Introduction
Cultured meat (CM), an advanced meat technology where animal-derived stem cells are cultured on a large scale in vitro to produce meat products, has attracted attention as a promising alternative meat source. CM arises from adverse points associated with conventional livestock involving greenhouse gas emissions, resource inefficiency, animal welfare, and public health (Post, 2012). The CM industry is expected to have higher eco-friendly impacts than conventional meat from livestock as technology develops (Hong et al., 2021, Sinke et al., 2023). Muscle satellite cells (MSCs), myogenic stem cells located in muscle fibers, serve as the primary cell source for CM production. MSCs progress through a cascade of myogenesis for muscle generation in response to injury (Lepper et al., 2011). MSCs are activated for myogenic progression, involving a cascade of proliferation, differentiation, and fusion to form matured myofibers. A train of process muscle generated is regulated by myogenic transcription factors such as paired box protein 7 (pax7) and myogenic regulatory factors (Schmidt et al., 2019).
Current CM production faces challenges in terms of cost and efficiency. The transition from traditional 2D culture to 3D culture has been proposed as a method to address the issue of CM production (Alam et al., 2024; Ben-Arye et al., 2020; Kang et al., 2021; Pasitka et al., 2023). In addition, efforts are being made to accelerate the construction of serum-free media to address high costs of CM production (Messmer et al., 2022; Stout et al., 2022; Yu et al., 2023). However, fundamentally, research on the capabilities of the cells central to CM production has not been extensively conducted. There are various types of cells that can be utilized in CM production, and it is important to understand the characteristics of each cell type to effectively utilize them in production (Kang et al., 2024). In particular, the quality of the final CM product as a food is expected to be determined by the diverse characteristics of the cells and the choice of cell types used in the industry.
Meat quality could be influenced by several factors, including breed, sex, growth rate, nutrition, muscle types, and temperature (Joo et al., 2013; Kim et al., 2023b). Similarly, the quality of CM can be determined by the characteristics of the cells used such as growth rate, nutrition and taste etc. Previous studies showed the difference in MSCs characteristic containing proliferation and differentiation capacities with bovine and porcine age (Kim et al., 2023a; Mesires and Doumit, 2002). These capacities of MSCs could be changed by the age of the livestock, potentially affecting the production and quality of CM. However, there is a lack of studies on the impact of age on characteristics of chicken muscle stem cells (cMSCs) relatively than those of mammal cells. Also, research exploring the nutritional value of the chicken CM