Introduction
Meat is a significant source of protein, offering essential nutrients and consequences for physiological improvements, wherein its constant consumption is greatly shaped by other various factors including functional properties, excellent taste and texture profiles, as well as freshness state (Barido and Lee, 2021). Chicken meat, in particular, enjoys popularity owing to its abundant nutritional value at relatively low cost. However, its rich nutritional content also makes it prone to spoilage during storage. Apart from protein denaturation and oxidation of lipid, the reduced shelf life of meat may also be attributed to the abundant percentage of poly- and mono-unsaturated fatty acids, which readily experienced an oxidative degradation (Lawrie and Ledward, 2014). In this matter, synthetic antioxidants are employed to tackle the short shelf life by suppressing the formation of pro-oxidant and act as bacteriostatic. Yet, although butylated hydroxyanisole, butylated hydroxytoluene, sorbate, nitrates, and nitrites serve essential role as synthetic preservative to delay meat discoloration and inhibit excessive colony forming of bacteria during retail display and storage, the increased consciousness from consumer on potential side effects urges the more utilization of natural antioxidants. Since, the naturally derived antioxidant not only acts as an antioxidant, but also provides foods with enhanced functionalities (Horbańczuk et al., 2019).
Bio-based techniques, such as enzymatic hydrolysis was reported to capable of enhancing essential parameters, including quality properties, functionalities, and flavour acceptances in meat and meat products, thus help in producing healthier foods (Barido et al., 2024; Thirumdas et al., 2020). Lee et al. (2025) reported strong intensification on pleasant umami taste following enzymatic hydrolyzation of beef M. semimembranosus using Flavorzyme®. In addition, Qin et al. (2022) elaborated the possibility of enzymatic tenderization using Flavorzyme® to produce a remarkably higher unique peptides, which functionally serve to counteract DPPIV, photoaging activities, and act as antithrombotic, immunomodulatory and promote to an osteoblast proliferation during gastric and gastrointestinal digestion of the cattle Longissimus dorsi. Moreover, Kosasih et al. (2021) reported improvement of essential fatty acids following enzymatic hydrolysis on lemuru fish by-product due to a higher quantity of releasing free fatty acid from its complexes. Further, our recent study reported the enhanced activity on antioxidative capacity and degree of proteolysis in chicken meat following marination treatment using exopeptidase (Barido et al., 2024). It was mentioned that short protein peptides resulting from hydrolyzation process, such as carnosine, anserine, and methyl histidine, exhibit antioxidant properties (Kim et al., 2024; Kobayashi et al., 2008). However, investigation study on the effect of exopeptidase hydrolyzation, mainly using Flavorzyme® on quality attributes of chicken meat during cold storage is absent.
Mushrooms are a popular ingredient for boosting the sensory quality of culinary products. The special and distinct properties, including abundant proteins, polysaccharides, along with bioactive compounds, calcium, phosphates, magnesium, and vitamins play a significant role in enhancing quality and promoting health improvements. These components are integral to the broad functionalities they provide (Barido and Lee, 2021; Guo et al., 2023). Our previous research discovered that mushrooms from the Cordycipitaceae family, particularly Cordyceps militaris (CM) mushrooms, have a pronounced functionality for improving the physicochemical properties and mouthfeel parametrical indexes in meat and meat products (Barido and Lee, 2021; Barido and Lee, 2024; Barido et al., 2022a). According to reports, CM mushrooms include metabolites such as adenine, adenosine, cordycepine, cordyheptapeptide, and polysaccharides, all of which have antifatigue, anti-inflammatory, immunomodulatory, and anticancer activities. Furthermore, the texture-enhancing capabilities of CM mushrooms are due to moderate activity metallo- and serine protease, which contribute to their strong tenderizing effects (Barido and Lee, 2021). This study is essential for exploring the use of serine and metalloproteases contained in CM mushrooms, prepared through enzymatic hydrolysis with Flavorzyme®, as alternatives to synthetic preservatives. It aims to offer crucial insights and practical application into the impact of enzymolyzed CM mushroom using Flavorzyme® on the quality attributes and functional properties of chicken breast during cold storage.
Materials and Methods
The enzymatic hydrolysis of CM mushroom was carried out referring protocols by Guo et al. (2023). Prior extraction, CM mushroom was brought to oven drying (Hauzen, Samsung Electronics, Suwon, Korea) at 50°C with 1.5 m/s air velocity for 18 h. It was then homogenized together with the deionized water (1:2, w/v) using a food blender (Hanil Electronics, Seongnam, Korea) at 1,200×g for 1 min, kept at a regulated temperature of 4±2°C for 6 h. Subsequently, the slurry was centrifuged (1248R, Labogene, Lynge, Denmark) at 2,400×g, 2±2°C for 30 min, wherein the supernatant was regarded as crude extracts. These extracts were then adjusted to pH 7.0 using 2 M NaOH for Flavorzyme® hydrolyzation. The utilized Flavorzyme® was obtained from Aspergillus oryzae, having enzyme activity at a range of 500 to 1,000 leucine aminopeptidase unit/g. The utilized Flavorzyme® characterized by having optimal hydrolyzation reaction at pH of 5.5 to 7.7, with temperature optimum at 50°C to 55°C. Therefore, the hydrolyzation was conducted at 55°C within the waterbath (Memmert WNB14RACK, Memmert, Bayern, Germany) for 2 h. The hydrolyzed extracts were then centrifuged at 3,500×g at 4°C for another 30 min and filtered through Whatman paper no. 1. The filtrate were brough to heating in waterbath at 85°C for 15 min to terminate proteolytic enzyme activities.
