Introduction
Cultivated meat technologies are environmentally sustainable and contribute to animal welfare compared with traditional meat production (Post, 2012; Stephens et al., 2018). Compared with traditionally produced beef, sheep and pork, the production of cultivated meat production uses approximately 82%–96% less water use, 78%–96% less greenhouse gas emissions, 7%–45% less energy use and involves 99% less land use (Stephens et al., 2018). Meat contains intramuscular fat (IMF). The IMF plays an important role in food quality, such as sensory properties and health. In general, IMF content has a positive effect on the sensory properties of meat such as flavor, juiciness, and tenderness (Hocquette et al., 2010). Since fat is a flavor compound and affects transient release of flavor (Elmore et al., 2002), increasing the IMF improves flavor (Thompson, 2004). Many cells that differentiate into fat are being studied to obtain IMF for cultivated meat. It is possible to differentiate fat for cultivated meat from various cells, such as dedifferentiated adipocytes, embryonic stem cells, and induced pluripotent stem cells (Fish et al., 2020; Hill et al., 2019; Wei et al., 2013). Among them, intramuscular adipose tissue is mainly derived from a stromal stem cell population known as fibro-adipogenic progenitors (FAPs; Guan et al., 2017; Huang et al., 2012; Loomis et al., 2022). FAPs are differentiated from satellite cells via platelet-derived growth factor receptor A (PDGFRA) expression (Fitzgerald et al., 2023; Joe et al., 2010; Uezumi et al., 2010). FAPs contribute to muscle regeneration under physiological conditions, and to fibrogenesis and adipogenesis during pathology (Heredia et al., 2013).
Citrus fruits contain beneficial phytochemicals (Satari and Karimi, 2018). It contains phenylpropanoids, coumarins, carotenoids, and flavonoids, including polymethoxylated flavones such as nobiletin, tangeretin, naringin, and hesperidin (Kang et al., 2005). Natural extracts derived from citrus fruits are rich in flavonoids and exhibit anti-inflammatory, antioxidant, and anti-cancer activities (Galati et al., 1994; Ko et al., 2010; Lu et al., 2010). Citrus sunki has been used in oriental medicine since ancient times (Kang et al., 2005). Nobiletin and tangeretin, which are unique components of citrus fruits, are particularly abundant in Citrus sunki, and have excellent antioxidant, anti-inflammatory, and anti-obesity effects (Kang et al., 2012; Pang et al., 2023; Shin et al., 2011). These bioactive substances are contained in the peel rather than the fruit pulp and are more physiologically effective (Chung et al., 2000; Wu et al., 2013; Yoshigai et al., 2013). However, one-third of citrus fruit is processed, and the large amount of peel produced during juice processing is generally considered agro-industrial waste (Negro et al., 2016). Incineration and landfilling for the disposal of citrus by-products, which are produced in tons per day, can cause environmental and economic problems (Casquete et al., 2015; Satari and Karimi, 2018). Increasing the utilization of citrus by-products may be a solution to the disposal problem. Few studies have investigated the role of natural products in enhancing the culture and differentiation of FAPs to IMF. Furthermore, the effects of citrus fruits on the differentiation of FAPs into fat have not been reported. The purpose of this study was to investigate the effect of Citrus sunki peel extract (CPE) on proliferation and differentiation of FAPs from Holstein cattle, a popular breed of cattle, in cultivated meat production.
Materials and Methods
The Holstein cattle used in the experiment was approved by the Institutional Animal Care and Use Committees (IACUC) of Chungbuk National University (CBNUA-2257-24-01). The cells were isolated using collagenase type II (600 units/mL DMEM) and centrifugation from Holstein cattle's buttock muscle. The obtained cells were suspended in freezing media (Gibco, Waltham, MA, USA) and stored in liquid nitrogen. For sorting of cells using fluorescence-activated cell sorting (FACS), cells were suspended in FACS buffer [0.1% bovine serum albumin (BSA; Roche, Basel, New Zealand) in PBS]. The cells were then stained with CD29 antibody (APC, 303008, BioLegend, San Diego, CA, USA), and CD56 antibody (PE-CyTM7, 335826, BD Bioscience, Franklin Lakes, NJ, USA). FAPs were sorted into the CD29+/CD56- population using the FACS Aria II Cell Sorter (BD Bioscience).
