Introduction
Chicken meat is commonly used for the production of burger patties and many other ready-to-eat (RTE) muscle foods owing to its notable nutritional value and distinctive flavor (Jahan et al., 2004; Patsias et al., 2008). However, poultry-based muscle foods are highly perishable products because of the onset of oxidative rancidity even at refrigeration temperatures (Al-Juhaimi et al., 2016; de Santana Neto et al., 2021). Preceding studies found that lipid oxidation can be controlled in muscle foods by means of the addition of natural or food-grade synthetic additives (Armenteros et al., 2016; Ferreira et al., 2017; Mielnik et al., 2003; Serra et al., 2021). Additives with antioxidant activity such as nitrites, ascorbate and sulphur compounds are regularly used in the food industry. However, there is a growing demand among consumers for the so-called “clean label” foods in which classical additives are replaced by bioactive compounds obtained from plant kingdom (Kola and Carvalho, 2023; Zhu, 2021). On this line, profuse research has been carried out for assessing the antioxidant effects of Mediterranean fruits and berries, such as rose hips (Rosa canina L.), oak nuts (Quercus ilex subsp. ballota), strawberry tree (Arbutus unedo L.) or common hawthorn (Crataegus monogyna Jacq.) in various meat and RTE products. Some examples are lamb chops (Morcuende et al., 2020), cooked pork hams (Armenteros et al., 2016), RTE pork (Ganhão et al., 2010), beef patties on high-oxygen modified-atmosphere packaging (Vallejo-Torres et al., 2023), chicken patties (Ferreira et al., 2017), smoked beef sausages (Zheleuova et al., 2021) and frankfurters (Vossen et al., 2012). Notably, one of the above-mentioned Mediterranean fruits, oak nut (Q. ilex subsp. ballota), was selected and applied as sprayed extract on lamb chops and prevented lipid and protein oxidation during chilled storage (Morcuende et al., 2020). By using an extract from oak nut, Ferreira et al. (2017) extended the shelf-life of RTE chicken patties and increased consumer acceptance. While acorns are a profuse and costless source of nutrients and bioactives for humans and animals in the Mediterranean forest, it is still underused and hence, their potential as food ingredient/additive worth further applications.
As noted by de Santana Neto et al. (2021), the means of antioxidant application may affect the antioxidant effectiveness of plant phenolics and currently, several innovative strategies are being introduced in the meat industry such as the manufacture of processed meat products with edible films. Edible films and coatings are key components in the food industry due to their ability to improve the quality, safety, and shelf life of foods (Tavassoli-Kafrani et al., 2016). They are composed of edible ingredients such as polysaccharides, proteins and lipids, which create a thin and flexible layer capable of wrapping or coating foods (Zhu, 2021). In the context of meat products, edible coatings offer notable advantages (Tavassoli-Kafrani et al., 2016). First, they serve as protective barriers, reducing moisture loss and, consequently, mitigating dryness, while also improving meat succulence (Xie et al., 2023). Additionally, these coatings function as protective layers against external contamination and oxidation, thus extending the shelf life of the meat product (Kandasamy et al., 2021; Xie et al., 2023). Alginate, commonly used in the formulation of edible coatings, is a polysaccharide obtained from seaweed (Song et al., 2011; Tavassoli-Kafrani et al., 2016). Alginate is a copolymer with a structure of (1→4)-α-L-guluronate with variable residues of (1→4)-β-D-mannuronate which has been shown the ability to form translucent, uniform, and resilient water soluble films (Mahcene et al., 2020). The ability of this biomolecule to form gels and flexible films is well known (Xie et al., 2023), and hence, it is a suitable candidate for the development of food coatings. In addition, alginate-based coatings can be enriched with natural extracts rich in polyphenols, bioactive compounds widely recognized for their antioxidant properties (Pei et al., 2022). For example, Song et al. (2011) applied edible alginate-based coatings to golden carp (Megalobrama amblycephala) meat showing remarkable reduction in moisture loss and lipid oxidation rate.
These innovative edible films, backed by scientific research, have substantial potential to revolutionize the food industry and meet the demands of contemporary consumers for safe and high-quality food products (Zhu, 2021). However, the utilization of edible films infused with plant-based antioxidants for safeguarding RTE muscle foods against oxidative reactions has remained relatively unexplored. In light of this gap, the current study was undertaken to analyze the efficacy of an alginate-based edible coating enriched with a high-content acorn phenol extract. The primary objective was to enhance oxidative stability, counteract discoloration, and evaluate the influence on sensory preferences, particularly in relation to the olfactory appeal, of RTE patties.
Materials and Methods
Chemical species and reagents for analytical procedures were acquired from Extrasynthese (Genay, France), Scharlau (Scharlab, Sentmenat, Spain) and Merck (Merk, Darmstadt, Germany).
All material for coating applications were commercial food grade ingredients and were supplied by Sosa Ingredients (Moià, Spain). Sodium alginate (Sosa Alginat®) from brown algae (Fucus, Laminaria, Macrocystis spp.) was used as a polysaccharide-based edible coating. Glycerol was applied as a plasticizer. Finally, a mixture of calcium lactate hydrate and calcium gluconate, commercially named as Gluconolactate®, was used for gel forming and cross-linking reactions.
Acorns from different specimens of evergreen oaks [Q. ilex L. subsp. ballota (Desf.) Samp.], were harvested as full ripened fruits in the Caceres region, Spain. Straightforwardly, the samples were transported to the laboratory, washed, and sorted to eliminate damaged fruits. They were subsequently frozen at –80°C. Fresh boneless and skinless chicken thighs for burger patties were acquired from the company Avinatur Producciones (El Viso del Alcor, Spain).