The emulsion capacity, including the emulsion stability index (ESI) and the emulsifying activity index (EAI), were assessed using a modified method by Giménez et al. (2009). The process involved combining 1 mL of soybean oil with 3 mL of extract solution at varying concentrations, followed by homogenization at 16,200×g for 1 min using a T25 Basic Ultra-Turrax homogenizer. Subsequently, a 100 μL sample of the emulsion was taken from the bottom of the container immediately and 10 min post-homogenization, diluted to 10 mL with a 0.3% SDS solution (1:200 dilution), and the absorbance was recorded at 500 nm using UV-spectrophotometer (UV-mini 1240 PC, Shimadzu, Kyoto, Japan) at both time points, designated as A0 and A10.
The total phenolic content (TPC) encompasses both total polyphenols and flavonoids. The quantification of TPC was utilizing Folin-Ciocalteu Reagent (FCR), wherein initially, 1 mL of samples was mixed with 50 μL of 2 N FCR. Following vigorous vortexing, the mixture was allowed to stand for 3–5 min at room temperature. Subsequently, 0.3 mL of a 20% sodium carbonate solution was added, and the reaction was left for 15 min. The solution was then diluted with 1 mL of distilled water and its absorbance was measured at 725 nm. The results were presented as milligrams of gallic acid equivalents (GAE mg/g) per gram of the sample, using gallic acid as a standard. Meanwhile, the TFC was determined by the method described by Choi et al. (2011). In this regard, 0.1 mL of extract solution was combined with 0.9 mL of 80% ethanol. Subsequently, an aliquot 0.2 mL sample was added to 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate, and 4.6 mL of 80% ethanol. The reaction mixture was then left to stand for 40 min at 30°C, after which the absorbance was measured at 415 nm. Quercetin served as the standard for the results, which were expressed in milligrams of quercetin equivalents (QE) per gram of the sample.
The 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity assay is conducted through a series of well-defined procedures aimed at evaluating the capacity of antioxidants to neutralize free radicals. Initially, the ABTS radical cation (ABTSo+) is generated by combining equal volumes of 7 mM ABTS stock solution and 2.4 mM potassium persulfate. This mixture is then allowed to incubate in darkness at ambient temperature for approximately 14 h, during which period it transforms into its stable greenish-blue form ABTSo+. Subsequently, the resultant solution is diluted with methanol to attain an optimal absorbance reading of 0.706±0.01 at 735 nm via spectroscopic analysis. The analysis was based on protocol by Nenadis et al. (2004), wherein 1 mL of samples were reacted at room temperature in the dark with the diluted ABTSo+ solutions for 30 min. The scavenging robustness against ABTS radicals were expressed as IC50 values (the concentration required to inhibit 50% of ABTS radical scavenging activity), which was detected at 735 nm.
Preparation of chicken breast sample was initiated by removing the connective tissues in the refrigerated room at 4±2°C. It was done to a total of ninety-six broiler chicken breast samples (112±2 g), obtained from the local market. Each treatment was prepared with 6 replications, consisting of saline buffer-treated samples (SBF), synthetic antioxidant treatment (SYT), CMs and Flavorzyme® hyrolyzed Cordyceps militaris extracts (FEM). The dipping marination was conducted at 4±2°C for 1 h. With regard to storage experiment, marinated samples were brought to a styrofoam plate, covered with a low-density polyethylene film and refrigerated for 9 days at 4±2°C. Sample analyses were carried out at day 0, 3, 6, and 9.
The proximate analysis of the extracts followed the method established by the AOAC (2012). In order to determine the proximate composition of a sample, the crude protein content is first assessed using the Kjeldahl method. A sample weighing 0.1 g is placed into a Kjeldahl flask, followed by the addition of a digestion mixture and 10 mL of concentrated sulfuric acid. The flask is then heated until boiling, after which it is cooled and diluted to a final volume of 100 mL. The resulting solution is titrated with standardized sulfuric acid until the endpoint is reached, indicated by the return of the original pink color. The protein content is subsequently calculated using a conversion factor of 6.25. For crude fat determination, 5 g of dried sample are placed in a thimble and subjected to solvent extraction using petroleum ether in a Soxhlet apparatus for 48 h. After extraction, the solvent is evaporated completely, and the flask containing the crude fat residue is re-weighed to determine the fat content gravimetrically. Meanwhile, the moisture content is measured by drying a known quantity of sample in a hot air oven at 105°C until a constant weight is achieved. The moisture content is calculated by subtracting the final mass from the initial mass. Regarding the ash content, samples were incinerated at a known quantity within a muffle furnace at approximately 550°C until complete combustion occurs. The initial weight of the crucible containing the sample is compared to its weight after ignition to quantify the residual ash, allowing for calculation of ash content based on mass differences. This systematic approach provides essential data on the nutritional composition and quality of the sample.
Instrumental color of the chicken breast’s surface was assessed using a Chroma meter (CR-400, Konica Minolta Sensing, Osaka, Japan) featuring an 8 mm aperture. Calibration was previously performed using a white calibration plate with values (Y=93.6, X=0.3134, Y=0.3194). Measurements of CIE L*, CIE a*, and CIE b* were recorded at five different points on each sample.
The pH level was ascertained by blending a 5 g sample with 45 mL of distilled water using a PH91 homogenizer (SMT, Chiba, Japan) at 12,500×g for 1 min. Subsequently, the pH was measured with a Seven Easy pH meter (Mettler-Toledo, Greifensee, Switzerland), which was calibrated using technical buffer solutions with pH values of 4.01, 9.00, and 7.00.
To evaluate lipid oxidation, the thiobarbituric acid reactive substances (TBARS) method was employed. A 0.5-gram meat part was placed into a TBARS test tube (25 mL). An antioxidant mixture was subsequently added into the tube, followed by addition of 1% thiobarbituric acid in 0.3% NaOH (3 mL). The mixture was then vortexed, added with trichloroacetic acid (2.5%, 17 mL) and sealed. The sample was heated at 100°C for 30 min, cooled in water for 15 min. A 5 mL aliquot of the aqueous phase was transferred to a clean centrifuge tube (15 mL). The mixture was then included with 3 mL chloroform. The mixtures were centrifuged at 2,400×g for 30 min at 4°C (model 1248R, Labogene), while absorbance was evaluated at 532 nm using a UV spectrophotometer against a distilled water blank. Each sample was analyzed in triplicate.
