Introduction
Mycotoxins are fungal secondary metabolites that frequently contaminate agricultural crops worldwide and adversely affect farm animals (Abdallah et al., 2015; Holanda and Kim, 2021). Deoxynivalenol (DON) is a type B trichothecene. It is produced by Fusarium species and is the most frequently detected mycotoxin in feed samples (Kwon et al., 2023). DON toxicity can lead to impairment of the immune system, oxidative stress, and damage to the gastrointestinal tract, which can affect the survival and productivity of livestock (Chen et al., 2024; Holanda and Kim, 2020). According to the European Food Safety Authority (EFSA), 75.2% of EU feed samples were contaminated with DON (EFSA, 2013). Furthermore, Gruber-Dorninger et al. (2019) reported DON contamination in 64.1% of feed samples from 2008 to 2017. In response, the maximum residue level for DON has been established by the US Food and Drug Administration (FDA) in grain and grain byproducts for swine at <5 mg/kg (FDA, 2018). In contrast, the European Commission has set strict limits for capping DON levels in compound feeds for pigs at 0.9 ppm (European Commission, 2016).
Pigs, followed by mice, rats, poultry, and ruminants, are most susceptible to DON toxicity (Zhao et al., 2016). This is likely because pigs consume cereal-rich diets and lack the rumen microorganisms required to break down mycotoxins (Jia et al., 2023; Pierron et al., 2016). Consequently, pigs exhibit a higher bioavailability of DON and a prolonged elimination period of the toxin from the body compared to other animals (Schelstraete et al., 2020; Sun et al., 2022). A notable consequence of DON toxicity in pigs is growth retardation (Pestka and Smolinski, 2005). Symptoms such as diarrhea, vomiting, and anorexia result from ingestion of feed containing high levels of DON, which reduces feed intake and efficiency (Pestka et al., 2017; Pinton et al., 2009). Furthermore, DON causes oxidative stress through the generation of reactive oxygen species (ROS), further compromising the immune system and causing histological alterations, including fibrosis and apoptosis (Chaytor et al., 2011; Kang et al., 2022). DON toxicity disrupts several metabolic processes, including glycolysis, protein biosynthesis, and cellular metabolism (Dänicke et al., 2006; Saenz et al., 2021; Wang et al., 2019).
Despite extensive research, the effects of DON may be highly variable and depend on several factors: the amount of toxin in the animal, its origin, the animal’s age, duration of exposure, and its simultaneous interaction with other substances (Serviento et al., 2018). Weaned piglets are more vulnerable to DON toxicity because their intestines are less adapted to sudden changes in feed. Many studies have focused on DON toxicity in piglets. However, as slow growth in pigs results in reduced profitability, knowledge of the harmful effects of feed to pigs is important to enable farmers to manage their diets effectively (López-Vergé et al., 2018). Determining the amount of DON in the diets of growing pigs is essential to reduce the risk of DON in pig production. However, studies on DON toxicity in growing pigs are limited. The toxicity of DON in this study was evaluated at concentrations higher than the maximum residue level during the growing period. We investigated the effects of different DON levels on histological alterations, growth characteristics, and blood biochemistry of growing pigs. Additionally, this study explored metabolites and their correlation with growth performance.
Materials and Methods
Castrated pigs were sourced from Taeheung (Yeonggwang, Korea). Twelve pigs (Landrace×Yorkshire) were housed in individual pens measuring 130×240 cm. Housing conditions were maintained throughout the study, including acclimation, according to the following specifications: a light-dark cycle of 12:12 h, a room temperature of 25±2°C, and a relative humidity of 60±5%. The pigs were divided into four groups as follows: the control group received a basal diet, the T1 group received a basal diet with 1 mg/kg added DON, the T2 group with 3 mg/kg, and the T3 group with 10 mg/kg (Table 1). Pigs had ad libitum access to water and food for 4 weeks. Diets were supplemented with DON (TripleBond, Guelph, ON, Canada) according to established experimental concentrations. Mycotoxins were dissolved with 1%–5% ethanol in an autoclaved sterilized beaker and stirred until completely dissolved. The solvent amount was tested to ensure no impact on feed fluidity despite moisture content. At the end of the experimental period, blood samples were taken 1 d before tissue sampling. T61 was used to anesthetize all animals. Samples were taken from the feces, ileum, liver, muscle, rectum, and urine immediately after exsanguination. Blood and debris were removed using phosphate-buffered saline (PBS) and sterile disposable wipes. Samples were rapidly frozen in liquid nitrogen for storage at –80°C. Additionally, tissue fixation for histologic analysis was performed with 10% neutral buffered formalin (NBF; Sigma-Aldrich, St. Louis, MO, USA). The average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated as follows:
| Ingredients | Percentage (%) |
|---|---|
| Corn | 57.30 |
| Soybean meal | 25.00 |
| Wheat bran | 11.50 |
| Molasses | 1.40 |
| Soybean oil | 2.00 |
| Limestone | 1.00 |
| L-Lysine | 0.40 |
| Salt | 0.40 |
| Sweet whey | 0.50 |
| Tricalcium phosphate | 0.50 |
| Total | 100 |
Ultra-performance liquid chromatography (UPLC) mass spectrometry was used to analyze DON in diets, as described previously (Jeong et al., 2024a). Briefly, a 1 g homogenized DON sample was extracted with water and diluted in PBS, then applied to the appropriate columns. The diets contained 0.73, 2.61, and 9.52 mg/kg DON. The same diet was used as previously described. The control sample had no DON contamination.