Extracts were produced following the procedure described by Cando et al. (2014) with some modifications. After defrosting, shells from acorns were removed, and the fruit was then cut into pieces, and finely grounded. One hundred grams of shredded acorn was divided equally between two 250 mL wide-mouth LDPE centrifuge bottles with closure (Nalgene, Ruchester, NY, USA), and dispersed with 180 mL of 80% food-grade acetone using an Omni-mixer homogenizer (model 5100). The ensuing homogenates were dispensed into an ultrasonic bath (Ultrasound, J.P. Selecta, Barcelona, Spain) for 30 min and subsequently stored in darkness for 12 h at 3°C. After maceration, samples were centrifuged at 150×g for 10 min at 4°C. The supernatants were collected and mixed in an Erlenmeyer flask and subsequently concentrated using a rotary evaporator at 40°C. The resultant aqueous residue was brought to volume (100 mL) with distilled water, analyzed for total phenolic contents (TPCs) and eventually stored at 1°C until subjected to all other analyses (<24 h). The extraction technique herewith explained was optimized in a previous study to maximize the amount of bioactive compounds.
The TPC of acorn aqueous extracts was assessed following the Folin-Ciocalteu procedure (Soong and Barlow, 2004) with minor modifications. An aliquot of 200 μL of diluted extract (1:100) was mixed with 1,000 μL of 1:10 diluted Folin-Ciocalteu’s phenol reagent, followed by 800 μL of 7.5% (w/v) sodium carbonate. The mixture was shaken and allowed to stand for 45 min at 20°C temperature in the dark. In due course, the absorbance was measured at 765 nm (Hitachi spectrophotometer, Hitachi, Tokyo, Japan). The concentration of total phenols was calculated using a standard curve of gallic acid.
An optimized food grade alginate/Ca2+ coating formulation was used, based on Song et al. (2011) with modifications. Alginate solution was prepared by slowly dissolving 30 g of sodium alginate in 1 L of sterile distilled water at 40°C. The mixture was initially blended for 10 min until a homogenous solution was achieved. Then, it was stirred for 2 h at 70°C until the mixture turned into a clear solution. After this was achieved, the temperature was decreased down to 30°C and then, 200 mL of glycerol was added under magnetic stirring. The basic film was prepared by adding 100 mL of solution containing distilled water. For the preparation of the film impregnated with acorn extract, 100 mL of such aqueous acorn extract (800 GAE equivalent according to TPC) was added. Thereafter, the mixed solution was made up to 2,000 mL with distilled water and mixed under magnetic stirring for 10 min and finally refrigerated (Ta<4°C) before coating applications.
The experimental patties were produced in a pilot plant. All patties were manufactured using the same basic formulation. For each replicate, 1.5 kg of chicken patties was prepared by using the general recipe as follows (g/kg raw batter): 800 g chicken thighs, 180 g distilled water and 20 g sodium chloride. Fresh boneless and skinless chicken thighs (5.29% fat and 19.87% protein, according to the manufacturer) were purchased from a local supermarket in Cáceres (Spain).
As previously made by Ganhão et al. (2010) for the manufacture of emulsified pork patties, chicken thighs were first cut into 2.5 cm3 pieces and minced through a 4.5 mm plate (Mainca mincer, Mainca, Barcelona, Spain). Next, all ingredients were minced using a bowl cutter (CM-14, Mainca) until a homogeneous batter was achieved (6 min/2,000 rpm/Ta<8°C). Chicken patties (43 g, 5 cm diameter and 1 cm thickness) were molded from the emulsion using a semi-automatic hamburger maker (MH-55, Mainca). Finally, patties were cooled down to 4°C for 2 h before coating applications.
Raw chicken patties were randomly divided into three groups depending on the coating strategy, namely, control patties without coating “CON”, patties with alginate coating “FILM”, and patties with alginate coating containing 800 GAE of acorn extract “FILM-ANTIOX”. This concentration was chosen based on previous studies (Ferreira et al., 2017) that guaranteed positive antioxidant outcomes under the tested conditions. Within each of these three experimental groups, three additional subgroups of samples were considered depending on the technological treatment and processing stages applied to patties, completing a 3×3 factorial design. The processing stages were: “COOKED”, cooked patties at day 0 after coating application, “CC”, cooked and chilled patties, and “CCR”, which correspond to cooked and refrigerated patties subjected to subsequent reheating in a microwave. Two chicken patties per experimental group and processing stage were produced (technical replicates) and the entire experimental procedures was repeated tree times in independent production batches (true replicates). Fig. 1 shows the entire technological process and the preparation and application of the edible films.