The Citrus sunki peel was broken into small pieces for extraction, and 50 g was extracted at 70°C for 6 h with 1 L 60% ethanol. It was then filtered through a 0.4 μm filter and concentrated using a rotary vacuum evaporator. The concentrated CPE was lyophilized and stored.
The high-performance liquid chromatography (HPLC) analysis was performed to analyze the nobiletin content of Citrus sunki extract. The sample to be analyzed was 2.4 mg CPE to which 12 mL of ethanol was added and separated at 30°C using a column (150 mm×4.6 mm, 5 μm Zorbax Eclipse XDB-C18; Agilent, Santa Clara, CA, USA). The mobile phase was methanol and distilled water (pH adjusted to 3 using glacial acetic acid). The gradient elution program was as follows: 25%–40% methanol from 0–24 min, 40%–62% methanol from 24–35 min, 62% methanol from 35–44 min, 62%–80% methanol from 44–50 min, and 85%–100% methanol from 50–60 min. The detection wavelength was 330 nm.
FAPs for differentiation were cultured in Matrigel-coated flasks. Cells were seeded at 2,000 cells/cm2 and cultured in Ham's F-10 medium (20% FBS, 1% PSA) for 6 days in an incubator (37°C, 5% CO2). After more than 80% confluence, cells were differentiated in DMEM (11995-065, Gibco) containing 1% FBS, 1% PSA, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; I5879, Sigma-Aldrich, Burlington, MA, USA), 1 μM dexamethasone (D-085, Sigma-Aldrich), and 10 μM insulin (I0516, Sigma-Aldrich) for 3 days. Then, replace the DMEM medium containing 1% FBS, 1% PSA, and 10 μM insulin once every 3 days for a total of 6 days.
Cell counting was measured with a cell counter (Countess®, Invitrogen, Waltham, MA, USA) using trypan blue. The MTS assay was performed to measure cell number via mitochondrial activity. The MTS assay was measured using MTS solution (G3582, Promega, Madison, WI, USA), and absorbance was measured a wavelength at 490 nm following 2 h of incubation at 37°C.
Immunofluorescence staining of FAPs was fixed using 2% paraformaldehyde and permeabilized using 0.1% Triton-X (in PBS). Permeabilized cells were blocked using 2% BSA (Roche) and stained with PDGARA primary antibody and secondary antibody (anti-rabbit IgG cross-labeled antibody; Invitrogen). After antibody staining, nuclei were counterstained using Hoechst 33342 (H3570, Invitrogen) and images were measured. The differentiated FAPs were fixed by incubation with 2% paraformaldehyde for 40 min. Fixed cells were incubated in 60% isopropanol for 5 min and then stained using Oil Red O solution. The Oil Red O solution was prepared using a 2:3 ratio of distilled water:Oil Red O.
RNA extraction was performed on day 6 of differentiation using TRIzol reagent, and Reverse Transcription Master Premix (Elpis-Biotech, Seongnam, Korea) was used to convert the extracted RNA into cDNA. The cDNA conversion was incubated at 60°C for 1 h and 94°C for 5 min. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed with 1 μL of primers and 1 μL of cDNA in a total volume of 20 μL using SYBR Green solution (A46109, Applied BiosystemsTM, Waltham, MA, USA). Table 1 lists the primer sequences used in the RT-qPCR.
Proteins from FAPs were collected using RIPA lysis buffer. The concentration of the obtained protein was measured using the Bradford assay and equilibrated. Proteins were separated by TGX Precast Gels (Bio-Rad Laboratories, Hercules, CA, USA), and the gel was transferred to a PVDF membrane. Affinity Purified Goat Anti-Mouse IgG (H+L) HRP-conjugated antibody was used as the secondary antibody.