The TVBN was determined based on a specified protocol given by Kobayashi et al. (2008). A 5 g sample was homogenized for 1 min with 90 mL of distilled water using an UltraTurrax T25 basic (Ika-Werke, Staufen, Germany). The resulting liquid was filtered through Whatman filter paper #1 (Whatman, Maidstone, UK). In the inner compartment of a Conway micro-diffusion cell (Shibata, Saitama, Japan), 0.01 N boric acid was placed. Additionally, 1 mL of the sample solution and 1 mL of saturated K2CO3 were placed into the outer compartment of the cell, and the lid was sealed immediately. The cell was then incubated at 37°C for 100 min and titrated against 0.02 N H2SO4. The volatile basic nitrogen (VBN) content was calculated and expressed as mg%.
The shear force value was determined to assess the tenderness of the meat. This was achieved by quantifying the softness assay using the Warner-Bratzler Shear Force test on the TA-XT2i Plus (Stable Micro Systems, Surrey, UK). The chicken breast sample was cut into a 1.5 cm×1.5 cm×1 cm size and positioned under the V blade with a parallel orientation to the fibers. The cutting was performed at a constant speed (pretest speed: 2.0 mm/s; test speed: 1.0 mm/s; posttest speed: 10 mm/s). Each sample was tested five times.
The composition of fatty acid after treatment with enzymolyzed CM mushroom extracts were according to protocol by Folch et al. (1957), wherein the fatty acid composition was determined using a gas chromatography (GC)/flame-ionization detection instrument (6890 N, Agilent, Santa Clara, CA, USA) equipped with an autosampler (7683, Agilent). The procedure began with the extraction of finely ground samples (20 g), performed in duplicate using a chloroform-methanol (2:1 v/v) solution. The fatty acids were then converted into methyl esters through methylation, employing 25% boron trifluoride in methanol at 80°C for 1 h. After methylation, the fatty acid methyl esters were combined with 1.5 mL of hexane, and 1 μL of this mixture was injected into the GC via the autosampler. The injector was set to a temperature of 250°C and a split ratio of 100:1. Separation of the fatty acid methyl esters was achieved using a WCOT-fused silica capillary column (100 m×0.25 mm i.d., 0.20 μm film thickness; Varian, Palo Alto, CA, USA) under a helium flow of 1.0 mL/min. The oven temperature program was detailed as follows: hold at 150°C for 1 min, ramp from 150°C to 200°C at 7°C/min, hold at 200°C for 5 min, increase from 200°C to 250°C at 5°C/min, and finally hold at 250°C for 10 min. The detector temperature was maintained at 275°C. Identification of fatty acids was based on the comparison of retention times with those of fatty acid standards (47,015-U, Sigma-Aldrich, St. Louis, MO, USA), and the proportion (%) of each fatty acid was calculated from the total identified peak area.
The specified variables were subjected to a two-way multivariate analysis of variance (MANOVA). Significant differences in emulsion capacity, ABTS, total phenol, and total flavonoid content, were assessed using a one-way analysis of variance (ANOVA) in R-version 3.6.1. Duncan’s multiple range test was employed to ascertain the mean values for each group. p-values less than 0.05 were considered indicative of significant differences.
Results and Discussion
Table 1 depicts the emulsifying properties of CMs and FEM treatments. Based on the presented table, this study revealed a notable difference between CMs and FEM, in which, the EAI under CMs was recorded at 14.79 m2/g, notably lower than that of FEM, detected at 27.11 m2/g (p<0.05). Accordingly, similar trends were also obtained for ESI parameter, wherein FEM treatment had markedly higher percentage at 15.77, than that of CMs at 14.79% (p<0.05). The hydrolyzation process creates an oil-in-water emulsion with surface-active materials that are characterized by an increase in hydrophilicity and hydrophobicity. Emulsion capacity in general is applied to measure the protein capacity by quantifying stabilized area in comparison to protein weight (m2/g; Noman et al., 2018). These properties comprise both EAI and ESI parameters evaluate proteins’ ability to act as stabilizers in newly formed emulsions and their effectiveness in emulsifying oil. Further, these two variables are key parameters in discovering proteases and protein hydrolysates, as they directly impact stability when proteins are mixed with specific solutions, preventing issues like flocculation, coalescence, or creaming. Therefore, understanding the emulsion’s properties aids in predicting developed products’ shelf life, since they provide a comprehensive illustration of textures and appearances, that would provide hand in quality and formulation optimization (Ang and Ismail-Fitry, 2019). The enhanced emulsifying properties observed in this study align with the findings of Padial-Domínguez et al. (2020), which suggest that the improvement results from synergistic effects between protein structure and properties post-enzymatic treatment. Smaller peptides have a better interaction with the oil and water phases, enhancing emulsion activity. Moreover, enzymatic hydrolysis reduces hydrophobicity, further contributing to the stabilization of emulsions.
| Parameters | Treatments | SEM | p-value | |||
|---|---|---|---|---|---|---|
| SBF | SYA | CMs | FEM | |||
| EAI (m2/g) | ND | ND | 14.79b | 27.11a | 1.43 | <0.05 |
| ESI (%) | ND | ND | 9.21b | 15.77a | 0.03 | <0.05 |
Regarding phenolic content in this study, the enzymatic hydrolyzation effect on CM mushroom is presented in Table 2. Enzymatic hydrolysis did significant alteration on both TPC and TFC content. In terms of TPCS, the highest concentration was recorded at 224.12 GAE mg/g, under FEM treatment, followed by CMs, synthetic antioxidant (SYA), and SBF with 201.45, 197.33, and 0.12 GAE mg/g. Similarly, the TFC quantification revealed that FEM group displayed the highest concentration at 35.81 QE mg/g, followed by numerically 29.61, 25.22, and 0.41 QE mg/g for CMs, SYA, and SBF, respectively (p<0.05). As demonstrated by prior research (Barido et al., 2024; Das et al., 2010), the elevated extractability and free state of polyphenols as a consequence of enzymatic hydrolyzation interventions are the probable causes of the higher documented TPC.