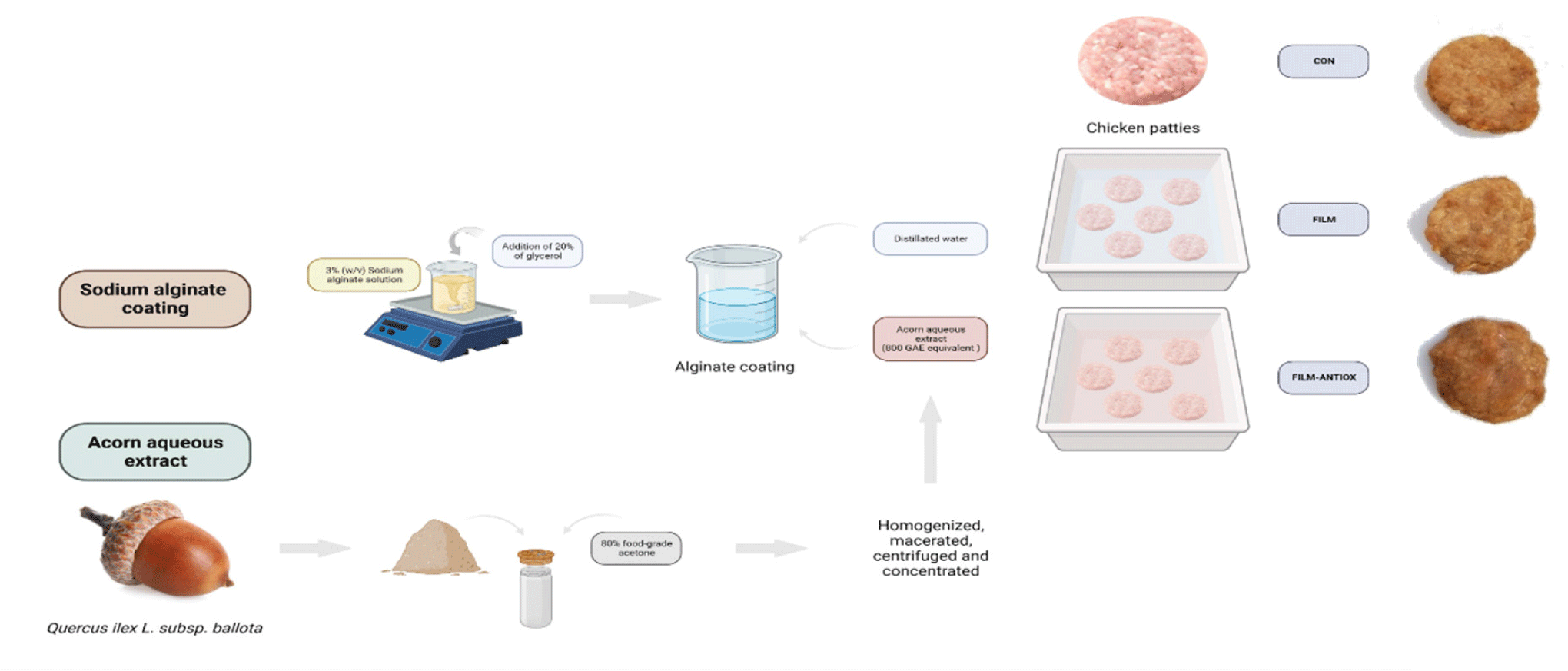
Coating applications were as follows: “FILM” and “FILM-ANTIOX” patties (6 units per batch) were placed in a polypropylene drain grate and immersed in 1.5 liters of their respective alginate solutions. After 2 minutes, patties were removed and allowed to drain for 1 minute and subsequently, immersed in 1.5 liters of 4% calcium gluconolactate for another two minutes to complete the formation of the coating. After 2 hours of refrigeration, coated and uncoated patties were cooked in an electric oven at 170°C for 18 min (9 min each side; GN2.1, Unox, Cadoneghe, Italy). Preliminary cooking trials were performed to establish the cooking conditions required to achieve a temperature of 73°C in the core of the product. Then, coated and uncoated patties belonging to the “COOKED” group were frozen at –80°C for further analysis. The remaining patties were dispensed in polypropylene trays, wrapped with polyvinyl chloride film (Tecnodur, Valencia, Spain) and stored at 4 1°C for 8 days in darkness. After this storage, two batches from each treatment were taken randomly; one was frozen at –80°C (“CC” patties), and the remaining patties were reheated in a microwave (MW 213 INOX, TEKA, Barcelona, Spain) for 1 minute at 600 W of net power (“CCR” patties). Upon reheating, “CCR” patties were also frozen at –80°C for further analysis.
The weight of the individual patties was recorded during the different stages of processing and storage. The percentage of weight gain in relation to coating absorption was calculated as follows: [(Wc – Wu) / Wu] × 100 where Wc and Wu are the weights of the chicken patties before and after alginate coating application, respectively. The weight loss during oven cooking or microwave reheating was calculated as follows: [(Wb – Wa) / Wb] × 100 where Wb and Wa are the weights of the chicken patties before and after thermal treatments, respectively. Storage loss was calculated as the weight loss during refrigerated storage of cooked chicken patties as follows: storage loss = [(W0 – W8) / W0] × 100 where W0 and W8 are the weight of the cooked patties at days 0 and 8, respectively.
Color analyses were made on the surface of cooked chicken patties using a Minolta CR-300 chromameter (Meter Division, Konica Minolta, Ramsey, NJ, USA). Previous to the assessment of color, the chromameter was calibrated on the CIE color space system using a white tile. The CIE L*-value, CIE a*-value and CIE b*-value values were recorded from the average of three random readings across each patty surfaces. Color measurements were made at room temperature (≈22°C) with illuminant D65 and a 0° angle observer.