Pig blood samples were taken in tubes from each growing pig via the jugular vein. Briefly, serum was centrifuged at 700×g for 15 min at 4°C, then stored at –80°C (Jeong et al., 2024b). Blood parameters, including glucose, creatine, blood urea nitrogen, phosphate, calcium, total protein, albumin, globulin, alanine aminotransferase, alkaline phosphatase, total bilirubin, cholesterol, amylase, and lipase levels, were analyzed using a VetTest chemistry analyzer (IDEXX, Westbrook, ME, USA).
Analysis of DON-induced fibrosis and apoptosis may improve our understanding of tissue damage and repair. Samples (5×5 mm) of liver, muscle, duodenal, ileal, rectal, jejunal, cecal, and colonic (ascending, transverse, and descending) tissues were collected as previously described (Jeong et al., 2024b). Each sample was fixed in 10% NBF, dehydrated, embedded in paraffin, and heated. Slides were deparaffinized, rehydrated, and stained. They were then observed under a microscope at 200× and 400× magnifications.
Ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF MS) was used to analyze changes in pig metabolites following DON-contaminated diets. Experimental pretreatment and analytical methods were carried out as previously described (Jeong et al., 2024a). Briefly, 100 μL serum was mixed with 400 μL acetone, shaken, and the 400 μL supernatant collected, lyophilized, then dissolved in 100 μL 20% methanol containing an internal standard. Urine was treated the same way, while liver, cecum, and feces samples were dissolved in 80% methanol with an internal standard. The resulting solutions were analyzed by UPLC-Q-TOF MS. After metabolomic analysis, samples were pooled. Samples were injected into an Acquity UPLC C18 column with a mobile phase of water and acetonitrile. Blood, liver, cecum, feces, and urine took 12 min; blood and urine at 40°C took 16 min. The eluted compounds were analyzed by MS in ESI mode. TOF-MS data were scanned between 100 and 1,500 m/z with 0.2 s scan time. Capillary and sample cones set at 3 and 40 V, 800 L/h desolvation flow, 300°C desolvation temperature, and 100°C source temperature. Leu-Enk was used as the reference compound due to its low mass and was analyzed every 10 s. QC samples were analyzed every 10 runs. MS/MS spectra were obtained at m/z 50–1,500 using a ramped collision energy. MS data were processed using MarkerLynx 4.1, including m/z, RT, and intensity calculations. LC-MS data were acquired using MarkerLynx. Peak data were identified using various parameters and normalized. Metabolites were identified using multiple databases and relevant literature.
Metabolite data were analyzed with SIMCA-P+. Partial least squares discriminant analysis (PLS-DA) was used to visualize the results. PLS-DA using R2, Q2, and permutation tests. R2X/Y assessed the model fit; Q2, future data. The PLS-DA results were validated using a permutation test. One-way analysis of variance (ANOVA) with Duncan test was used to analyze metabolite abundances (p<0.05). Heatmaps of identified compounds were created in R using a color scale based on z-scores. Prism 9.5.1 was used to perform a one-way ANOVA and Tukey’s tests. The results are expressed as the mean±SEM. Statistical significance was set at p<0.05.
Results
Table 2 shows the impact of DON intake on 10-week-old pigs over 28 days. The control and DON treatment groups had similar initial body weights (BWs; 34.5±0.53 kg). The T3 group had the lowest final BW (46.4±0.84 kg). ADFI, ADG, and FCR were not significantly different among the four diet groups.
CTL, control (basal diet); T1, basal diet+DON 1 mg/kg feed; T2, basal diet+DON 3 mg/kg feed; T3, basal diet+DON 10 mg/kg feed.
The effects of DON treatment on the biochemical parameters of the blood of growing pigs over a 28-day period are presented in Table 3. Blood parameters that did not differ significantly among dietary treatments were not reported. The levels of alkaline phosphate (ALKP) in the blood of growing pigs in the T3 group were the highest among the diet treatment groups (p=0.003). However, in the T3 group, the level of lipase (LIPA) was significantly lower than that of the other treatments (p=0.007).