| Parameters | Treatments | SEM | p-value | |||
|---|---|---|---|---|---|---|
| SBF | SYA | CMs | FEM | |||
| Total polyphenol content (GAE) mg/g | 0.12d | 197.33c | 201.45b | 224.12a | 3.02 | <0.05 |
| Total flavonoid content (QE) mg/g | 0.41d | 25.22c | 29.61b | 35.81a | 0.04 | <0.05 |
Enzymatic hydrolysis is a crucial technique for increasing the total phenolic and flavonoid content in plant extracts. This higher observation is due to various distinctly independent mechanisms. It is presumably that complex polyphenol structures and glycosidically-bound flavonoids are broken down through specific enzymatic reactions, including Flavorzyme®, which belong to the hydrolase class (Rawdkuen et al., 2013). This reaction consequently disassembles cell matrices, releasing phenolic acids and flavonoids that were previously bound and making them more bioavailable. This process enhances the yield of extraction in later stages. Additionally, the resulting lower molecular weight phenolics from hydrolysis have improved solubility in water-based solutions, which allows for more accurate measurement in subsequent quantification methods. This discovery is consistent with the previous investigation done by Cho et al. (2023), who discovered that a suitable substrate for enzymatic hydrolysis resulted in an increased number of phenolic acids. The modifications are correlated with both anti-microbial and antioxidant activity of food, as higher concentrations of phenolic acids demonstrate enhanced strength of antioxidant capacity (Cho et al., 2023). The antioxidant properties of both polyphenols are primarily attributed to oxidation-reduction properties, which allow them to operate as hydrogen donors, singlet oxygen quenchers, and provision of redox catalyst (Cho et al., 2023). The findings suggested that enzymatic hydrolysis treatment may be essential for the enhancement of the phenolic compounds in CM mushroom extract and the potential contribution to functional properties, mainly antioxidant capacity.
Evaluation of the antioxidant activity in CMs post-enzymatic hydrolysis treatment was primarily quantified using ABTS scavenging activity. As illustrated in Fig. 1, SBF treatment exhibited the lowest ABTS scavenging activity, with CMs, FEM, and SYA following suit based on the IC50 value (where a lower score indicates higher reduction activity; p<0.05). Notably, the study did not observe significant differences between SYA and Flavorzyme® enzymolysed samples, suggesting comparable antioxidant properties. The IC50 for SYA and FEM were 20.08 and 21.79 mmol/L respectively. The alleviations in ABTS radical scavenging activity following enzymatic hydrolysis treatment utilizing exopeptidases can be elucidated through several mechanistic pathways. Initially, the hydrolytic process liberates bioactive peptides from larger proteinaceous substrates, which inherently possess potent antioxidant properties. These diminutive peptides exhibit heightened solubility and reactivity, enabling them to efficaciously interact with and neutralize ABTS radicals (Sun et al., 2021). Secondly, the enzymatic treatment considerably increases the solubility of the resulting hydrolysate in aqueous settings, allowing for faster diffusion rates and greater interaction with ABTS radicals. The hydrophilic structure of these smaller peptides increases their ability to engage in redox processes, resulting in increased antioxidant activity. Additionally, the enhanced liberation of polyphenol and flavonoid bonds due to enzymatic hydrolysis treatment led to the dominance of proteases, deactivating endogenous oxidative enzymes that could potentially degrade antioxidative compounds in their natural state (Barido et al., 2024). The result is in accordance with previous research by Cho et al. (2023) indicated that fermented CMs displayed a notably higher antioxidant activity in comparison to extract derived from both fresh and dried CM mushroom.