Results and Discussion
Cells from Holstein cattle muscle tissue were gated for the CD29+/56- population and these cells represented FAPs (Fig. 1A). To confirm that the cells sorted by FACS were FAPs, fluorescence staining was performed with a PDGFRA antibody (Fig. 1B). Progenitors of the non-myogenic lineage have the potential to become adipogenic (Joe et al., 2010; Wosczyna et al., 2012). PDGFRA is a specific surface marker for these non-myogenic precursor cells (also called FAPs; Uezumi et al., 2010). PDGFRA positivity has establishes the presence of FAPs in various experiments (Guan et al., 2017).
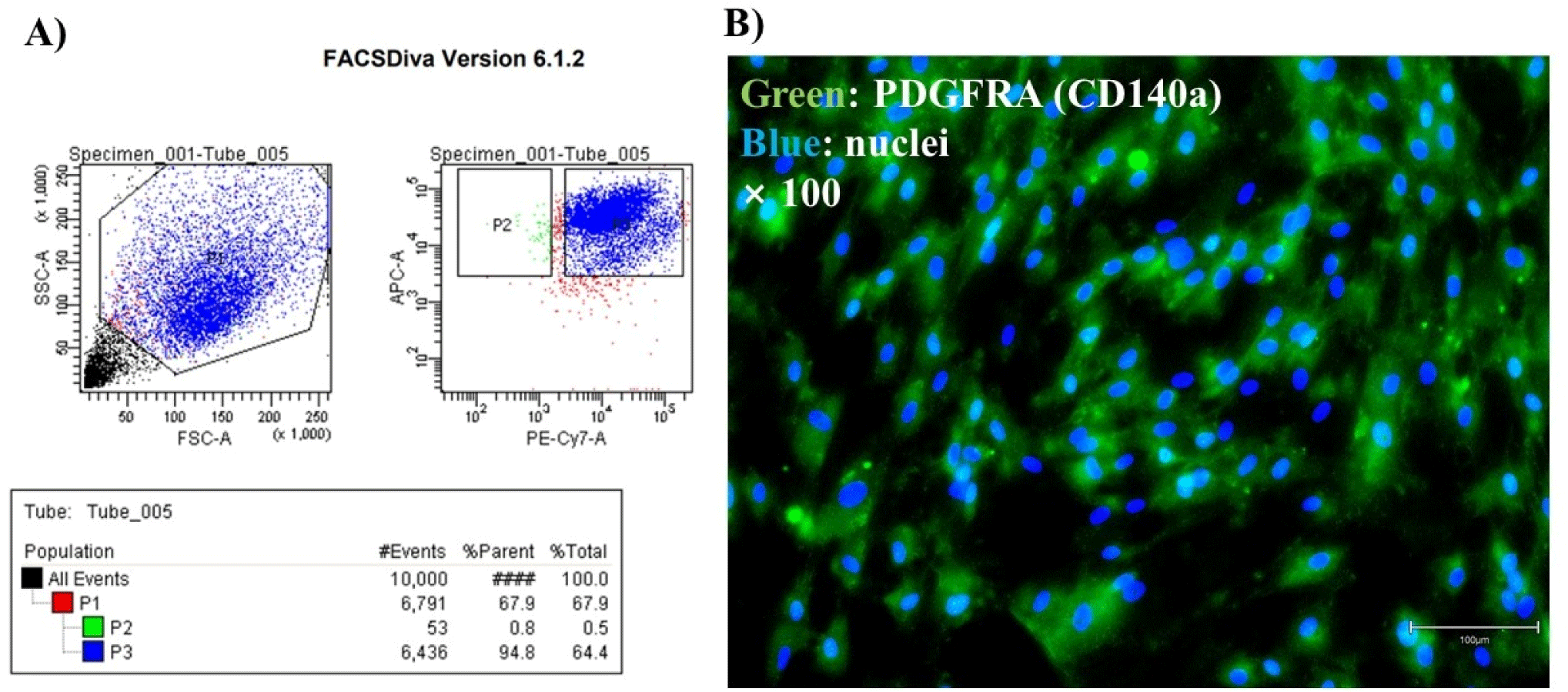
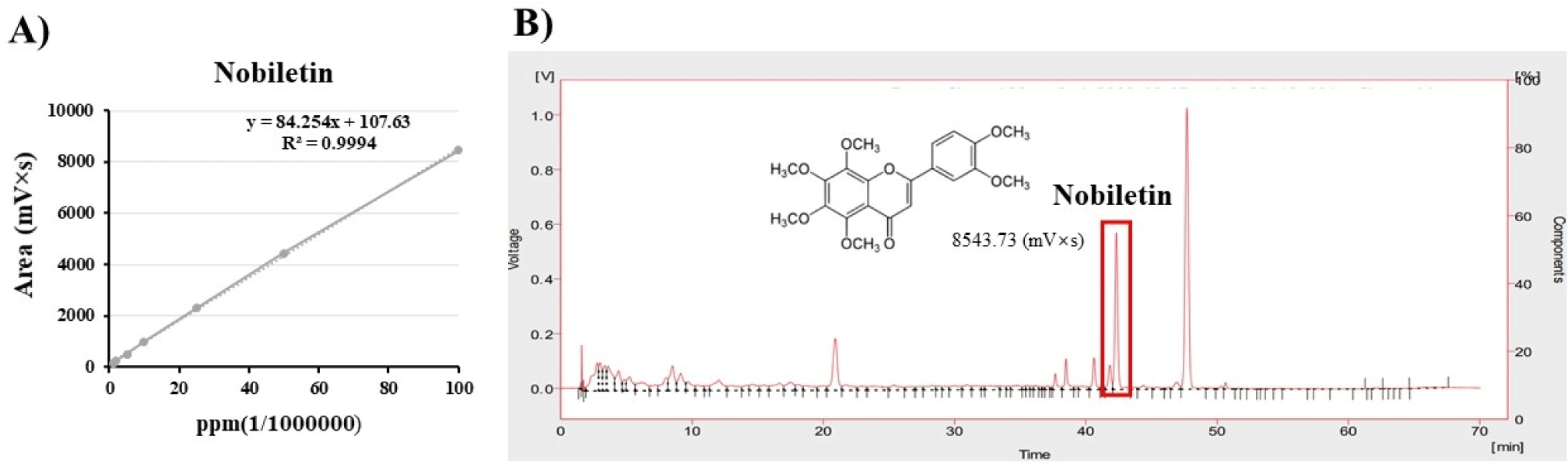
Fig. 2A presents the CPE chromatogram and the nobiletin structure. The marked portion in Fig. 2B represents nobiletin (Li et al., 2021) and the area indicates 8,543.73 mVs. Substituting the standard curve, CPE contains 5,006.35 ppm of nobiletin.
FAPs were cultured for 6 days in each experimental design (C, CDMSO, CPE50, CPE100, CPE200, CPE300, and CPE400). Since the concentration of DMSO in the medium with CPE was 0.05%, it was compared with the medium containing only 0.05% DMSO without CPE (Fig. 3A). The live cell count and viability of FAPs were measured (Figs. 3B and C). The live cell count of FAPs was higher in FAPs with 100 μg/mL CPE compared with control groups (p<0.05). CPE50 and CPE200 were significantly higher than CDMSO (p<0.05). Fig. 3D shows the results of the MTS analysis, where the FAP of CPE50 and 100 were significantly higher than the control group (p<0.05).