| Parameters | CTL (n=3) | T1 (n=3) | T2 (n=3) | T3 (n=3) | SEM | p-value |
|---|---|---|---|---|---|---|
| Alkaline phosphatase (U/L) | 150.0b | 201.3a | 175.7ab | 221.3a | 7.94 | 0.003 |
| Lipase (U/L) | 25.7a | 25.2a | 10.5b | 11.3b | 1.27 | 0.007 |
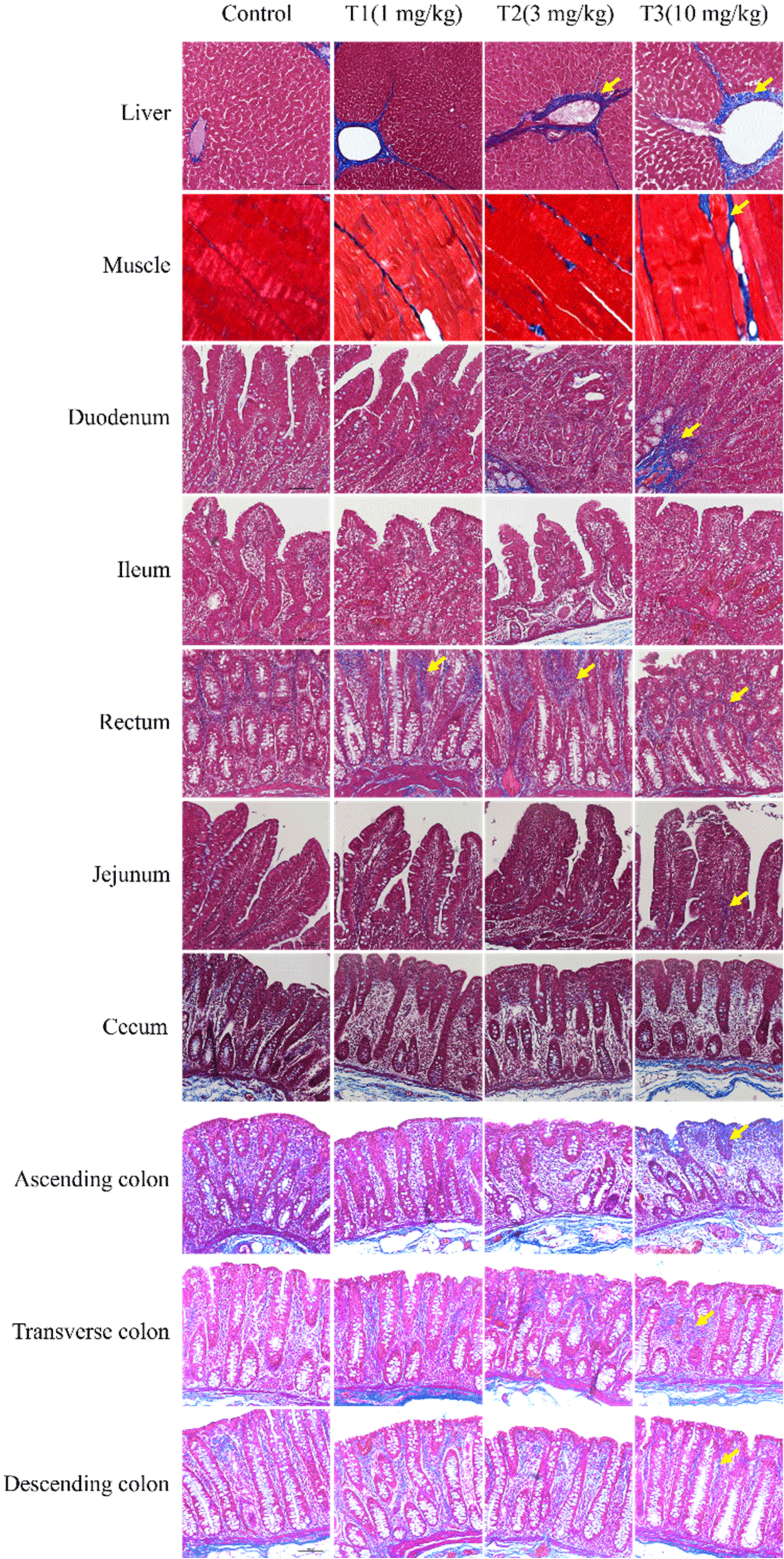
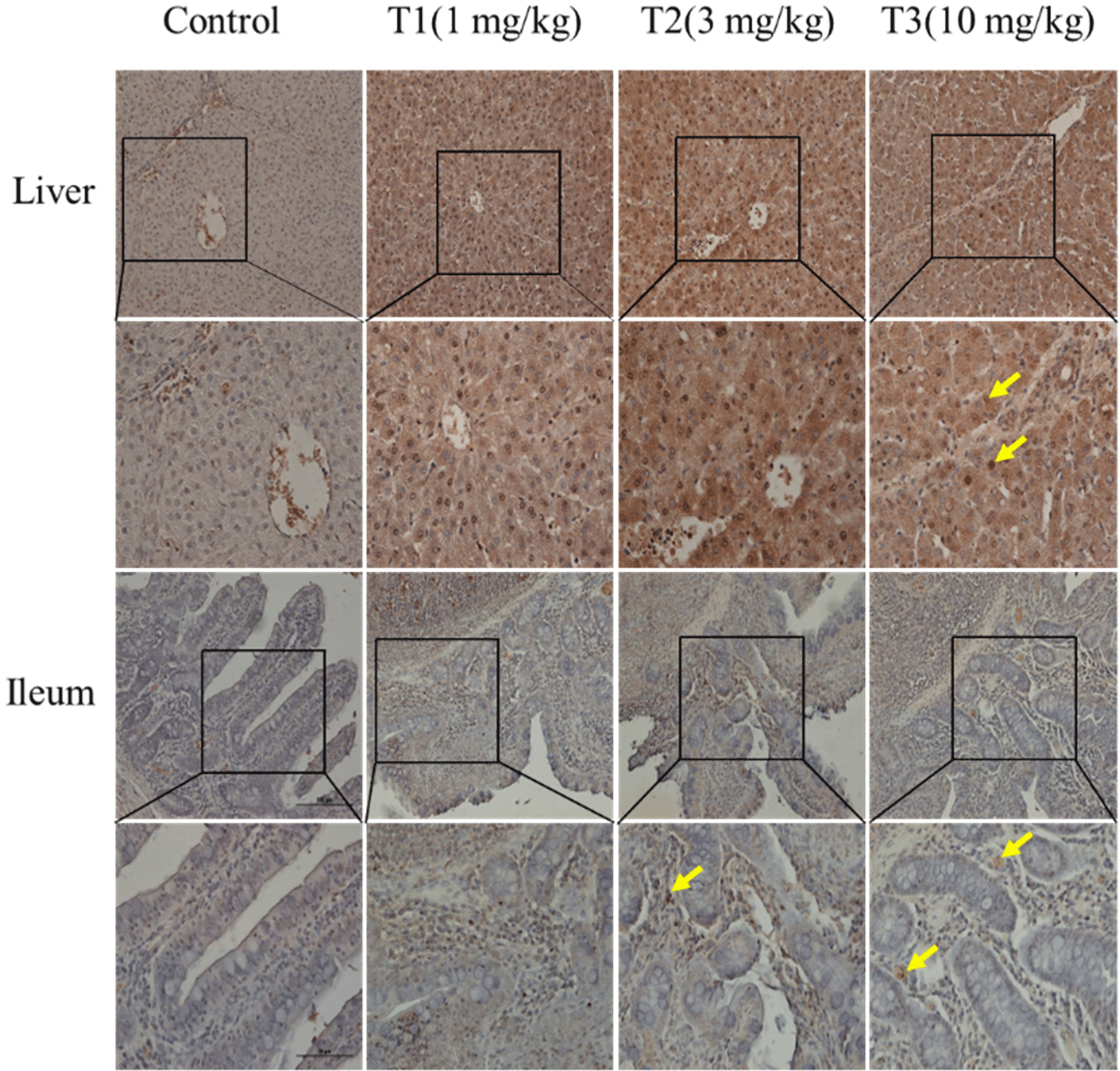
Masson’s trichrome staining was used to observe histological changes, including fibrosis, in the liver, muscle, duodenum, ileum, rectum, jejunum, cecum, and colon (ascending, transverse, and descending; Fig. 1). Increased fibrosis was observed in the portal areas of the liver lobules formed by an envelope of fibrous connective tissue. In skeletal muscle, fibrosis was caused by DON in the endomysium and blood vessels. In the duodenum, fibrosis was observed in the muscularis mucosa and the submucosa. Blue staining was observed in the ascending colonic mucosa. Fibrosis was also observed in other tissues. However, these differences were minimal and difficult to detect. The TUNEL staining results, performed to observe apoptosis in the liver and ileum, are shown in Fig. 2. The figures represent 200× and 400× magnified images. The DON group showed more TUNEL-positive staining than the control group, suggesting severe apoptosis. DON increased fibrosis and apoptosis; however, the effects were minimal or insignificant in some tissues.