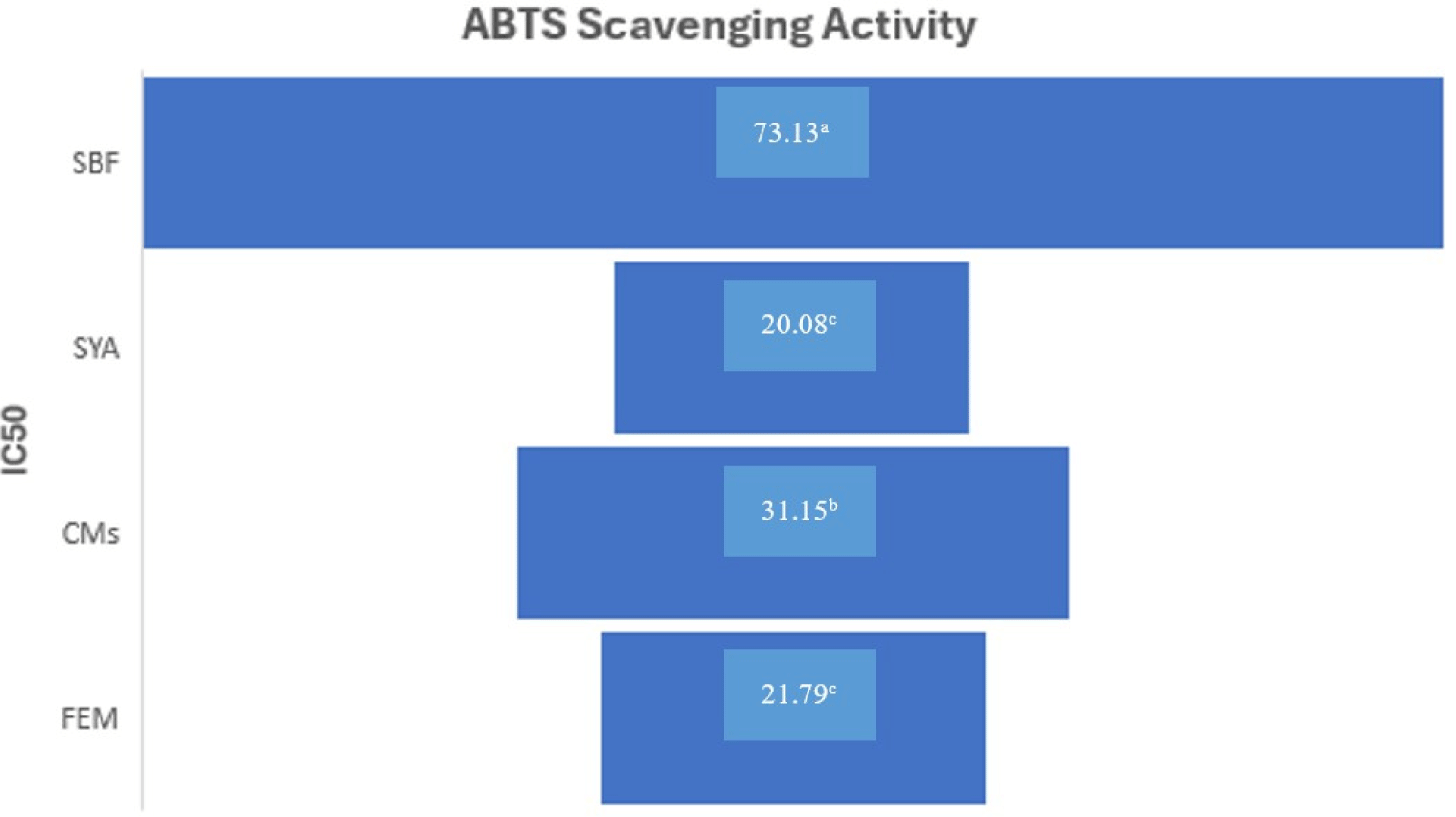
Table 3 visualized notable alteration on proximate composition of chicken breast after enzymatic hydrolysis using Flavorzyme®. Specifically, moisture content was recorded ranging from 70.20%–75.32%, with SYA treatment exhibiting the highest percentages (p<0.05). Treatment of chicken breast with FEM significantly elevated its protein content, rising from 20.69% with SBF treatment to a peak of 27.67% under FEM (p<0.05). This value surpassed even that of CMs, which recorded a protein content of 24.19% (p<0.05). Meanwhile, the Soxhlet extracted fat content following enzymolysis was not in significant manner for all treatment, whilst the ash content was closely similar (p>0.05). The enzymatic hydrolysis of chicken breast with proteolytic enzymes, including endo- and exopeptidase, possibly changes its proximate composition and improves its nutritional and functional qualities. The process stimulates an increase in the degree of hydrolysis, leading to the conversion of larger protein molecules into smaller peptides and amino acids. In addition, enzymatic hydrolysis not only boosts peptide content but also enhances the solubility and bioavailability of these compounds. Prior studies showed the proximate composition to experience a modest rise in protein concentrations due to the release of bioactive peptides, whereas the fat content tends to stay relatively minimal, at approximately 1%–2% (Barido et al., 2024; Dong et al., 2020; Kotlar et al., 2013; Maky and Zendo, 2023). Further, this study also propose a CM mushrooms as a compelling subject for experimenting with protease or protein hydrolysate production, as the protein content plays a crucial role in determining their functional use.
Table 4 illustrates alterations in the chicken breast’s surface color following immersion into enzymolyzed CMs. During cold storage, both variables of storage and treatments significantly influenced color formation (p<0.05). The CIE L* value tended to be lower under FEM treatment when compared to that of SYA. Yet, no differences were observed among SBF, CMs, and FEM throughout storage periods (p>0.05). Regarding the intensity of red color, a notable increament was seen under SYA, CMs and FEM treatment groups, accounted for 1.91, 1.82, and 1.86 respectively (p<0.05). Furthermore, basic yellow color of CMs led to enhanced yellow coloration in the breast meat, as evidenced by significantly higher CIE b* values, regardless of the enzymolysis process using Flavorzyme® (p<0.05). Alterations in meat color have been attributed to various factors, including the natural color of the marinades. Previous research has established that phytochemicals from plant extracts can penetrate into meat muscle, resulting in modifications to the measured color parameters (Barido and Lee, 2021; Suman and Joseph, 2013). In addition, over the time, myoglobin oxidation the meat discoloration, transforming oxymyoglobin with tendency of creating red color into metmyoglobin with brown color profiles, while the increased red color profile appears to enhance consumers’ preference toward displayed meat in retail (Suman and Joseph, 2013). This study aligns with previous investigation, showing a decrease in the CIE L* value of various mushrooms, namely bunashimeji, enoki, oyster, and shiitake after enzymolysis treatment using endo- exopeptidase (Ang and Ismail-Fitry, 2019). Furthermore, this finding is also consistent to that of Nenadis et al. (2004), investigating the effect of hydrolysis on squid head. The reason comes from the implication of increased release of peptides during enzymolysis, which causes change in measured parameters.