produced has to be conducted, which could be critical factors for CM. In particular, since the taste and nutritional components of CM differ significantly from those of conventional meat, analysing amino acid profiles is necessary for good quality of CM as food (Joo et al., 2022). We hypothesized that there would be variations in proliferation and differentiation capacities of MSCs and amino acid contents of chicken cultured meat tissue (CMT) based on the chicken age. Therefore, this study aimed to investigate effect of chicken age on proliferation and differentiation capacities and amino acid contents of CMT.
Materials and Methods
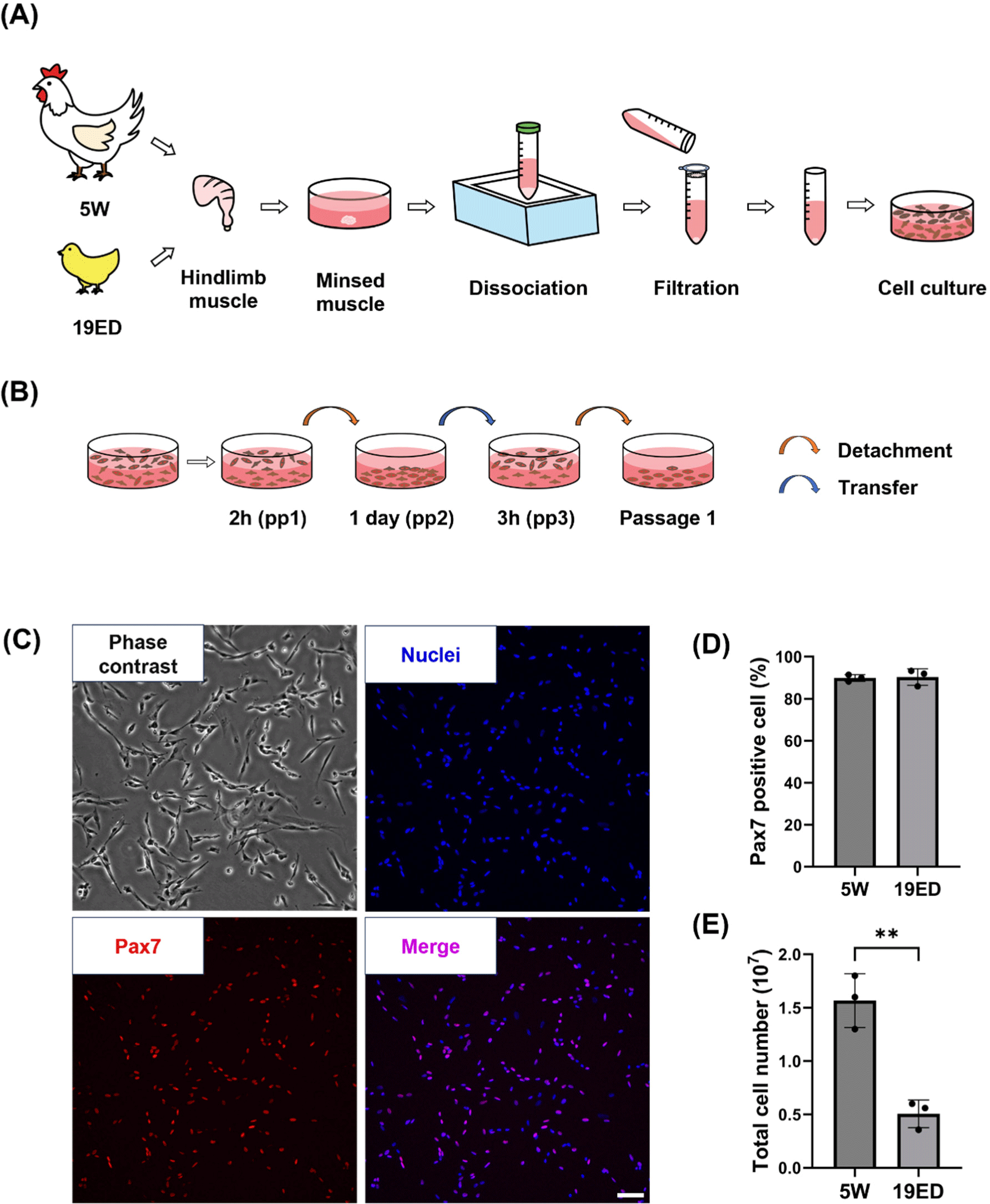
Broiler chickens (Cobb) and their fertilized eggs were supplied by a local farm. The fertilized eggs were incubated under controlled conditions at 37.8°C and 80% relative humidity for a period of 19 d. As described below, chicken primary cells were isolated from 19-embryonic-day (19ED) and 5-week-old (5W) chickens each (Fig. 1A). Briefly, the hindlimb muscle was thoroughly sterilized with 70% ethanol and dissected into small pieces (~1 mm). These pieces were then treated with 0.1% collagenase type 1 (LS004194, Worthington Biochemical, Lakewood, NJ, USA) at 37°C for 1 h with gentle shaking every 15 min. Dissociated tissues were then digested with 0.25% Trypsin-EDTA (25200072, Gibco, Grand Island, NY, USA) for 10 min at 37°C. Dulbecco’s Modified Eagle Medium (DMEM; LM 001-08, Welgene, Gyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS; S1-004, Welgene) was added to cell suspension to block enzymatic digestion. The cell suspension was filtered with 100 and 40 μm cell strainers, centrifuged at 800×g for 5 min, and treated with red blood cell lysis buffer (00443357, Invitrogen, Waltham, MA, USA) for 10 min at 4°C to isolate primary cells. These cells were seeded into cell culture flasks in a CO2 incubator, incubated at 41°C with 5% CO2, and purified with a pre-plating method (Kim et al., 2022; Fig. 1B). In brief, isolated primary cells (pp1) were plated into a culture flask at a density of 5×106 cell/cm2 and cultured for two hours. Suspension cells (pp2) were transferred into a new culture flask and cultured for one day. Next, adhesion cells (pp3) were detached with 0.05% trypsin-EDTA and cultured for two hours. Suspension cells (passage 1) were cultured for further sub-culture or cryopreservation.
cMSCs were cultured in growth media (GM) added with 20% FBS, 1% GlutaMAXTM supplement (35050061, Gibco), 1% antibiotic-antimycotic (15240062, Gibco), 5 ng/mL basic fibroblast growth factor (233-FB-025, R&D Systems, Minneapolis, MN, USA), and 10 μM p38 inhibitor (SB203580, MedChemExpress, Monmouth Junction, NJ, USA) in DMEM. These cells were seeded at a density of 5×103 cells/cm2 on 0.1% collagen (354236, Corning, Corning, NY, USA) coated flask. Cells were then subcultured or induced for differentiation when confluency reached over 70%.
