Introduction
Lacticaseibacillus casei, previously known as Lactobacillus casei, is traditionally recognized as a probiotic and has numerous applications in the food fermentation industry (Bellaver et al., 2024; Sultana et al., 2023). L. casei has been reported to show several health-promoting and nutritional functions, such as immune system regulation, intestinal pathogen inhibition, obesity treatment, cardiovascular disease prevention, and microbiota-gut-brain axis modulation (Balasubramanian et al., 2024; Hill et al., 2018; Ibrahim et al., 2023; Pimentel et al., 2021). These promising functions have contributed to a gradual increase in consumer demand for this probiotic. Fermented milk has been widely used to produce probiotic products due to its favourable taste and high nutritional value. However, L. casei strains have a slow growth rate in milk; thus, producing fermented dairy products using pure L. casei strains is time-consuming (Ma et al., 2015b; Zhang et al., 2020). Therefore, determining and optimizing the fermentation conditions of probiotic L. casei in milk have important practical application value.
L. casei is one of the most commonly used lactic acid bacteria (LAB), a general term for bacteria that can ferment lactose to produce lactic acid, and they have similar physiological characteristics and metabolic pathways. LAB are typically auxotrophic to some amino acids and vitamins and cannot biosynthesize several nutrients (Koduru et al., 2022). Thus, LAB strains are nutritionally fastidious and require many free amino acid (FAA) or peptides that are present in trace amounts in milk, which is insufficient to sustain growth (Lin et al., 2021; Playford and Weiser, 2021). In the field of milk fermentation, the proteolytic system of LAB has gained considerable attention due to its ability to hydrolyze milk casein into peptides or amino acids, ensuring successful reproduction. The first step in milk casein utilization by LAB is performed by cell envelope proteases (CEP), as the production of amino acids depends on the extracellular protease activity (Koduru et al., 2022). Although L. casei and Lacticaseibacillus paracasei possess a complete proteolytic system like CEP, the protease activity is low (Satılmış et al., 2023; Solieri et al., 2018), which may be a limiting factor for culture in milk. Boulay et al. (2020) found that Streptococcus thermophilus CEP PrtS involved the proteolysis of soya proteins, and the deletion of PrtS gene from S. thermophilus resulted in slow acidification and low growth levels. In another study, peptides served as a potential nitrogen source for Bifidobacterium animalis ssp. lactis during fermentation (Zhang et al., 2024), which could be effectively utilized by different bifidobacterial strains through the oligopeptide transport systems (Cui et al., 2022). A previous study reporting the effect of enzymatic hydrolysis of milk proteins on the growth of Lactobacillus gasseri found that oligopeptides were the optimal nitrogen source rather than FAAs or proteins (Arakawa et al., 2015). In summary, peptides play a key role in the fermentation process of LAB. Therefore, utilizing exogenous proteolytic enzymes to improve the growth and propagation of probiotic L. casei during milk fermentation has high significance.
Currently, research on peptides initially present in milk and their effect on the metabolism and propagation of L. casei is scarce. Therefore, this study aimed to obtain milk protein peptides using different proteases (neutral protease and flavourzyme), determine the effects of these peptides on L. casei, evaluate the properties of fermented milk, and explore whether peptides may act as a growth-stimulatory factor for L. casei.
Materials and Methods
L. casei LC2W (China General Microbiological Culture Collection Center, No. 0828) was isolated from traditional dairy products in Inner Mongolia, China, and obtained from Bright Dairy & Food (Shanghai, China). L. casei 01 was obtained from Chr. Hansens (Hørsholm, Denmark). L. casei strains were cultured in MRS agar (Oxoid, Basingstoke, UK) under anaerobic conditions (Bugbox Anaerobic System, Ruskinn, Bridgend, UK) with 95% N2 and 5% CO2 at 37°C for 36 h. Single colonies were sub-cultured twice in MRS broth overnight in an MIR-253 incubator (Sanyo, Osaka, Japan) for starter preparation. Both L. casei strains were prepared as direct vat-set cultures (2×1011 CFU/g) in State Key Laboratory of Dairy Biotechnology (Shanghai, China).