Thiobarbituric acid reactive substances (TBARS) and other reactive secondary products of lipid oxidation were quantified in chicken patties using the 2-thiobarbituric acid (TBA) methods following the procedure reported by Ganhão et al. (2011) with slight modifications. Briefly, 5 g of chicken patty were dispensed into 50 mL polypropylene tubes and homogenized with 15 mL of perchloric acid (3.86%) and 0.5 mL butylated hydroxytoluene (4.2% in ethanol). Two mL of the filtered and centrifuged suspension (260×g for 5 minutes) were mixed with 2 mL of TBA (0.02 M) in screw cap test tubes. The tubes were placed in a boiling water bath for 45 minutes together with the standard curve tubes. After cooling, the sample were centrifuged at 260×g for 5 min. Absorbance was measured at 532 nm against a blank containing 2 mL of the extraction solution and 2 mL TBA solution. The standard curve was prepared using a solution of 1,1,3,3-tetraethoxypropane in 3.86% perchloric acid. Results were calculated as mg malondialdehyde per kg of chicken patty.
The total carbonyl content was used as a marker of the extent of protein oxidation and analyzed using 2,4-dinitrophenylhydrazine (DNPH) method according to the procedure reported by Ganhão et al. (2010), with minor changes. Chicken patties (1 g) were ground, mixed with 1:10 (w/v) ratio 10 mL 0.6 M NaCl in 20 mM sodium phosphate buffer (pH 6.5) and then homogenized for 30 s using an Ultra-Turrax homogenizer. Two aliquots of 150 μL, each, of this homogenate were used to quantify total protein concentration and total protein carbonyls, respectively. In both cases, the proteins were precipitated with 1 mL of cold 10% trichloroacetic acid (TCA) after centrifugation (4°C) at 2,000×g. For the determination of carbonyls, 1 mL of 0.2 % DNPH in 2N HCl was added. For protein concentration, 1 mL of 2N HCl was added. After incubation at room temperature for 1 h, the proteins were again precipitated with 1 mL of cold 10 % TCA and centrifuged at 1,800×g for 10 minutes. After two washes with 1 mL of ethanol/ethyl acetate (1:1, v/v) followed by centrifugation at 1,800×g for 5 minutes, the precipitated proteins were dissolved in 1.5 mL of 20 mM phosphate buffer (pH 6.5) with 6 M guanidine HCl solution. The protein concentration of the samples was calculated from the absorbance read at 280 nm using a five-point of albumin standard curve. The amount of carbonyl was expressed as nmol of carbonyls per mg of protein using a hydrazone molar extinction coefficient (21.0 nM–1 cm–1) with absorbance reading at 370 nm.
The trained sensory panel consisted in 19 assessors aged between 23 and 60 and all were regular consumers of chicken products. Prior to the assessment of samples, panelists attended 3 training sessions during which they assessed similar products with increasing intensity levels of the attributes under investigation (rancidity and warmed-over favor). Assessors provided a written consent for their involvement in the study which was approved by the Ethical Committee from the University of Extremadura (IRB: 2516/23). They carried out an odor analysis of “CCR” patties as described as follows. All tests were conducted at room temperature (20°C±1°C) and in individual booths located in standardized sensory cabins. Coated and uncoated patties were evaluated for the intensity of rancidity and warmed-over-flavor (WOF) using a 10-point scale (1=non perceptible; 10=extremely intense), and overall acceptability employing a 7-point scale (1=extremely dislike; 7=extremely like). Five grams of each sample were finely minced, dispensed in falcon tubes, sealed, and wrapped with aluminum foil and offered to the panelists after being warmed up to 37°C in a thermostatic chamber. All samples were blind coded with 3-digit random numbers and the orders of serving samples were randomized.
The application of alginate coatings with acorn extracts (main variable under study) was repeated three times in three independent processing batches. Two chicken patties per experimental group (“CON”, “FILM”, and “FILM-ANTIOX”) and per processing stage (“COOKED”, “CC” and “CCR”), were produced in each batch, totaling 54 chicken patties, and consequently, means and SDs were calculated from 6 data (3 technical replicates×2 true replicates). Data were evaluated using a two-way analysis of variance (ANOVA) to assess the effect of coating (3 levels) and processing stage (3 levels) along with interaction. Tukey’s test was performed when ANOVA revealed significant differences (p<0.05) among treatments. The significance level was set at p<0.05. The SPSS computer program (v. 21.0, IBM, Armonk, NY, USA) was used to perform the statistical test.
Results and Discussion
The concentration of bioactive compounds and antioxidant activity of the acorn extract have been detailed in previous work (Morcuende et al., 2020). Briefly, remarkable contents in total phenolics (~2,055 mg GAE/100 fruits) were found in the extracts within these quantities exceeding those reported by Ganhão et al. (2010); Ferreira et al. (2017) and Cantos et al. (2003) in the same fruits, Among the specific phenols of interest, we identified hydroxybenzoic acids (~41.8 mg/100 g dry matter) procyanidins (~904 mg/100 g dry matter), ellagitannins (~317 mg/100 g dry matter) and flavonoids (~1.50 mg/100 g of dry matter), and to a lesser extent, tocopherols (~0.58 mg/100 g of dry matter) and ascorbic acid (~0.05 mg/g of dry matter). Acorn extract showed significant antioxidant activity under DPPH, ABTS and CUPRAC assays as mentioned by Morcuende et al. (2020). As already stated in that previous paper, the acorn extract contains an interesting variety of phenolic compounds, tocopherols and ascorbic acid, which have been shown to display intense antioxidant activities (Morcuende et al., 2020). Since each type of antioxidant displays different modes of action, the combination is likely to perform efficient antioxidant protection against both lipids and proteins (de Santana Neto et al., 2021; Lund, 2021).