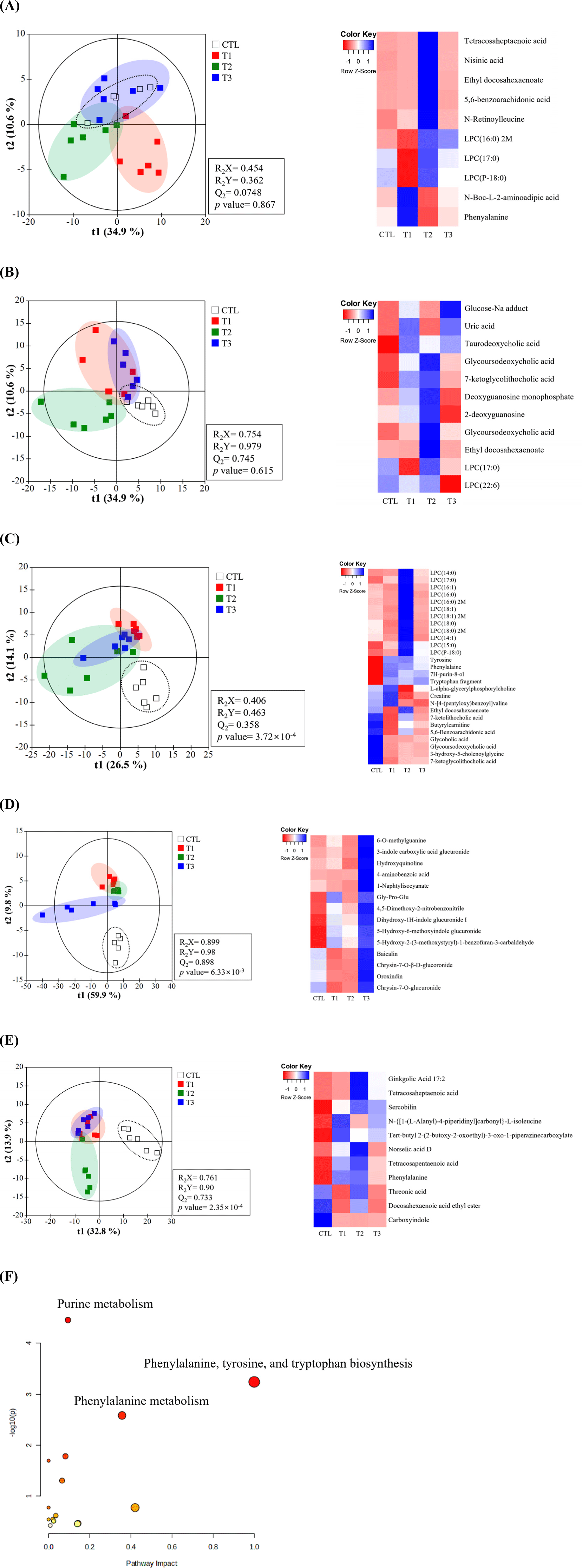
To understand the metabolic impact of DON toxicity at different levels, liquid chromatography-mass spectrometry was used to characterize the metabolites in the blood, cecum, feces, liver, and urine of growing pigs. PLS-DA indicated that metabolites in the DON and control groups were significantly separated in the cecum, urine, and feces compared to those in the control but not in the blood or liver (Figs. 3A, B, C, D, and E). Additional analyses were performed on DON-contaminated pigs to identify biomarkers. Based on VIP values>1.0 and p<0.05, the metabolites in tissues including blood, liver, cecum, urine, and feces were as follows. In blood, levels of N-Boc-L-2-aminoadipic acid, phenylalanine, N-retinoylleucine, tetracosaheptaenoic acid, nisinic acid, benzoic acid, ethyl docosahexaenoate, LPC(P-18:0), LPC(16:0) 2M, and LPC(17:0) were significantly altered. The levels of several compounds in the liver were also significantly altered. In the cecum, L-alpha-glycerylphosphorylcholine, creatine, 7H-purin-8-ol, tyrosine, phenylalanine, butyrylcarnitine, tryptophan fragment, glycolic acid, glycoursodeoxycholic acid, 3-hydroxy-5-cholenoylglycine, 7-ketoglycolithocholic acid, 5,6-benzoarachidonic acid, ethyl docosahexaenoate, LPC(14:0), LPC(14:1), LPC(15:0), LPC(16:0), LPC(16:0), LPC(17:0), LPC(18:0), and LPC(18:1) were all significantly altered. Urine showed significant changes in 4-aminobenzoic acid, Gly-Pro-Glu, chrysin-7-O-β-D-glucuronide, oroxindin, chrysin-7-O-glucuronide, baicalin, and 5-hydroxy-2-(3-methoxystyryl)-1-benzofuran-3-carbaldehyde. In feces, levels of threonic acid, phenylalanine, N-[{1-(L-alanyl)-4-piperidinyl}carbonyl]-L-isoleucine, and tert-butyl 2-(2-butoxy ginkgolic acid, tetracosaheptaenoic acid, and tetracosapentaenoic acid were significantly changed. Fig. 3G shows changes in purine metabolism, phenylalanine-tyrosine, tryptophan biosynthesis, and phenylalanine metabolism in the DON-treated group. Most candidate metabolites increased at 3 mg/kg (T2) and decreased at higher concentrations (10 mg/kg; T3), but urinary levels only increased at higher concentrations.