5-Ethynyl-2'-deoxyuridine (EdU) assay was conducted with a Click-iT Plus EdU Imaging Kit (C10640, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's manual. Briefly, cMSCs were cultured on culture slides overnight and treated with 10 μM EdU for 2 h. Fluorescence images were obtained with a confocal laser scanning microscope (AX R, Nikon, Tokyo, Japan). Data were presented as means of five image fields randomly selected for statistical analysis.
cMSCs were seeded into T25 flasks at a density of 5×103 cells/cm2 and cultured for 3 d. The population doubling time (PDT) was calculated using Eq. (1):
Where PDT was population doubling time, N0 was the number of cells at the beginning of culture, N1 was the final number of cells following culture, and dT was the time of culture.
cMSCs were seeded into 96-well plates at a density of 3×103 cells/well and cultured overnight. The next day, 20 μL cell counting kit-8 (CCK-8; KTA1020, Abbkine, Atlanta, GA, USA) was added to each well and the plate was incubated for 3 h. UV absorbance was measured at 450 nm using a UV spectrophotometer (Multiskan Sky, Thermo Fisher Scientific).
cMSCs with confluency over 70% were differentiated with differentiation media (DM) containing 2% FBS, 1% GlutaMAXTM supplement, and 1% antibiotic-antimycotic in DMEM. The differentiation ability was calculated as fusion index indicating the percentage of nuclei in myosin heavy chain (MHC)-positive cells. Data are presented as mean of five randomly selected image fields for statistical analysis.
cMSCs were fixed with 4% formaldehyde and permeabilized with 0.5% Triton X-100 for 15 min. These cells were blocked using 3% bovine serum albumin (BSA) in Dulbecco's Phosphate Buffered Saline (DPBS; LB 001-02, Welgene) at room temperature (RT) for 1 h. They were then incubated with primary antibodies, mouse anti-pax7 (1:200; sc-81648, Santa Cruz Biotechnology, Dallas, TX, USA) and mouse anti-myosin heavy chain (1:40; MF20, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), at 4°C overnight. They were then incubated with a secondary antibody at RT for 2 h. Finally, cells were mounted using mounting media and DAPI and visualized using a fluorescence microscope (CKX53, Olympus, Tokyo, Japan).
cMSCs were lysed with RIPA lysis buffer (R4100-010, GenDEPOT, Katy, TX, USA) and harvested with a cell scraper on ice. Protein samples were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to 0.45 μm polyvinylidene fluoride membrane using semi-dry blotting (PB0013, Thermo Fisher Scientific). The membrane was blocked using 3% BSA for 1 h at RT and incubated with primary antibodies against myosin heavy chain (1:20) and beta actin (1:10,000; AC-15, Thermo Fisher Scientific) overnight at 4°C. The membrane was incubated with HRP-conjugated secondary antibodies (1:10,000; SA001-500, GenDEPOT) at RT for 2 h. The signal band was detected by ECL solution and visualized with a chemiluminescence imager (A44116, Thermo Fisher Scientific).
cMSCs were sub-cultured to reach 7 passages. They were then plated into ten 175T flasks at a density of 5×103 cells/cm2 to obtain CMT. When the cell confluency reached over 70%, the culture medium was replaced with DM and cells were cultured for 3 d to induce myogenic differentiation. The CMT was harvested by physically detaching it using a cell scraper, followed by centrifugation at 1,000×g for 5 min and washing three times with DPBS.
The CMT was freeze-dried for 24 h. Then 10 mg of lyophilized sample was hydrolyzed with 6N HCl in a dry oven at 110°C for 16 h. After evaporating the solution, the hydrolysate was diluted with 0.1 N HCl and filtered through a 0.22 μm membrane filter. Total amino acid was analyzed according to the amino acid analysis manual produced by Agilent (Henderson et al., 2000).
All statistical analyses were conducted using GraphPad Prism 10 version 10.0.2 (GraphPad, San Diego, CA, USA). Student’s t-test was performed to compare two groups of data. Data were collected from three independent experiments and presented as mean±SD. Statistical significance was considered when p-value was less than 0.05 (p<0.05).