Treating breast meat samples with CMs and FEM significantly altered their biochemistry, as evidenced by the pH values presented in Table 5. On the first day of storage, the saline buffer treatment resulted in the lowest pH value at 5.91, compared to the pH values of 6.02 for SYA, and 6.02 and 6.13 for CMs and FEM treatments, respectively (p<0.05). Throughout the storage period, pH values fluctuated in most treatment groups except for the FEM treatment. Moreover, enzymatic hydrolysis was observed to substantially reduce the pH value, starting at 6.13 on the first day and decreasing significantly from the third day, maintaining this lower level until the end of the storage period (5.98). The measured pH value of breast meat ranged between 5.91 to 6.13 at initial storage day, within the range for normal pH as reported by previous studies (Barido and Lee, 2022; Lawrie and Ledward, 2014; Sujiwo et al., 2018). In addition, apart from exhibiting the highest pH value, the present study revealed that enzymolysis notably displayed a significantly higher pH value than that of non-enzymolysed ones (CMs; p<0.05). The observed pH elevation in this study could be ascribed to the basic nature and high hydrogen ion concentration in rich-polyphenols CMs (Bozkurt, 2006). All experimental groups, including those treated with SYA, sustained pH levels within the acceptable range, not surpassing 6.15, wherein breast meat with a pH exceeding 6.20 is deemed spoiled, a condition linked to protein degradation and pathogenic bacterial growth (Giménez et al., 2009; Kotlar et al., 2013). The pH value is a vital biochemical indicator in meat quality evaluation, closely associated with water retention, color, texture, and consumption safety (Lawrie and Ledward, 2014). Further, an increase in meat pH is an unavoided nature during storage due to bacterial activity that produces biogenic amine, and protein denaturation. Also, although clear mechanisms are not well elaborated, increased pH value after enzymatic hydrolysis are presumably due to the more liberation of individual amino acid like lysine and arginine, leading to an increase in the overall pH of meat. At the same time, the meat’s buffering capacity causes a pH rise when alkaline conditions prevail during hydrolysis, due to protein solubilization and the release of basic amino acids (Kurozawa et al., 2008).
Table 5 displays the shear force and myofibrillar fragmentation index (MFI) values of chicken breast following treatment with CMs, FEM and both control groups. The breast meat demonstrated significant tenderization after exposure to the hydrolyzed extract (FEM), which exhibited the lowest shear force at 2.25 kgf at the initial storage period (p<0.05). Subsequently, samples marinated using CMs were the second most tender, recording a shear force of 2.45 kgf, followed by SBF and SYA at 2.76 kgf and 2.89 kgf, respectively (p<0.05). In addition, storage experiments revealed significant influences, in which ultimate storage day (9 d) possessed markedly lower value in comparison to that of day 0 (p<0.05). The result aligns with our previous study, documenting possible improvement in texture properties after marination using CMs (Barido and Lee, 2021). The serine and metalloprotease from CMs may synergistically act to upregulate endogenous proteolytic activity in chicken meat, promoting larger solubility of key proteins and more fragmentation of myofibrillar proteins. Accordingly, as also observed in this present study, the MFI values of breast meat experienced the largest fragmentation under FEM, indicated by the highest MFI score at 50.02 (p<0.05). The subsequent order was CMs with MFI at 39.91, while SBF and SYA groups having 31.15 and 17.11, each (p<0.05). The myofibrillar protein experienced continuously higher fragmentation until the end of storage period, regardless of treatment groups (p<0.05). The fragmentation of myofibrils is a key indicator of postmortem tenderization, quantifying the degradation of myofibrillar proteins. This degradation is generally linked to the activity of both endogenous and exogenous enzymes, as well as fluctuations in nucleotide concentrations (Bekhit et al., 2014). As also measured by this study, utilizing casein as a substrate, the proteolytic activity of fresh CMs extract and Flavorzyme® hydrolyzed CMs extract was at 2.88 and 4.14 unit/mL respectively (data not shown), which possibly support the higher fragmentation under FEM-treated samples. The level of fragmented protein signifies structural changes and the degradation of key proteins such as desmin, nebulin, troponin-t, vinculin, and titin, which result from hydrolytic reactions driven by endogenous enzymes (Bekhit et al., 2014). Furthermore, the improved tenderness observed under treatment with both CMs, and FEM suggest to broader binding sites for substrate interaction, wherein CM mushroom is known to possibly bind to specific sites within myofibrillar proteins. The hydrolysis process by proteases within meat matrices involves the structural modification of protein molecules via specific amino acids, act as functional regulator and utilize water, lowering the harmful effect from the occurrence of cancer cell and toxicity in comparison to chemical methods (Bekhit et al., 2014). Besides, the alterations in molecular architecture resulted in an increase in umami-related compounds and improved protein solubility. In this manner, Flavorzyme®, developed from A. oryzae, is an enzyme with endo and exopeptidase activity. This enzyme type has sparked widespread attention in the biotechnology industry due to its various biological features. These qualities include antibacterial, antifungal, and insecticidal actions. Furthermore, studies have demonstrated that Flavorzyme® originated from A. oryzae possesses cardioprotective, immunomodulatory, anti-inflammatory, and antioxidant properties (O’Sullivan et al., 2019). This study suggested that the incorporation of Flavorzyme® in the hydrolysis process may further enhance the CM protease’s ability to degrade a more diverse array of substrates (Das et al., 2010).
Fig. 2 illustrates the variations in total VBN levels following marination in different solutions. Initially, VBN levels were between 6.08 and 7.04 mg/100 g, which is typical for fresh meat. From the third day of storage, chicken breast meat marinated with FEM consistently showed significantly lower VBN levels compared to the control group, a trend that persisted until the end of the storage period (p<0.05). In contrast, there was no significant difference in VBN levels compared to CMs until day 3. However, by days 6 and 9, FEM-treated samples exhibited lower VBN concentrations of 6.99 and 7.02 mg/100 g, respectively, compared to CMs, which had levels of 7.26 and 8.21 mg/100 g. Additionally, the study found that all treatment groups, with the exception of SBF, maintained VBN values below 10 mg/100 g, indicating the meat remained within the acceptable freshness range. As expected, storage period did significant influence on the intensification of VBN concentrations, since it quantifies the total concentration of nitrogenous compounds, primarily ammonia and amines, that are produced during the degradation of proteins as meat spoil (Sujiwo et al., 2018). In addition, although FEM group displayed a tendency of lower VBN value among treatment groups, no significant differences were found when compared to SYA, suggesting potential utilization of FEM as a natural preservative, replacing synthetic additives for extending chicken meat’s shelf life.