The peptides were obtained through hydrolysis of skim milk according to the method described by Nongonierma and FitzGerald (2013) with slight modifications. Skim milk powder (33.4% protein, Fonterra, Auckland, New Zealand) was reconstituted in distilled water to produce reconstituted skim milk (RSM; 12%, w/w). Neutral protease NP (Danisco Company, Copenhagen, Denmark) or flavourzyme PB03 (a protease/peptidase complex; Pangbo Biological Engineering, Nanning, China) were then added to RSM at a concentration of 0.1% (w/w) and dispersed under agitation at 50°C for 60 min using an overhead stirrer (RW20, IKA, Staufenim, Germany). The protease-added RSM was sequentially hydrolyzed at 50°C for 240 min without stirring. Later, the enzyme was inactivated by heating the hydrolysis sample at 90°C for 20 min. The NPMP and FPMP were stored at 4°C until use within 30 d.
NPMP or FPMP was added to the 12% RSM base, replacing 5%, 10%, 20%, or 40% of the base (w/w). Twelve percent of RSM without peptide supplementation was used as the control. The media was heated to 95°C for 90 min using a GFL1002 water bath (GFL Company, Burgwedel, Germany) and then cooled to 37°C. The prepared media was then inoculated with L. casei LC2W or L. casei 01 (5×106 CFU/g) and incubated at 37°C. The pH values of fermented milk were monitored and measured using a Cinac system (Alliance Instruments, Mery-Sur-Oise, France), with automatic recording every 5 min during milk fermentation. The number of viable L. casei cells was determined every 24 h for 72 h during fermentation by culturing on MRS agar, and the cell numbers in 72 h-fermented milk samples were further monitored each week during cold storage (4°C) for one month.
The fermented milk samples supplemented with 20% NPMP (NPMP20%) and 20% FPMP (FPMP20%) and FM were incubated at 37°C until the pH value reached 3.70. All samples were then cooled and stored at 4°C for 24 h. Finally, the volatile flavour compounds and FAA compositions were determined.
The peptide contents in the three fermented milk samples (FM, NPMP20%, and FPMP20%) at 0, 24, 48, and 72 h were measured according to the method described by Dhakal et al. (2024) with some modifications. The reagent was prepared by mixing 25 mL of 100 mM borax, 2.5 mL of 20% (w/w) sodium dodecyl sulfate, 40 mg of o-phthaldialdehyde solution (dissolved in 1 mL of methanol), and 100 μL of β-mercaptoethanol and adjusting the final volume to 50 mL with deionized water. The samples were filtered using an ultrafiltration tube with a molecular weight cut-off at 10 kDa (Millipore, Billerica, MA, USA), and 50 μL was mixed with 2 mL of reagent. This reaction mixture was combined for 2 min at room temperature (about 25°C), and the absorbance at 340 nm was measured using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The peptide concentrations were quantified using casein tryptone (Difco Laboratories, Sparks, MD, USA) as a standard.
The fermented milk samples were pre-treated to remove the proteins, and the amino acids were extracted following the method described by Ma et al. (2015a). The amino acid content of the samples was analyzed using a high-performance liquid chromatography fitted with a sodium cation exchange amino acid analysis column (4×150 mm) and an o-phthalaldehyde post-column derivation system (Pickering, Mountain View, CA, USA). The equipment was coupled with a Waters 510 pump, a 7725i manual injector, and a 363-fluorescence detector (Varian, Walnut Creek, CA, USA). The flow rate was 1.7 mL min−1. The elution was performed by applying a linear gradient of 100% solution A for 1 min, followed by 0%–100% solution B over the subsequent 48 min (solution A: 0.2 M sodium citrate, pH=3.0; solution B: 0.2 M sodium borate, pH=9.8).