Results and Discussion
To purify cMSCs, chicken hindlimb muscles were dissociated with collagenase. Isolated cells were sorted using a pre-plating technique (Figs. 1A and B). These isolated cMSCs were identified with pax7, a specific marker of muscle stem cells, to confirm the purity of cMSCs in isolated groups (Fig. 1C). The purity of cMSCs was observed to be at 90% in both of groups (p>0.05). Pre-plating is a simple and low-damaging method of isolating cells using different cell adhesion speeds of cells to be purified. This technique allows purification of MSCs from many species by slightly applying various time processes. In this study, cMSCs with a high purity in both groups were obtained with the above method. The total cell number after isolation showed a significant difference (p<0.001) between 19ED and 5W groups (Fig. 1E).
MSCs are currently the main cell source used to produce CM (Kang et al., 2024; Lee and Choi, 2024). In previous studies, pools of MSCs varied with age. Previous studies have reported that the number of MSCs is decreased with age in mammals, including humans, mice, porcine, and bovine (Kim et al., 2023a; Mesires and Doumit, 2002; Ogura et al., 2020; Verdijk et al., 2014). The present study showed that the number of MSCs decreased with age in chickens. This indicates that MSCs from embryos could allow more MSCs to be obtained from the same muscle mass. Thus, less time is required to obtain sufficient MSCs. MSCs of avians can be obtained with relatively easier preparation than mammals even at the embryonic stage. However, despite the variety of biopsy methods available, performing a biopsy on small chick embryos can inflict fatal damage. From an animal ethics perspective, which method is more ethical between aged animals at post-natal and embryos at pre-natal to obtain avian cell sources for CM production should be further discussed.
To measure the proliferation ability of cMSCs, we cultured cMSCs in GM for 3 d (Fig. 2A). The percentage of EdU-positive cells among total cells was not significantly (p>0.05) higher in the 19ED group than in the 5W group (Figs. 2B and C). However, the PDT was significantly (p<0.05) lower in the 19ED group than in the 5W group (Fig. 2D). There was no significant difference in absorbance at 450 nm between 1 d of culture and 2 d of culture, whereas a significant difference (p<0.05) was found after 3 d of culture between 19ED and 5W groups. This result indicated that cMSCs isolated from 19ED showed a higher proliferative ability than those isolated from 5W.
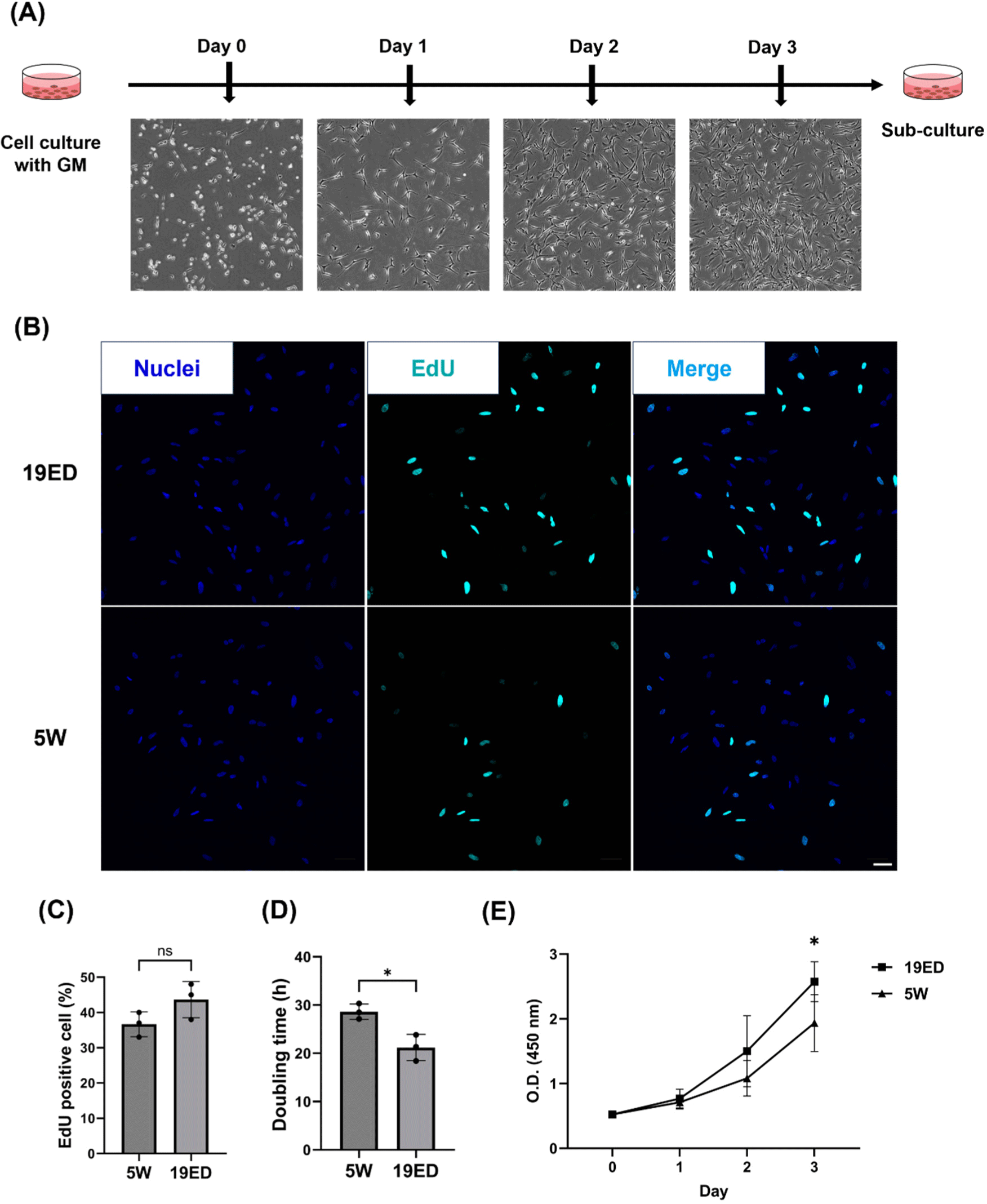