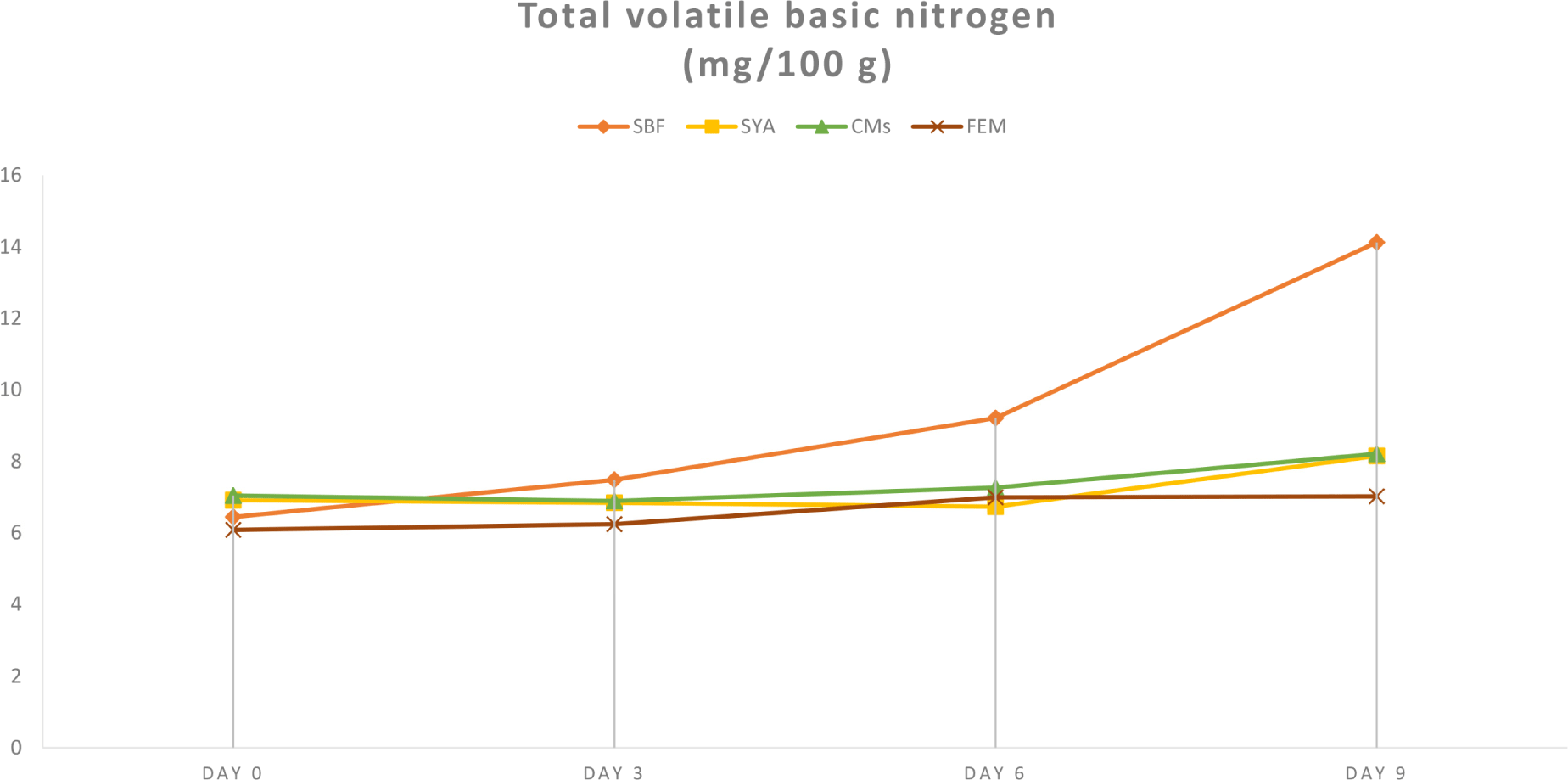
The lowering effect from utilization of lavorzyme on VBN is presumably achieved through breaking down proteins into smaller peptides and amino acids, which are less prone to causing spoilage. The process targets peptide bonds, reducing the amount of protein that can be converted into ammonia and other volatile bases during decomposition. As hydrolysis continues, the peptides produced help inhibit microbial growth and the enzymatic actions that increase total VBN levels. Prior studies indicate that with proper optimization, this enzymatic approach can significantly reduce VBN, extend the shelf life of meat, and produce bioactive peptides with antioxidant and antimicrobial qualities, thereby enhancing meat preservation and reducing spoilage (Kong et al., 2017; Noman et al., 2018; Zhang et al., 2016). Moreover, our previous study demonstrated that microorganisms’ protein degradation was delayed in chicken breast meat treated with detoxified Rhus verniciflua extract, resulting in lower VBN values (Barido and Lee, 2022). Similarly, another study found that high polyphenol and flavonoid plant extracts significantly reduced total VBN levels in treated meat (Qin et al., 2022).
The TBARS assay quantifies reactive substances that contribute to undesirable flavors, especially malondialdehyde (MDA), which is produced by lipid autoxidation during storage. Table 6 illustrates that the TBARS value of chicken breast following treatment in various marinade solutions. During the initial storage period, the concentration of formed MDA did not differ significantly among the treatment groups, ranging from 0.051 mg MDA/kg in the SYA group to 0.059 mg MDA/kg in the SBF group (p>0.05). On the third day of storage, FEM showed a robust inhibition of MDA formation comparable to SYA, with concentrations of 0.056 mg MDA/kg and 0.054 mg MDA/kg, respectively. By the sixth day, the concentration was even lower in the FEM-treated samples at 0.069 mg MDA/kg, compared to 0.076 mg MDA/kg in the SYA-treated samples (p<0.05). However, there was no significant difference in MDA formation between FEM and SYA during the final storage periods. It indicates similar efficacy of enzymolyzed CMs to counteract lipid oxidation as SYA does. Furthermore, this study recorded that FEM treatments demonstrated stronger suppressive activity against MDA formation compared to SBF and CMs, starting from day three and continuing until the end of the storage period.