The effect of edible coating with and without acorn extract on the formation of TBARS on cooked chicken patties (C); refrigerated and cooked chicken patties (CC); and cooked, refrigerated, and reheated chicken patties (CCR) compared to the control group of samples (patties without coating) is shown in Table 1. The TBARS levels in C patties were considerably low irrespective of the treatment (<1 mg TBARS/kg sample in all samples). The extent of lipid oxidation significantly increased between 2 and 4-fold times during refrigerated storage of all types of cooked patties. While a certain increase in TBARS occurred during the subsequent reheating, this processing stage had no significant effect on TBARS numbers. These results, which are in agreement with previous works (Akcan et al., 2017b; Fernandes et al., 2017; Nitteranon and Sayompark, 2021), indicates that cooking induce changes in patties which makes these samples appear to be highly susceptible to oxidation during the following chilled storage. In fact, the final microwave reheating had a negligible effect on the extent of lipid oxidation as compared to the previous chilled storage. According to literature, some of the pro-oxidative mechanisms of cooking, may involve denaturation of proteins and structural damage of structural lipids (phospholipids), release of pro-oxidant metals (heme iron), depletion of endogenous antioxidant defenses from muscle, and formation, during heating, of early oxidation products able to induce further oxidative damage (Domínguez et al., 2019; Soladoye et al., 2015). This same behavior, albeit to a lesser extent, was observed in FILM patties and even milder in the FILM-ANTIOX counterparts.
1) Significance level in Tukey test for evaluating the impact of Alginate-based edible coating (CONTROL, FILM & FILM-ANTIOX) within a processing stage. * p<0.05; ** p<0.01; *** p<0.001.
The application of alginate-based coatings (FILM and FILM-ANTIOX) significantly reduced (p<0.05) TBARS values during C, CC, and CCR compared to the CONTROL. These results suggest that, irrespective of the incorporation of antioxidant extracts in the coating, the edible film acted as a physical barrier against oxidative reactions. Polysaccharide-based edible films, such as those produced from alginate, are reported to display effective properties against oxygen diffusion, as a result of their well-structured hydrogen-bonded linkage (Matloob et al., 2023; Song et al., 2011). As expected, the incorporation of the acorn extract provided additional protection against lipid oxidation as TBARS values were lower in FILM-ANTIOX patties compared to FILM treatment. This effect was particularly observed in patties subjected to CC and CCR treatments. These results indicate the effectiveness of this acorn extract in controlling lipid oxidation in cooked, refrigerated, and reheated chicken patties. This effectiveness can be compared with the study by Song et al. (2011), where alginate coating with antioxidants such as vitamin C and tea polyphenols contributed to inhibiting lipid oxidation in refrigerated fish fillets. Similar results were obtained in muscle foods such as chicken nuggets coated with pomegranate peel powder and sodium alginate (Bashir et al., 2022), chicken breasts coated with alginate and enriched with Ferulago angulata (Schlecht.) essential oil (Panahi and Mohsenzadeh, 2022) and lamb patties coated with alginate film impregnated with oregano essential oil (Vital et al., 2021). In agreement with all these previous papers, this study originally report the effectiveness of an acorn extract to improve the antioxidant properties of an alginate-based edible film. Taking into account the manifold antioxidant components of the acorn extract applied, the protection could be attributed to the combination of polyphenols, tocopherols and ascorbic acid. Whereas some other previous studies have reported the antioxidant potential of acorn extracts when directly applied as ingredients in processed meat products (Fernandes et al., 2017; Özdemir et al., 2022), this new study proves the efficiency of incorporating the antioxidant extract from acorn to edible-films. This protective effect could have positive consequences in terms of nutritional value and sensory properties since lipid oxidation products are responsible for rancidity (Patil et al., 2023). This extent would be confirmed by the sensory evaluation discussed in due course.
The effect of edible coating with and without acorn extract on the formation of protein carbonyls in cooked (C), refrigerated and cooked (CC), and cooked, refrigerated and reheated (CCR) chicken patties, compared to the control group of samples (uncoated patties), is shown in Table 1. Protein carbonyl levels in C patties were considerably low regardless of treatment (<3.6 nmol/mg protein). Protein oxidation increased significantly during storage and remained so during reheating, with this latter process having no significant effect on the extent of protein oxidation. These findings, along with those reported in previous works (Ferreira et al., 2017; Nitteranon and Sayompark, 2021; Raeisi et al., 2019), indicate that cooking causes alterations in patties, generating conditions that make them prone to protein oxidation during refrigerated storage. In agreement with the aforementioned results for lipid oxidation, reheating of patties had a minimal impact on the oxidation of proteins. As reported above, cooking may cause damage to phospholipids, lead to the release of heme iron and the depletion of endogenous antioxidant defenses that could eventually facilitate the oxidative damage to proteins (Domínguez et al., 2019; Soladoye et al., 2015).
Application of alginate-based coatings did not reduce protein oxidation levels during cooking (C), storage (CC) and subsequent reheating (CCR) of chicken patties, which indicates that the effectiveness of the barrier effect of the edible film against lipid oxidation was not efficient for protecting meat proteins. These results can be explained by the different mechanisms implicated in lipid oxidation and protein carbonylation. While the former requires molecular oxygen for the propagation of the reactions and hence exert the degradation of unsaturated fatty acids into TBARS, the formation of protein carbonyls is an oxygen-independent processed as reported by Estévez et al. (2022). In fact, Ferreira et al. (2017) reported that the extent of protein carbonylation in modified atmosphere packaged chicken patties was independent of the concentration of molecular oxygen. Hence, the limitation in oxygen diffusion and the protection of lipids did not contribute to inhibiting protein oxidation in FILM patties.