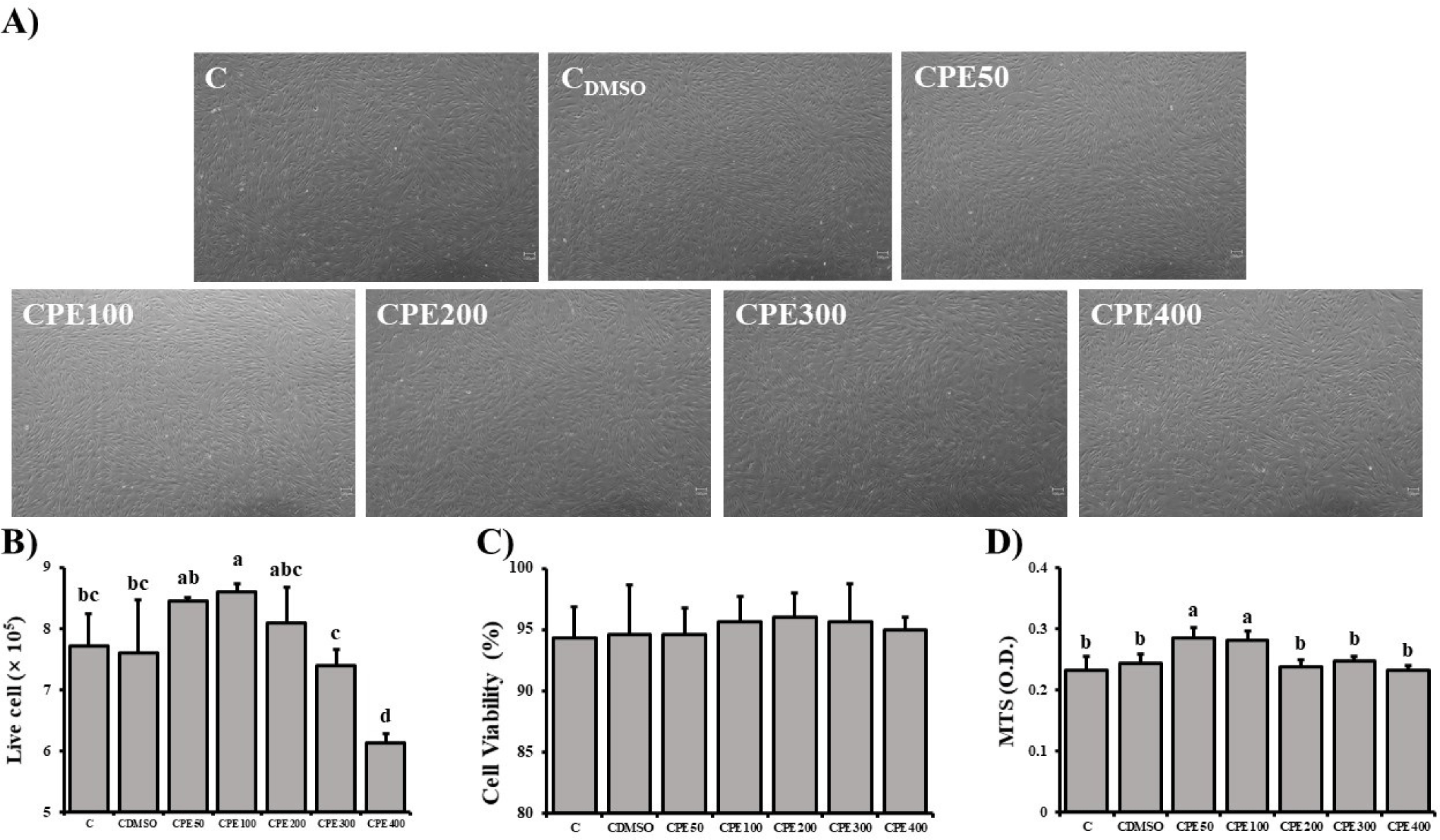
Reactive oxygen species (ROS) contribute to cell death, necrosis, and inhibition of cell proliferation (Matés et al., 2008). Excessive ROS induces cell death through activation of MAPK, AMPK, and BNI, inactivation of ATG4, and mitochondrial damage. Such ROS-induced cell death can be suppressed by antioxidant action (Villalpando-Rodriguez and Gibson, 2021). Flavonoids are antioxidants present in citrus peel and protect cells from free radical damage (Ashraf et al., 2017), and this effect is expected to increase the proliferative capacity of FAPs. Also, because citrus peel is rich in polyphenols, and its antioxidant capacity is higher than that of other edible fruits (Singh et al., 2020). In the study of Ikawati et al. (2019), C. reticulata extract at concentrations as low as 100 μg/mL induced cell proliferation, while higher concentrations decreased cell viability.