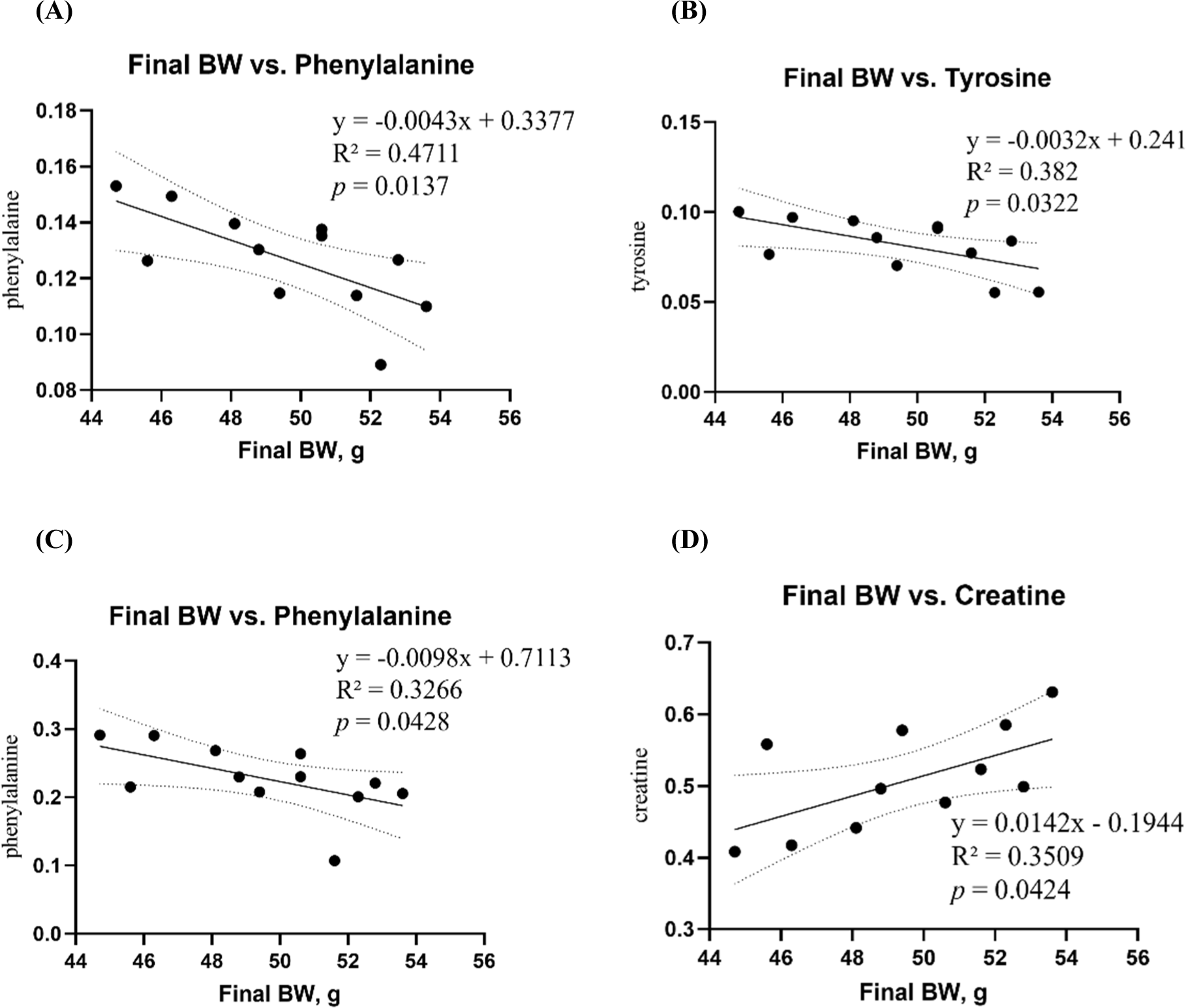
A linear regression model was used to analyze the correlation between final BW and blood and tissue metabolites (Fig. 4). Phenylalanine, tyrosine, and creatine levels were significantly correlated with the final BW. Phenylalanine in both cecum (R2=0.4771, p=0.0137) and feces (R2=0.3266, p=0.0428) metabolites, and tyrosine (R2=0.3820, p=0.0322) in cecum metabolites were negatively correlated with the mean final BWs (Figs. 4A, B, and C). Among the cecal metabolites, creatine (R2=0.3509, p=0.0424) was positively correlated with mean final BW (Fig. 4D). However, correlations with blood biochemical variables did not differ.
Discussion
The FDA has recommended a maximum residue level of DON of <5 mg/kg for grain and grain by-products within 20% of the total swine diet, which is equivalent to 1 mg/kg when converted to complete feed (FDA, 2018). The Canadian Food Inspection Agency (CFIA) also recommends the same standards as the FDA (CFIA, 2024). European Commission’s minimum residue level for DON in pig feed is 0.9 mg/kg (European Commission, 2016). China’s Feed Safety Standard sets the standard for DON in complete feed for pigs at 1 ppm (Zhao et al., 2021). The Korean Feed Standards and Specifications recommend a DON management level of 0.9 mg/kg in pig feed. Based on these recommendations and regulatory standards from these different countries, the minimum standard for DON treatment was set at 1 mg/kg in this study. Furthermore, we anticipated that DON levels above 1 mg/kg would adversely affect the health of growing pigs and thus focused on evaluating the effects of graded levels of DON. Although Wu et al. (2015) found that a DON concentration of 3 mg/kg had no significant effects on the health of growing pigs, Wellington et al. (2021) reported serious effects at the same concentration. Therefore, 3 mg/kg was used as the intermediate level to clarify the effects of DON toxicity in growing pigs. Additionally, to assess the effect of high levels of DON toxicity during the growth period, the maximum treatment was set at 10 mg/kg (Jeong et al., 2024b). Thus, the effects of graded levels of DON (1, 3, and 10 mg/kg feed) on the growth performance, blood biochemistry, histology, and metabolite levels were examined in growing pigs over a 4-week period.