The volatile compounds in the fermented milk were extracted through headspace solid-phase micro-extraction according to the method described by Ma et al. (2015a). The fiber used for manual extraction was DVB/CAR/PDMS (Supelco, Bellefonte, PA, USA). The volatile compounds were analyzed using an Agilent 7890 (II) gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to an Agilent 5975 series mass selective detector. The SPME fiber was inserted into the injection port that was held at 250°C, and the compounds were thermally desorbed for 4 min under the split (1:10) conditions. A DB-Wax column (30 m×0.25 mm×0.25 μm; Agilent Technologies) was used to separate the volatile compounds. The temperature of the column was maintained at 45°C for 5 min, ramped at 10°C min−1 to 80°C, and then further increased to 240°C at the rate of 5°C min−1. The carrier gas was helium (1 mL min−1). The mass spectrometer was run in the electron impact mode at 70 eV. The mass scan range was 25 to 400 m/z. The concentrations of volatile flavour compounds in the samples were expressed as the peak area of each compound.
Results and Discussion
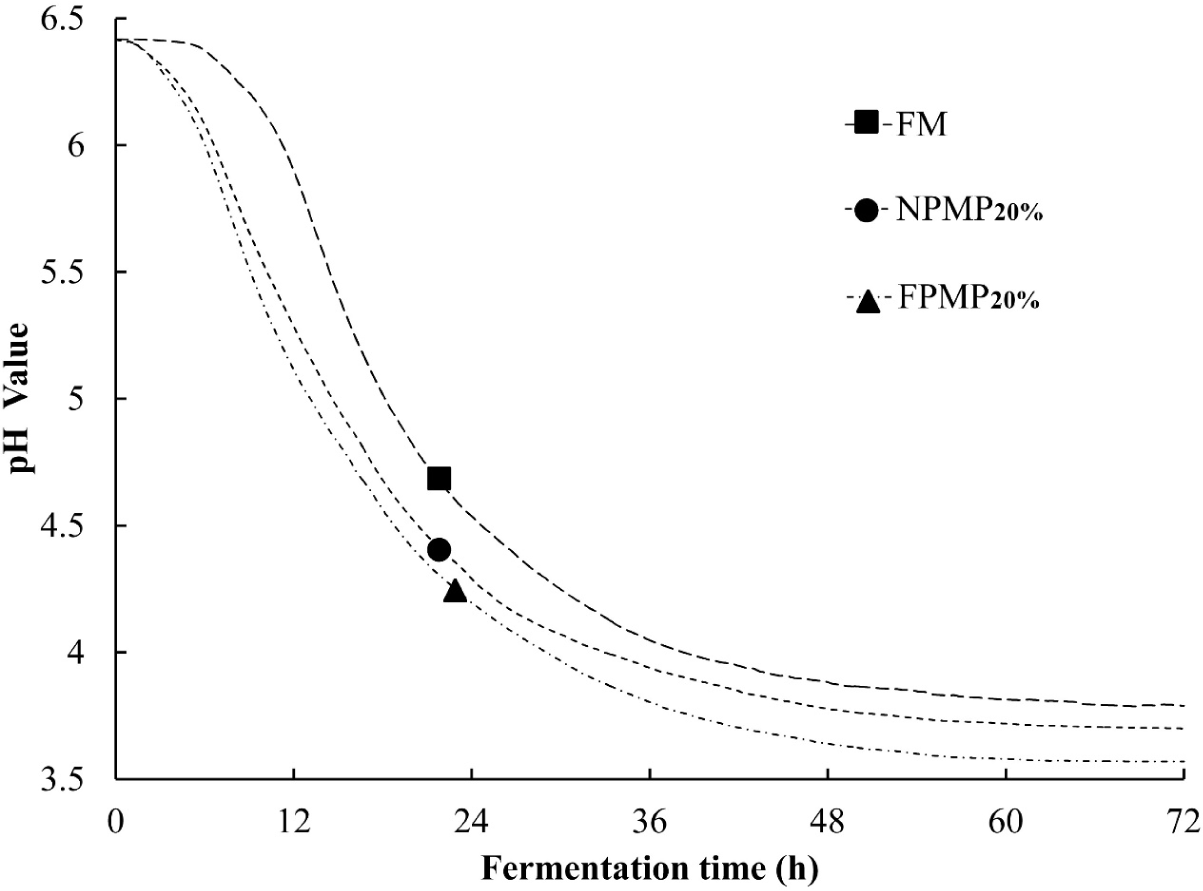
After hydrolysis for 5 h, the pH values of the neutral protease and flavourzyme-supplemented RSM samples were changed slightly from 6.65 to 6.41 and 6.37, respectively. A small protein precipitate was observed, indicating that enzyme hydrolysis destabilized the skim milk emulsion. A large protein precipitate was observed in the heat-sterilized media when over 40% NPMP or FPMP was added to the RSM (data not shown). Thus, 5%, 10%, and 20% NPMP or FPMP supplementation were used to evaluate the optimal growth conditions. Compared to the control FM, in the early stage of fermentation (0–24 h), 5%–20% of NPMP or FPMP significantly promoted the acidification of L. casei. In the middle and late stages of fermentation (24–72 h), FPMP10% and FPMP20% still had a significant stimulating effect on fermentation and acid production of L. casei (Table 1). FM reached the pH end-point (3.70) at 90 h, while FPMP20% and NPMP20% reached the same pH end-point only at 42 h and 67 h, respectively (Fig. 1). These results indicated that FPMP had a positive impact on the acidification ability of L. casei strains, suggesting that peptide supplementation shortened the production time, improved production efficiency and reduced the risk of microbial contamination.