Yet, compared to the control and FILM patties, the extent of protein carbonylation was significantly lower in samples coated with the extract of acorn (FILM-ANTIOX), suggesting that bioactive compounds from the this extract were effective against the onset of protein oxidation during chilling of cooked patties and the subsequent reheating. In a previous study (Ferreira et al., 2017), we tested the ability of phenolic-rich acorns extract to control protein oxidation in RTE chicken patties and found consistent results. It is, however, worth to emphasize that in that study the extract was simply mixed as an additional ingredient within the food matrix. The present study proves that bioactive compounds from this fruit are also efficient in inhibiting protein oxidation when impregnated in an edible coating. In line with the mechanisms proposed by Ferreira et al. (2017), the radical scavenging ability of condensed tannins and polyphenols naturally present in the acorn could explain the antioxidant protection of meat lipids.
The scientific literature is scarce in articles describing the benefits of plant phenolics against protein oxidation in muscle foods protected by edible films, which highlights the original contribution of the current study. In the study conducted by Pei et al. (2022), tragacanth gum-sodium alginate coatings containing epigallocatechin gallate were effective in delaying protein oxidation by inhibiting hydrogen peroxide generation and maintaining the activity of key enzymes. In addition, these coatings preserved the secondary and tertiary structure of proteins during storage. Charoenphun et al. (2023) presented significant findings with regard to the remarkable protection against protein oxidation in shrimp coated with alginate and Longkong pericarp extract during storage at 4°C. The positive results in controlling protein oxidation by plant phenolics in edible films reported in of those studies agree with the findings of the present study. Altogether, this protection may have consequences in terms of improved nutritional value and health benefits since the intake of oxidized proteins from ultra-processed muscle foods has been linked to impaired digestibility (Estévez et al., 2022; Ferreira et al., 2018) and risk of suffering oxidative stress and certain pathological conditions (Estévez and Xiong, 2019; Wang et al., 2023; Yin et al., 2022).
During the storage process (CC), all chicken patties experienced weight loss, as shown in Fig. 2. No significant differences in weight loss was found between CONTROL and FILM patties during storage and reheating though a clear trend in inhibiting such loss. In fact, the calculation of the global weight loss from the beginning until the end of the processing, led to significant differences in which using edible films revealed to be effective against weight loss. Furthermore, the incorporation of the acorn extract to FILM-ANTIOX patties improved this protection as significant differences were found for the reheating process and for the overall weight loss calculation. The impact of the alginate-based coating on this parameter may be explained by the ability of the film to minimize moisture loss during processing. Furthermore, polyphenolics from acorn extract such as procyanidins and ellagitannins are known to interact with biomaterials from films and form macromolecular complexes via covalent linkages (Engin et al., 2022). The incorporation of the acorn extract could have contributed to a denser coating structure and greater impermeability though this speculation needs further experimental proof. Additionally, the antioxidant protection of the phenolic-rich extract on muscle proteins may have also contributed to increase the water holding ability of these proteins and hence, inhibiting the moisture loss. The connection between the integrity and extent of protein oxidation in myofibrillar proteins and the water-holding capacity of muscle foods is well-documented (Bao and Ertbjerg, 2019).
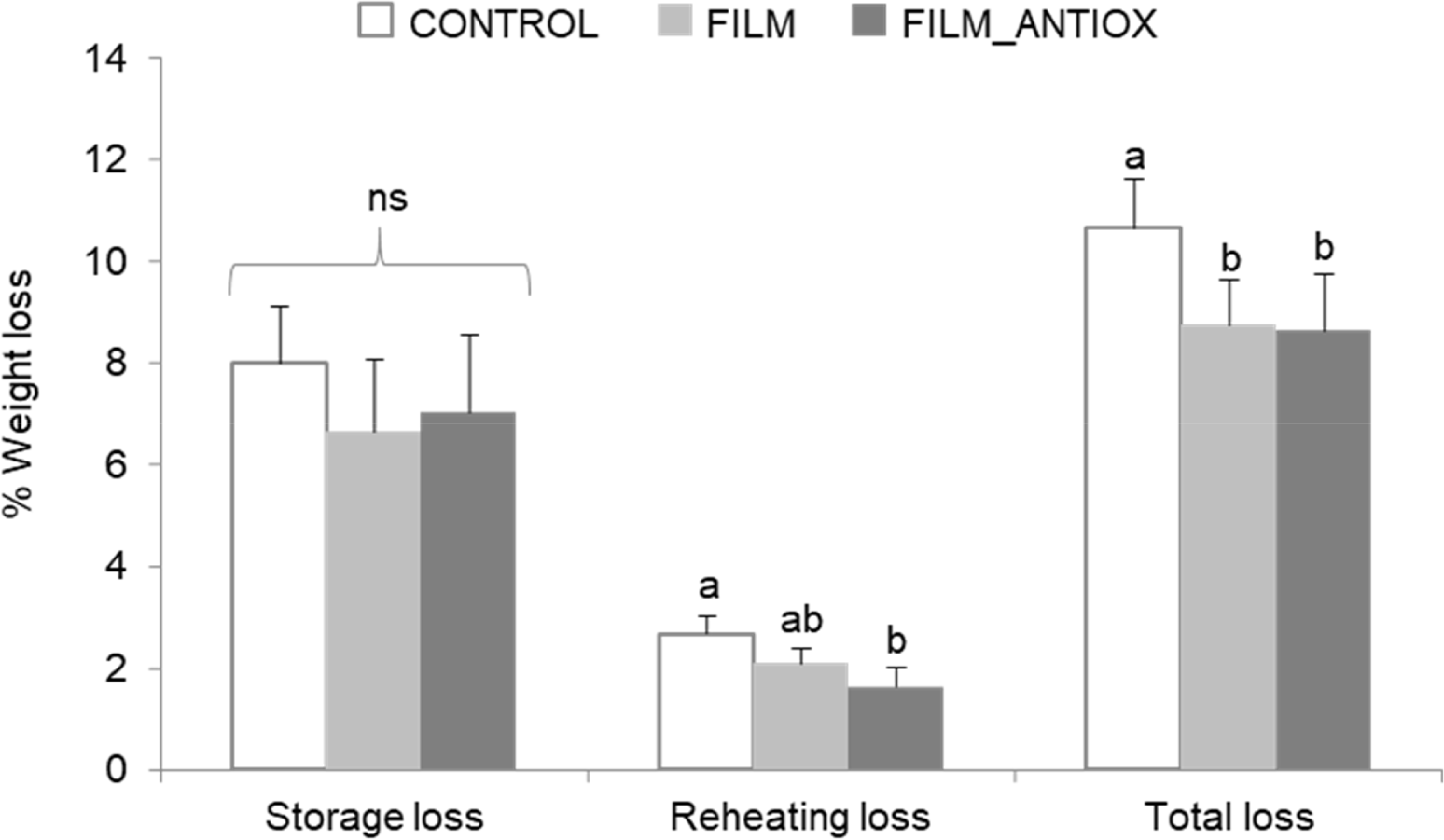