The proliferative ability of cMSCs is a crucial parameter as it is correlated with the yield and cost of CM production. The proliferative ability of cMSCs is a crucial parameter as it is correlated with the yield and cost of CM production. According to Zelenyuk, productivity is negatively correlated with the cost and time required for production (Zelenyuk, 2023). This implies that higher productivity can be achieved from the greater proliferative capacity of cMSCs, indicating in lower input of time and materials. The proliferative capacity of the cells could be directly related to productivity as time input means doubling time of the cells in this study (Hauser et al., 2024). When CM was first introduced in 2013 by Mark Post, the production cost was about $300,000 (Post, 2014). The culture medium accounts for the largest portion of CM production cost. As the technology and process of cultured media production are further developed, the production cost of cultured media could reach a maximum of $0.24/L (Garrison et al., 2022; Specht, 2020). However, for the CM market to grow into a competitive industry, not only culture media, but also labor costs and equipment should be significantly reduced (Garrison et al., 2022). A strategy to reduce production costs could include selecting cell sources with high proliferative capacity as a cell source for CM. MSCs with high proliferative ability could produce a greater quantity of CM per unit of culture medium, reducing the cost of production per kilogram. In studies using primary MSCs, animal age could directly affect CM production yield. This is because regenerative and proliferative abilities of MSCs are decreased with increasing age due to age-related loss of function (Liu et al., 2022). Some research studies about CM have argued about the relation between animal age and the proliferation capacity of MSC (Ono et al., 2010; Parker, 2015; Yin et al., 2013). In bovine, the proliferative capacity of MSCs derived from younger calves exhibits markedly high proliferative potential with up-regulated expression levels of key myogenic genes including pax7, myoD, and myoG (Kim et al., 2023a). NCAM+ MSCs showed a decrease in proliferative capacity as the age of porcine increased from 1 to 21 wk old (Mesires and Doumit, 2002). In this study, embryonic-derived cMSCs had a higher proliferative capacity than adult-derived ones. It means that cMSCs have potential to enhance the production efficiency of CM products.
Growth medium for cultured cMSCs was replaced with DM and cells were induced to undergo myogenic differentiation for three days. MSCs were fused and elongated on day 1 and differentiated into myotubes over three days (Fig. 3A). Myogenic differentiation exhibited accelerated progression for MSCs derived from 19ED. Differentiated myofibers were stained with MHC, a muscle-specific marker. Myotube formation area (MFA) was then measured to determine myogenic differentiation ability. Results showed a significantly (p<0.05) higher MFA in MSCs derived from 19ED than those from 5W (Figs. 3B and C). In addition, the relative expression level of MHC was observed to be significantly (p<0.05) lower in 19ED than in 5W (Figs. 3D and E).