FM: fermented milk without supplementation; NPMP5%, NPMP10%, NPMP20%, FPMP5%, FPMP10% and FPMP20%: fermented milk containing 5% NPMP, 10% NPMP, 20% NPMP, 5% FPMP, 10% FPMP and 20% FPMP.
LAB are microorganisms with fastidious requirements. In this study, FPMP or NPMP supplementation compensated for the weak proteolytic capacity of L. casei, notably in the early growth phase to overcome the low concentrations of FAA and peptides in milk. These results were consistent with the previous studies reporting that supplemental peptides could be well utilized by Lactobacillus rhamnosus and Bifidobacterium bifidum (Cui et al., 2022; Zhang et al., 2021).
The nutrient composition of the medium might affect the viable cell counts in the fermented milk culture. Herein, FPMP supplementation significantly enhanced the propagation of L. casei. For instance, the numbers of viable L. casei cells at 24, 48, and 72 h in the FPMP20% samples were 8.67, 9.39, and 9.58 Log CFU/g, respectively, with a significant increase of 0.44, 0.47, and 0.73 Log CFU/g (p<0.05), compared with the control (Fig. 2A). These results indicated that FPMP strongly stimulated both the acidification and propagation of L. casei. However, NPMP supplementation did not promote the reproduction of L. casei in milk fermentation. There was no significant difference in the number of L. casei cells between NPMP20% supplemented sample and the control FM at 24 h, 48 h and 72 h (Fig. 2A). Moreover, cell loss during cold storage in the NPMP20% samples was the highest among the three samples (FM, FPMP20%, and NPMP20%). The FPMP20% samples maintained the highest number of viable L. casei cells throughout the 28 day cold storage. During 14–28 day storage time, the cell counts of the NPMP20% samples were significantly lower (p<0.05) than that of the control FM and FPMP20% samples (Fig. 2B).