DON adversely affects the growth performance of pigs (Reddy et al., 2018; Wu et al., 2015). The main symptoms of DON toxicity include vomiting, diarrhea, and anorexia (Pestka et al., 2017; Pinton et al., 2009), possibly resulting in intestinal damage, reducing overall nutrient absorption and utilization, which leads to decreased growth performance (Ghareeb et al., 2014). In this study, high levels of DON (10 mg/kg) significantly decreased growth performance, with an 11.6% decrease in final BW compared with the control group. However, our results did not show any effect of DON toxicity on ADFI or FCR. Similar to our results, Reddy et al. (2018) reported that the final BW of growing pigs fed 8 mg/kg DON decreased by 17% compared with that of the control group, whereas DON supplementation had no significant effect on the ADFI of growing pigs compared with the control group. Sayyari et al. (2018) also administered high doses of 5 mg/kg DON during the growth phase, but neither ADFI nor FCR showed significant differences compared to the control group. In contrast, Wu et al. (2015) reported that ADFI in growing pigs fed a high level of DON (12 mg/kg) was reduced by 41.6% compared with the control group. Although approximately 85% of weight loss due to mycotoxicosis is attributed to reduced feed intake, various factors, such as the contamination level, pig health status, and feeding period, can also have an effect (Pastorelli et al., 2012; Weaver et al., 2013). Therefore, further research that considers internal and external factors is required to determine the exact causes of weight loss.
In the present study, ALKP and LIPA levels were affected by high DON levels. We observed increased serum ALKP levels in growing pigs in the high-level DON treatment groups, which is consistent with the findings of Wu et al. (2015), who administered 12 mg/kg DON to 60–88-d-old pigs. Serum ALKP is secreted by mucosal cells lining the biliary tract of the liver and can leak into the blood when the liver cells are damaged (Ji et al., 2023; Wu et al., 2013). Therefore, the increase in serum ALKP levels in the high DON treatment groups may indicate liver damage owing to DON-induced systemic toxicity, which may be explained by the abnormal excretion of hepatic metabolites (Chaytor et al., 2011). Our results also showed reduced blood LIPA levels in growing pigs fed 3 mg/kg DON. LIPA is a hydrolytic enzyme secreted by the pancreas that breaks down fatty acids, and its activity is an important indicator of intestinal digestive function (Long et al., 2021; Qin et al., 2023). To date, no study has reported a direct relationship between DON and blood lipid levels in growing pigs. However, DON intake damages the intestinal mucosa and increases intestinal permeability, reducing intestinal absorption and impairing digestive organ function (Pierron et al., 2016). This may result in the suppression of digestive enzyme secretion. Additionally, abnormalities in the biliary tract tissue may impair bile flow from hepatocytes during cholestasis, leading to the accumulation of bile acids in the liver, which may cause abnormal secretion of ALKP (Reyer et al., 2019; Tannergren et al., 2006). In all mammals, the hepatopancreatic biliary system consists of branching ducts linking the liver and pancreas to the duodenum (Zhang et al., 2023). Thus, abnormalities in these biliary tracts can cause pancreatitis and inhibit the secretion of digestive enzymes, including LIPA, from the pancreas (Tsomidis et al., 2024; Yin et al., 2023). Consequently, our results suggest that DON negatively affects the digestive processes of growing pigs, which may explain the decline in growth performance observed in the DON treatment group.
The liver is the primary organ affected by DON exposure, as it is crucial in detoxifying and metabolizing mycotoxins following the ingestion of DON-contaminated feed (Hasuda et al., 2022). Mycotoxins and their metabolites are primarily absorbed in the small intestine, with 51% of ingested DON absorbed in the small intestine (Lewczuk et al., 2016). Additionally, DON may be more susceptible to break down by microorganisms in the large intestine of pigs than by those residing in the initial segments of the intestine (Kollarczik et al., 1994; Lewczuk et al., 2016). In the present study, histological alterations, including fibrosis and apoptosis, were observed in liver and intestinal tissues in a dose-dependent manner. Our previous studies and several others have also observed fibrosis and apoptosis in porcine liver and intestinal tissues due to DON toxicity (Jeong et al., 2024a; Skiepko et al., 2020). Histological liver and small intestine damage may explain the abnormal secretion of ALKP and LIPA from the blood in this study. These changes may be closely related to DON-induced oxidative stress. Several studies have shown that DON induces oxidative stress by increasing the accumulation of ROS, impairing the function of key antioxidant enzymes such as superoxide dismutase, GSH-Px and catalase, and increasing the levels of malondialdehyde (MDA) and 8-OHdG, a marker of oxidative damage (Ji et al., 2023; Xu et al., 2020). Ji et al. (2023) found that increased levels of ROS, MDA and 8-OHdG were strongly correlated with an increase in the number of apoptotic cells in pig liver, demonstrating that hepatocyte apoptosis is induced by DON-mediated oxidative damage. Furthermore, DON-induced oxidative stress increased the expression of apoptosis-related genes and proteins, such as interleukin-1 beta (IL-1β), cyclooxgenase-2, interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), caspase-3, caspase-8 and caspase-9 in porcine intestinal epithelial cells (IPEC-J2 cells; Kang et al., 2019). DON-induced oxidative stress can lead to fibrosis (Lan et al., 2015). Fibrosis refers to the excessive accumulation of fibrous connective tissue in the extracellular matrix of a damaged tissue (Antar et al., 2023). Oxidative stress boosts fibrotic factors like TGF-β1, leading to fibrosis by amassing extracellular matter (Antar et al., 2023; Yao et al., 2021). In addition, oxidative stress can trigger the release of inflammatory cytokines such as TNF-α, monocyte chemoattractant protein, IL-6 and IL-8, which can lead to tissue fibrosis (Antar et al., 2023; Ranneh et al., 2017; Yao et al., 2021). Our results show the risk of increased DON concentrations in pig diets due to tissue damage. However, as this study did not analyse DON-induced oxidative stress, future research investigating histological changes and DON-induced oxidative stress will be necessary.