Differentiation of FAPs was confirmed by Oil Red O staining (Fig. 4A). RT-qPCR results of gene expression of FASN, CEBPA, CEBPB, and PPARG were compared (Fig. 4B). No significant difference in FASN gene expression was detected in all treatment and control groups. The expression of CEBPA gene was significantly higher in CPE50 and CPE100 compared with control groups (p<0.05). The expression of CEBPB gene was significantly higher in CPE100 and CPE200 than in control groups.
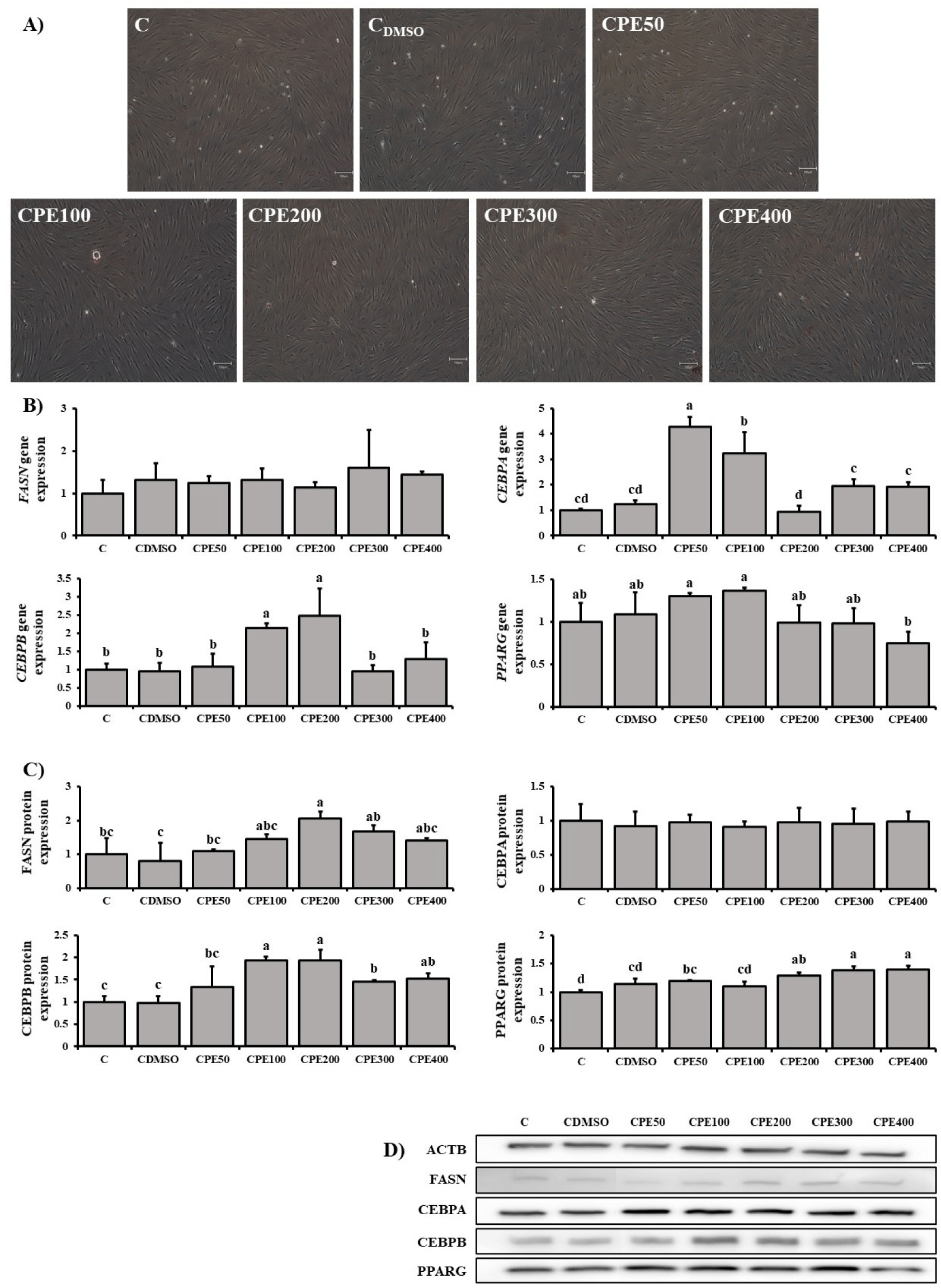
The protein expression of FASN, CEBPA, CEBPB, and PPARG was compared via Western blotting analysis (Fig. 4C).