Previous studies demonstrated how phenol-rich extracts affect the structure of edible films, improving their barrier effect and solid content (Engin et al., 2022). Similar results were reported using sodium alginate incorporating purple onion peel extract in food (Santos et al., 2021), rosemary and oregano essential oils in beef loin (Vital et al., 2016), and the use of nanocapsules of cinnamon essential oil and nisin in beef fillets (Zhang et al., 2022). This effect is crucial, as weight loss in meat could influence the perception of its sensory quality and freshness. The reduction in weight loss suggests that the sodium alginate coating with acorn extract helped in retaining some moisture in the meat, potentially contributing to positive sensory features such as juiciness. The aforementioned hypothesis of certain polyphenolics complexing to film materials could explain the observed effects but further research is required for clarification. This result is relevant to the food industry, demonstrating the potential use of extracts alongside alginate-based coatings to preserve certain meat quality features during storage.
The color of meat and processed meat products is a significant factor for consumers’ assessment of freshness and likeability, directly influencing their purchasing decisions (Tomasevic et al., 2021). The characteristics of an edible coating are linked to its constituent materials and have the ability to influence meat color (Vital et al., 2018). The effect of alginate coating, with and without acorn extract, was evaluated against the control (without coating) in the color parameters CIE L*, CIE a*, and CIE b* at different stages of pre-cooking (COOKED), storage (CC), and reheating (CCR). Table 2 shows that CIE a* decreased significantly (p<0.05) in chicken patties from the CONTROL and FILM groups during processing while CIE b* displayed the opposite trend and increased over time (from C to CC and finally CCR). This evolution of color parameters is typical for meat and chicken products subjected to consecutive processing technologies and is commonly associated to undesirable discoloration mechanisms. To this conclusion came de Santana Neto et al. (2021) and Santos et al. (2020), who observed CIE a* decline and increase of CIE b* in RTE chicken patties during chilled storage. The authors reported that this discoloration may be due to the oxidation of myoglobin pigments and the accretion of brownish pigments formed from advanced oxidative reactions. Similar hypotheses were formulated by Akcan et al. (2017a) after the assessment of the color evolution of RTE pork patties subjected to chilled storage. It is reasonable to attribute these changes to oxidative reactions since it is known that cooking (C) of muscle foods induces certain physicochemical changes that make the meat system very prone to oxidation (release of pro-oxidative iron, depletion of antioxidants etc.) during the subsequent technological stages (CC and CCR; Estévez, 2011; Soladoye et al., 2015). This hypothesis seems plausible since the addition of the phenolic-rich extract to edible films protected the chicken products against discoloration at the CC and CCR stages. The color displayed by FILM-ANTIOX samples in the CCR stage was redder (higher CIE a* values) and darker (lower CIE L* values) than that from FILM and CONTROL counterparts. The antioxidant effects of acorn extract rich in polyphenols (especially procyanidins and anthocyanins) may be behind the differences between treatments. Similar results were reported using sodium alginate with nanocapsules of cinnamon essential oil and nisin in refrigerated meat slices (Zhang et al., 2022). Santos et al. (2021) determined that the optical properties of an alginate-based film with added polyphenols from purple onion peel (Allium cepa) promote the development of red color, leading to better interaction between polymeric networks. Similar results were reported by Rojas-Bravo et al. (2019) when applying polyphenols from mango peel (Mangifera indica L. cv Manila) in films and edible coatings. These findings support the viability of utilizing coatings in terms of visual quality and their potential antioxidant properties.