Metabolomic analysis was conducted to explore the biological processes related to DON toxicity. Metabolites are the final products or intermediates of cellular activities and represent the overall response of organs or biological systems to various pathophysiological conditions (Wishart, 2019). Therefore, our results show the changes in pathways linked to DON toxicity. Metabolites from different tissues showed distinct profiles, indicating differences in metabolic profiles between the control and DON groups. These results are similar to those of our previous study that evaluated DON toxicity in weaned piglets (Jeong et al., 2024b). Additionally, the correlation between the metabolites that contributed to the separation among treatment groups and final BW was analyzed to identify metabolic biomarkers associated with growth in pigs exposed to DON toxicity. In the present study, increases in phenylalanine and tyrosine contributed to weight loss in growing pigs, whereas increases in creatine were associated with weight gain in growing pigs. Phenylalanine is an essential amino acid that is a substrate for protein synthesis and other biochemical pathways (Xian et al., 2018). Phenylalanine suppresses food intake by inducing the secretion of satiety hormones (Alamshah et al., 2017). Tyrosine is the main metabolite of phenylalanine and is converted into other compounds, including dopamine, serotonin, and epinephrine. These are involved in biological processes such as stress response, appetite control, and behavioral regulation (Jeong et al., 2024a). Creatine is endogenously synthesized from glycine, arginine, and methionine, primarily in the kidneys and pancreas (McBreairty et al., 2015; Wallimann et al., 2011). It is crucial in energy metabolism by providing the adenosine triphosphate required for cellular functions (Li et al., 2015). Additionally, creatine transporter mRNA expression is associated with the regulation of food intake, suggesting that creatine is closely related to feed intake and BW gain (Li et al., 2015). Therefore, this suggests the possibility that creatine supplementation may improve growth performance in pigs impaired by DON toxicity. Indeed, several studies have shown that creatine monohydrate supplementation improves growth performance in pigs by stimulating muscle energy metabolism and increasing protein synthesis (Li et al., 2015; Young et al., 2007). However, few studies have directly linked DON toxicity to creatine supplementation. Therefore, further studies are needed to elucidate the effect of creatine supplementation on DON toxicity. Consequently, our findings suggest that DON toxicity affects the imbalance of various metabolic pathways in the body of growing pigs, which affected the weight loss of growing pigs in the high DON group. In this study, phenylalanine, tyrosine, and creatine synthesis were considered as potential biomarkers of DON toxicity affecting growth performance.
Conclusion
In conclusion, we demonstrated that there are no significant health effects at low DON levels for growing pigs, whereas high DON levels decreased growth performance and altered blood biochemical characteristics. Furthermore, our results showed that DON toxicity caused significant dose-related histological changes, including fibrosis and apoptosis, in specific organs of growing pigs. Additionally, DON toxicity induced metabolic changes in growing pigs, which were linked to their final BWs. Therefore, our findings suggest that DON levels above the maximum residue limits cause adverse health effects in growing pigs, with these effects intensifying as DON levels increase. However, because DON toxicity can manifest differently in chronic versus acute exposure, we will conduct future studies to clarify its effects throughout the lifespan of pigs (Pestka and Smolinski, 2005). In addition, a substantial proportion of feed is contaminated with multiple mycotoxins. DON toxicity may be exacerbated by interactions with other mycotoxins—such as zearalenone, fumonisin, and aflatoxin B1—that are frequently detected in animal feeds (Holanda and Kim, 2021; Lei et al., 2013; Weaver et al., 2013). In this context, several studies have reported that mitigation strategies, including inorganic compounds, adsorption, antioxidants, yeast, and bacteria, can help alleviate the toxic effects of these mycotoxins (Holanda and Kim, 2021; Zhu et al., 2016). In particular, biological detoxifiers such as probiotics and yeast are considered a promising approach to reduce toxic effects without compromising the nutritional value of feed, compared to physical and chemical methods (Recharla et al., 2022). Therefore, we will conduct further research to elucidate how DON interacts with other major mycotoxins commonly found in feed, while additional investigations are also needed to develop the most effective biological detoxifiers for application in conventional farming systems. Although further research is needed, this study can be used as a basis for toxicity studies and as a criterion for DON-contaminated diets for growing pigs.