A–D Means with different superscripts in the same column indicate a significant difference from storage period (p<0.05).
SBF, saline buffer-treated samples; SYA, synthetic antioxidant treatment with butylated hydroxyanisole; CMs, Cordyceps militaris extracts; FEM, Flavorzyme® hydrolyzed Cordyceps militaris extracts; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde; MFI, myofibrillar fragmentation index.
Enzymatic hydrolysis plays a crucial role in inhibiting the formation of MDA, a byproduct of lipid peroxidation, by breaking down polyunsaturated fatty acids (PUFAs) and modifying the oxidative conditions within biological systems, particularly meat proteins. The process is driven by the activity of enzymes including proteases, which target triglycerides and proteins for hydrolysis. This action limits the substrates needed for free radical reactions that cause lipid peroxidation, thus reducing MDA concentrations. Furthermore, the enzymatic hydrolysis produces bioactive peptides and fatty acids with antioxidant capabilities, which help alleviate oxidative stress and block MDA formation by neutralizing reactive oxygen species. Research has shown that enzymatic treatment can markedly decrease MDA content in different food products, suggesting a safeguard against oxidative harm (Das et al., 2010; Elias et al., 2006). For example, studies indicate that oils processed enzymatically have lower MDA amounts than those untreated, underscoring the effectiveness of this method in improving food preservation and safety (Baena et al., 2022). In essence, enzymatic hydrolysis acts through a dual mechanism, namely diminishes the substrates available for MDA production and bolsters the antioxidant defense of the system. Further, the result of this study aligns with Guo et al. (2023) on morel mushrooms, wherein the enzymatically-hydrolyzed extract showed a robust antioxidant capacity. The increased formation of shorter chain peptides due to the degradation of intact proteins by enzymes was noted to enhance the efficiency of the protease powder in reducing interfacial tension and stabilizing emulsions, attributed to the rapid diffusion and adsorption at the interface (Ang and Ismail-Fitry, 2019; Das et al., 2010). Moreover, these bioactive compounds, known for their health benefits, can also reduce lipid oxidation rates through their potent reducing power, reductone activity, and strong radical-scavenging capabilities.
The fatty acid profiles following treatment using various marinade solutions are illustrated in Table 7. Its percentages were dominated by monounsaturated fatty acid (MUFA), saturated fatty acid (SFA), and PUFA, respectively, with the main individual fatty acid composed of oleic acid, linoleic acid, palmitic acid, stearic acid, and palmitoleic acid. The results were in accordance with that of previous studies in chicken breast (Barido et al., 2022b). In particular, percentages of SFA were directly influenced by treatment under marination using enzymolyzed CM mushroom, wherein FEM-treated groups showed the lowest proportion, followed by CMs, SYA, and SBF, respectively (p<0.05). There were no differences in SFA between FEM and CMs obtained by this present study (p>0.05). Retained concentration of stearic and palmitic acid under SYA and SBF were the reason for emergingly higher SFA percentages. Although main mechanism was not clearly elaborated, the conversion of SFA into unsaturated form of fatty acid, via SFA desaturation were thought to influence the lower SFA level. The unsaturated fatty acid desaturases from various sources including plant extracts and hydrolysate are capable of transforming SFAs into MUFAs and PUFAs through the insertion of double bonds within the acyl chain. This alteration is vital for the modification of fatty acid structures, influencing their biological roles and implications for health (Wereńska et al., 2021). Besides, enzymatic hydrolysis influences fatty acid composition in meat by breaking down triglycerides into their constituent parts, namely fatty acids, monoacylglycerols (MAG), and glycerol. This process is mediated by both endopeptidase and exopeptidase, which specifically target the ester bonds linking fatty acids to glycerol within triglycerides. During this process, the protease catalyzes stepwise cleavage of these esters, releasing fatty acids into solution while leaving behind MAG and eventually glycerol. In addition, the specific mechanism involves an initial complex formation between the enzyme and substrate (triglyceride), followed by the release of one or more fatty acid residues through intermediate complexes involving MAG. This sequential process ensures that each fatty acid chain is released individually, potentially altering their distribution and concentration within the meat matrix (Das et al., 2010; Larsen et al., 2010; Wereńska et al., 2021). In addition, protection of lipid passage from oxidation is presumed to influence a higher percentage of MUFA and PUFA in this study. A consequence from meat environmental stress due to oxidation is the structural modification of SFA carbon chain formation, leading to the formation of other compounds, like the 2-octenal, 2-nonenal, 2-heptanal, and benzaldehyde of the aldehydes (Kim et al., 2020; Larsen et al., 2010). Therefore, aggregating meat and meat products with antioxidant-rich herbs and spices can prolong the beneficial impact of unsaturated fatty acids by inhibiting the rate of lipid oxidation. As observed under this present study, FEM-treated samples possessed a MUFA ranged from 47.35% to 48.98%, not differed with that of CMs treated samples, which having 46.38%–47.12%. Meanwhile, a MUFA percentage at the range of 45.44%–46.96% was observed in SYA treated groups and range was seen under SBF treated samples. Besides, the PUFA percentages shared a similar pattern to that of MUFA. This reduction in total PUFA percentage was primarily due to a lower proportion of individual PUFAs, especially that of linoleic acid. Accordingly, the notable decrease of oleic acid resulted in a marked differences of MUFA percentages under various treatment subjections.
Conclusion
The findings of this study suggest that enzymatic hydrolysis with Flavorzyme® is an effective method for enhancing the quality and functional properties of chicken breast meat. The higher bioactive compounds and antioxidant properties can contribute to the development of healthier meat products, addressing consumer preferences for safer and more nutritious food options. This study suggested that enzymolysis of CM, prior addition to chicken breast meat have significant implications for enhancement of functional meat products.