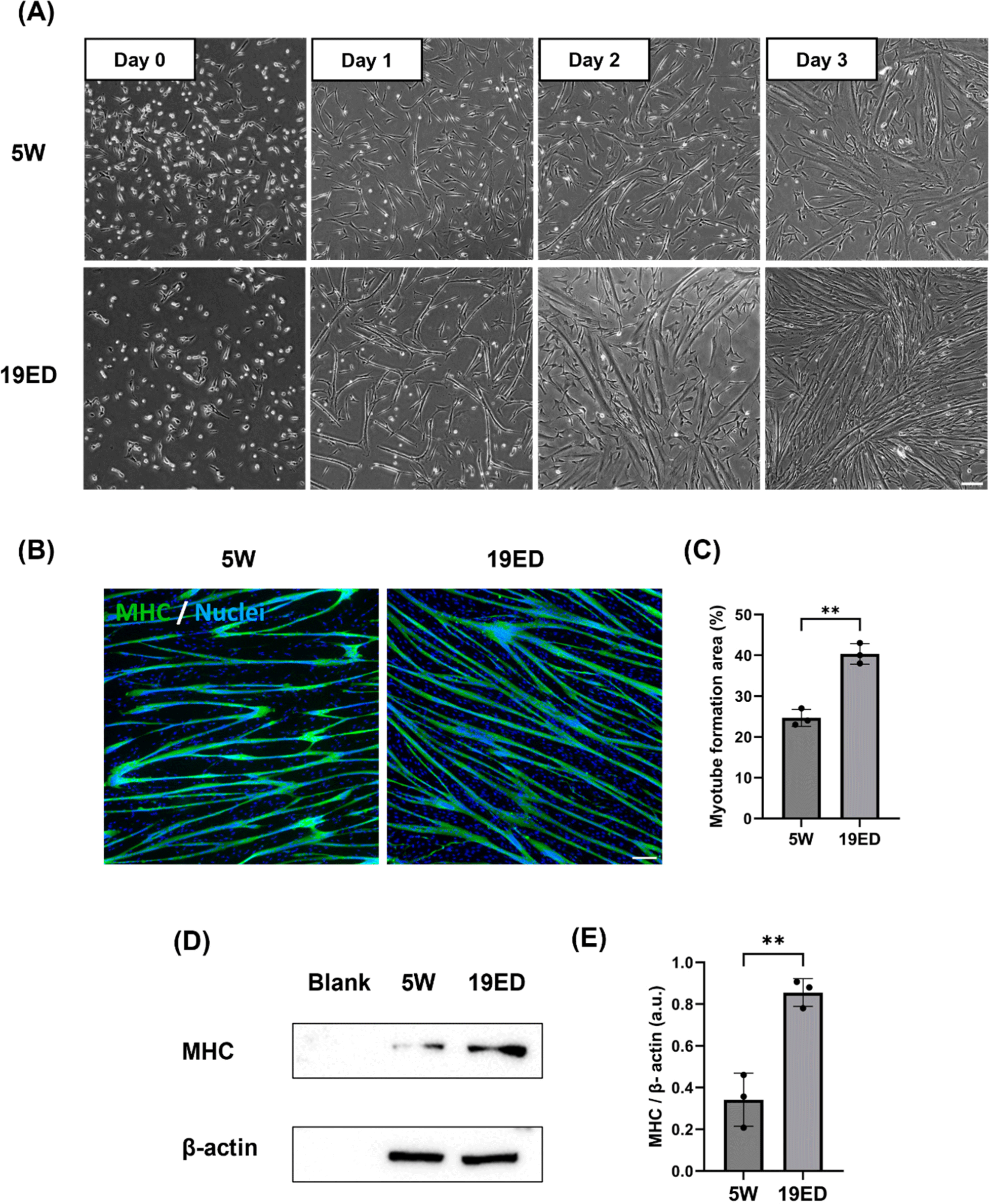
The age at which animals are slaughtered impacts meat quality such as texture, sensory, and nutritional value (Baéza et al., 2012; Li et al., 2021). Broiler chickens generally undergo muscle growth, differentiation, and development during a rearing period of 5–7 wk after hatching, ultimately being processed into meat. Over the duration of chicken growth, both elongation and enlargement of myofiber could control muscle growth, especially enlargement of muscle fibers in diameter of muscle fibers, which continued until 35 wk of age (Ono et al., 2010). Additionally, meat quality could vary depending on the growth stage and slaughter time of chicken (Baéza et al., 2012). A significant portion of differences in muscle fiber growth and meat quality characteristics can be attributed to the growth and differentiation of myofibers. Therefore, muscle fiber growth and differentiation might also play a crucial role in CM. However, there is a lack of research on how these differentiation properties affect the quality of CM and whether they play a pivotal role in precisely mimicking the flavor of conventional meat, as meat taste does not merely arise from muscle protein (Melzener et al., 2023). Thus, this study aimed to explore whether the level of muscle fiber growth and differentiation also could play a significant role in CM.
