal resources.
Conclusion
In this study, bixin and norbixin were analyzed by HPLC-DAD, and the method was verified by specificity, linearity, accuracy, and precision. Accordingly, it was proved that the analysis method complies with the standard validation guidelines. In addition, the reliability of the analysis results was confirmed by evaluating the factors influencing the analysis through measurement uncertainty, and the applicability of the proposed method to quantify bixin and norbixin in various commercial products, such as cheese and ham, was demonstrated. The analysis method employed in this study demonstrated its applicability for expedient and widespread determination of bixin and norbixin in diverse food matrices. Moreover, the analytical outcomes furnished crucial information on the levels of bixin and norbixin present in commercial food products, which can be used for further evaluation of intake and risk assessment.