The expression of FASN protein was significantly higher in CPE200 compared with control groups (p<0.05). The CEBPB protein expression in the other treatment groups varied significantly from that of the control groups (p<0.05), and the difference was the greatest in CPE100 and CPE200. The expression of PPARG protein was significantly higher in CPE300 and CPE400 than in control groups.
The CEBP family members exert a multifaceted influence on the differentiation of preadipocytes, playing a pivotal role in the processes of adipogenesis and differentiation. The transcription factors CEBPB and CEBPG are the initial mediators of differentiation in preadipocytes following exposure to differentiation factors, thereby initiating the differentiation process (Darlington et al., 1998). During the process of adipocyte differentiation, the transcription factors and CEBPD and CEBPB induce the expression of CEBPA (Rosen and MacDougald, 2006). As reported by Lin and Lane (1992), the inhibition of CEBPA expression in preadipocytes resulted in the absence of triacylglycerol accumulation and the lack of expression of fat-specific genes. This evidence supports the assertion that CEBPA is a crucial factor in the differentiation of preadipocytes. CEBPB induces CEBPA and acts as a transcriptional regulator of PPARG, a master regulator of adipogenesis (Hamm et al., 2001; Rosen et al., 2000). Adipogenesis is regulated not only by CEBP but also by PPARG (Gupta et al., 2010; Spiegelman and Flier, 1996). In addition, PPARG is required for adipogenesis and maintenance of differentiation status (Imai et al., 2004). PPARG is also important for adipocyte function, including insulin sensitivity, adipokine secretion, and lipid metabolism (Rangwala and Lazar, 2004). Consequently, two transcription factors in preadipocytes, CEBPA and PPARG, have been characterized as critical regulators of adipogenic differentiation (Lin and Lane, 1994; Tontonoz et al., 1994). The mutual expression of CEBPA and PPARG is enhanced, and these factors promote the differentiation of adipose tissue and increase the accumulation of lipids (Rosen et al., 2002; Tanaka et al., 1997). PPARG plays an important role in IMF in cattle, and the expression of CEBPA and PPARG in skeletal muscle is higher in cattle breeds with a high capacity for IMF deposition (Duarte et al., 2013; Moisá et al., 2014). FASN represents a delayed adipogenic marker and is a pivotal enzyme in the metabolic pathway of fatty acids. (Habinowski and Witters, 2001; Kim and Spiegelman, 1996).
The results of this study showed that CPE positively regulates CEBPA, CEBPB, PPARG, and FANS during the differentiation of FAPs. Another study showed that nobiletin increased the CEBPB expression of 3T3-L1 cells (Saito et al., 2007). The activation of extra cellular signal-regulated kinase and cAMP-responsive element-binding protein enhanced by nobiletin activated CEBPB and PPARG to promote adipogenesis (Saito et al., 2007). In addition to nobiletin, tangeretin makes up a large portion of citrus peel and is a potential insulin sensitizer (Guo et al., 2020). In addition to nobiletin, citrus fruits contain a variety of other flavonoids that enhance and positively affect adipogenesis (Bae et al., 2022; Kuroyanagi et al., 2008; Onda et al., 2013). Therefore, it is thought that CPE may enhance adipogenesis.
Conclusion
In our study, CPE enhanced the proliferation and differentiation of Holstein cattle FAPs and resulted in positive effects. In particular, the addition of 100 μg/mL of CPE was the most effective in terms of proliferation and differentiation induction for efficient cell culture-based meat production.