It was observed that after MSCs were induced to differentiate into myotubes in vitro, myotubes reached their maximum formation at 3–4 d of differentiation. However, myotubes were detached from culture flasks after 3–4 d, notwithstanding repeated experiments. This occurred because myotubes struggled to develop into fully matured muscle fibers in an in vitro culture. They face challenges for long-term myogenic maturation due to either minor physical impacts or spontaneous detachment over time (Denes et al., 2019; Romagnoli et al., 2021). Thus, rapid differentiation could be time-efficient and economically advantageous for CM production by quickly forming a large number of myotubes. The process of CM production involves differentiation and maturation of animal cells, following a large-scale culture of cells proliferating in a bioreactor to fabricate the CM. A limit of our investigation lies in the absence of comparative analysis regarding differentiation into fully matured muscle fibers due to an insufficient duration of differentiation resulting from myotube detachment. In this aspect, we plan to research the differentiation potential when muscle fibers are fully matured through a long-term culture. Further studies are needed to develop techniques for differentiating MSCs into muscle fibers for long-term maturation, along with investigating culture duration required to limit.
The CMT was fabricated on large-scale culturing in 175T flasks and then harvested as described below (Figs. 4A and B). The relative production yield (RPY) is a parameter comparing the relative weight of CMT produced per identical culture area. The RPY of CMT was significantly (p<0.05) higher in the 5W group than in the 19ED group (Fig. 4D). Subsequently, we measured total amino acid contents in CMT produced by each group. Results revealed that amino acid contents showed no significant (p>0.05) difference between the two groups (Fig. 4E).
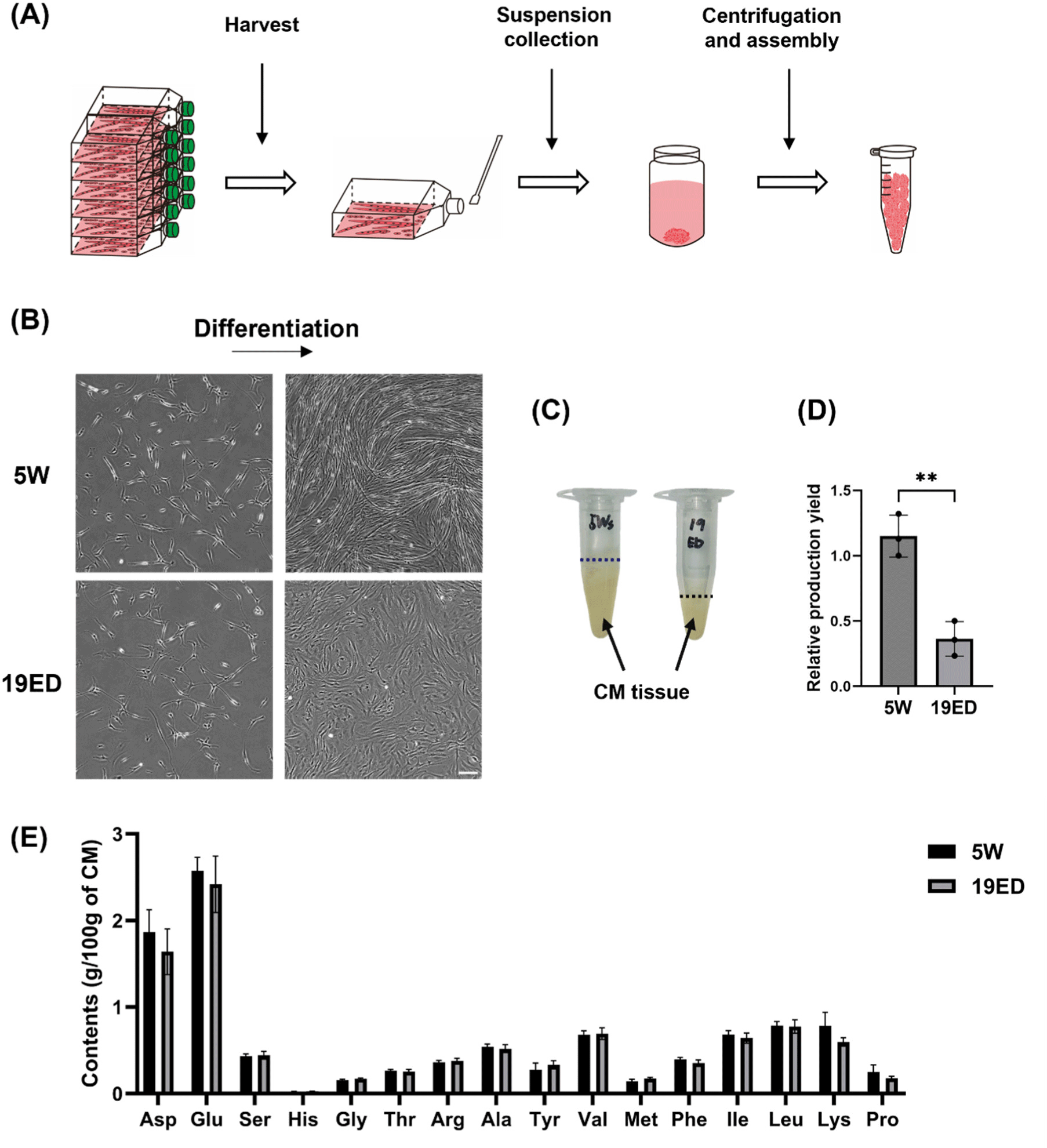
Remarkably, the yield of CMT per culture area from 19ED chickens was lower than that from 5W, and the differentiation into muscle fibers was also less progressed relatively than 5W. This observation could potentially be ascribed to the fact that cMSCs from 19ED exhibited rapidly declined stemness and myogenic potential compared to those at 5W during long-term culture. However, this rationale could not entirely elucidate the pronounced decline in differentiation capabilities observed in cMSCs from 19ED. The main cause could be fibroblast contamination during cell sorting. The pre-plating isolation method revealed that fibroblasts proliferated at a pace exceeding that of muscle stem cells. In this study, extended culture periods for