1) Significance level in Tukey test for evaluating the impact of Alginate-based edible coating (CONTROL, FILM & FILM-ANTIOX) within a processing stage. * p<0.05; ** p<0.01; *** p<0.001
The ability of plant antioxidants to neutralize the adverse effects of lipid and protein oxidation may not only be limited to a reduction in oxidation products since the benefits of such antioxidants may be manifested in terms of better sensory and/or nutritional quality (de Santana Neto et al., 2021). In this study, the intensity of odor (rancidity and WOF) and overall acceptability of chicken patties coated with an edible film, both with and without acorn extract (FILM and FILM-ANTIOX), were evaluated in comparison to CONTROL patties. In this regard, the antioxidant protection displayed by the acorns extract added to the edible films had significant effects in terms of sensory profile and overall acceptability (Fig. 3). Considering that all samples were evaluated right after reheating; simulating the stage at which RTE patties may be consumed, the results indicate that the undesirable oxidation-driven sensory deterioration of chicken patties were efficiently controlled by the application of edible films impregnated with plant antioxidants. The rancidity and WOF were perceived as “weak” and “very weak” in FILM-ANTIOX samples while such sensory traits were identified as “moderately intense” in samples from CONTROL group (p<0.05). Samples from the FILM had intermediate positions reflecting that, in line with the biochemical analysis, the application of edible films, alone, was not efficient enough to counteract completely the harmful effects of lipid and protein oxidation.
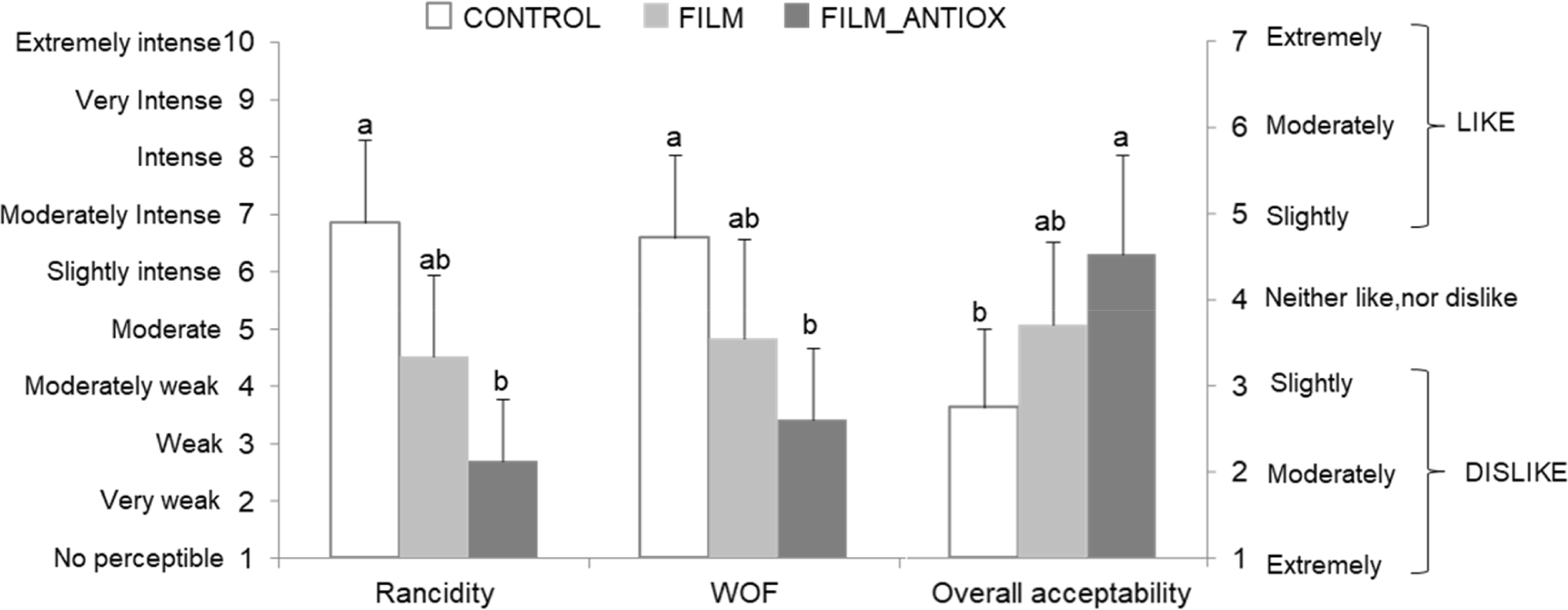
In a recent study, Panahi and Mohsenzadeh (2022) assessed the impact of a sodium alginate coating containing essential oil from F. angulata, nisin, and NaCl on the sensory characteristics of refrigerated chicken, and the results are consistent with the aforementioned. Similarly, Zhang et al. (2022) reported sensory improvements in beef fillets using a sodium alginate edible coating with nanocapsules of cinnamon essential oil (Cinnamomum zeylanicum), while Vital et al. (2016), Vital et al. (2018) incorporated essential oils from rosemary and oregano into alginate to coat beef fillets, highlighting the effectiveness of these combinations in enhancing the sensory quality of food products. These findings underscore the promising application of sodium alginate coatings in the food industry, offering significant potential to improve sensory quality and extend the shelf life of various meat products.
Conclusion
The use of alginate-based edible coatings impregnated with a phenol-rich extract from acorns (Q. ilex subsp. Ballota) resulted in a positive reduction in oxidative changes at both biochemical, and sensory levels. The barrier effect of the edible film was found to diminish the intensity of certain reactions but that was not reflected in a better sensory quality. Moreover, the coating method could be tested on an industrial scale, as it is a simple and relatively inexpensive technique. This strategy is in line with current trends linked to the usage of plant materials as sources of bioactive compounds to extend commercial shelf life in RTE muscle foods.