promoting excessive growth of these quicker proliferating fibroblasts might elucidate distinctive outcomes recorded for 19ED. Regarding the fabrication of CM, it is critical that stem cells retain their differentiation characteristics during long-term culture. Recent studies have focused on inducing spontaneous immortalization of primary cells or pluripotent stem cells as a strategy to overcome these limitations. In 2022, CM produced from immortalized chicken fibroblasts received approval from the FDA as no harmful factors for consumption were identified (Fasano et al., 2022). Therefore, further discussion should be given to the use of immortalized cell lines to ensure consistent maintenance of myogenic potential during long-term cultivation for CM production.
Without a doubt, high-quality meat is one of the most nutritious foods available, containing 20%–30% protein generally. It is also rich in essential amino acids that human beings require (Ahmad et al., 2018). In this study, total amino acid contents in CMT have been found to be lower than those of traditional meat in previous study (Bhawana et al., 2023). The differences are thought to be due to varying differentiation periods and levels of myogenic developmen of myofiber. However, in this experiment, there was no difference in amino acid content between 19ED and 5W, which were differentiated over the same period. This could be attributed to the relatively short-term cultivation for in vitro differentiation and maturation of MSCs. Namely, the period is too short to differentiate the MSCs and make the difference in amino acid contents with age. To bridge this nutritional gap, it becomes imperative to pioneer and implement technologies that ensure complete maturation of muscle stem cells into fully developed muscle fibers subsequent to their differentiation phase. Furthermore, since there was no significant difference in the amino acid composition between cultured tissues produced using the same amount of 19ED and 5W cells, it may be advantageous to use MSCs from 19ED, which proliferate and differentiate more rapidly, for CM production.
Conclusion
cMSCs from 19ED had higher proliferation and differentiation capacities than those derived from 5W chickens, although there were no significant differences in total amino acid contents produced between the two groups. This finding suggests that cMSCs from 19ED could be suitable for enhancing the production efficiency of CMT. However, the stemness and myogenic potential of cMSCs from 19ED diminished more rapidly than those from 5W during long-term cultivation. Therefore, it is necessary to maintain characteristics of MSCs over prolonged culture for CMT fabrication.