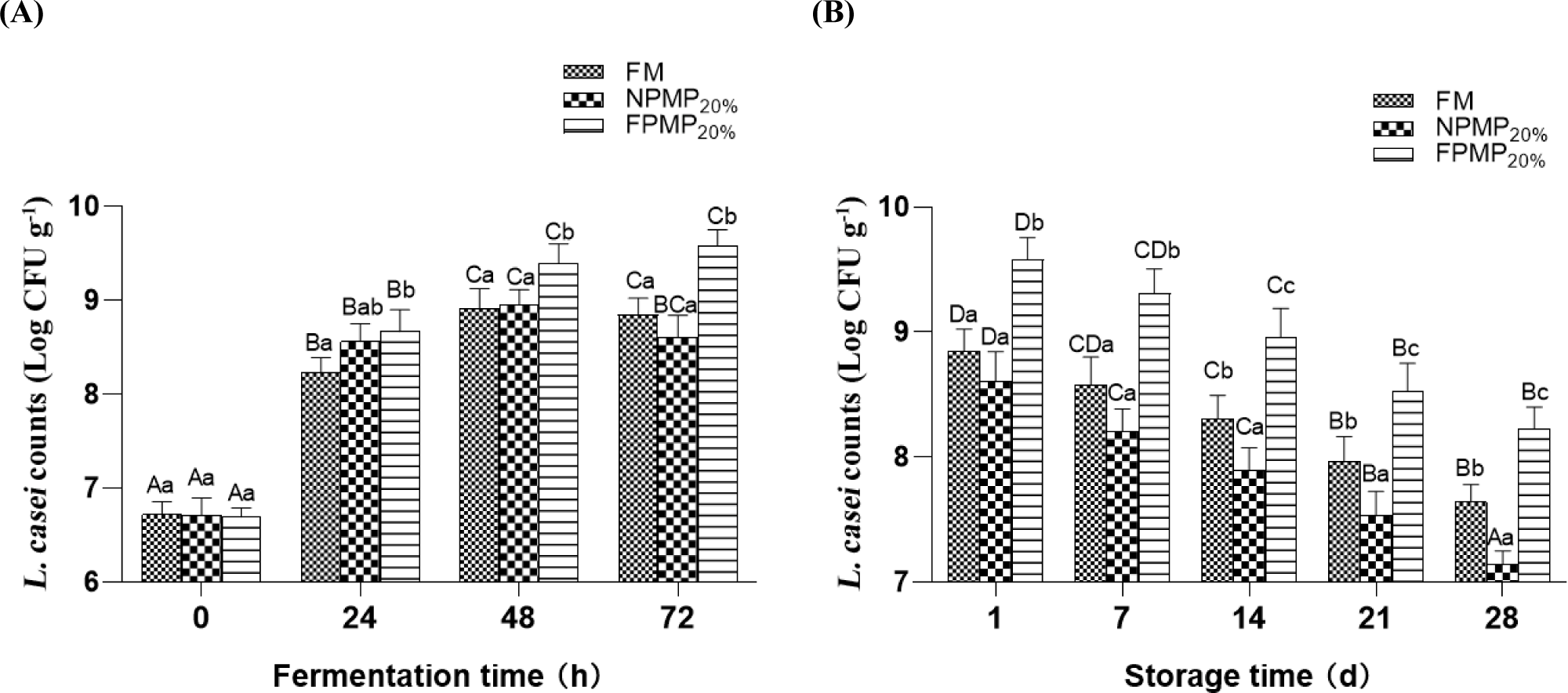
NPMP promoted acidification (0–24 h), suggesting that these peptides could be utilized by L. casei. However, NPMP might impair the propagation during fermentation and survival during the storage of L. casei. Therefore, it was speculated that some peptides in NPMP could stimulate the acidification and growth of L. casei, while others might inhibit the growth of L. casei. The antibacterial activity of peptides derived from milk has been given special attention. Hydrophobic peptides derived from sheep milk could severely damage the cell membrane integrity (Yang et al., 2024) and the peptides released from yak milk casein using flavourzyme showed strong activity against Staphylococcus aureus (Zhang et al., 2023a). Zhang et al. (2010) reported that peptides with different molecular weights had significantly different influences on the growth of yoghurt starter strains (Lactobacillus delbrueckii subsp. bulgaricus and S. thermophilus), and the peptides with molecular weights below 3 kDa might effectively promote the growth and metabolism of LAB. In another study, different bifidobacteria transported and utilized peptides with different residues due to the specificity and variability of their peptide transport systems (Cui et al., 2022). In this study, the use of different types of enzymes for hydrolysis resulted in different peptide profiles for each hydrolysate. NPMP likely contained protein hydrolysates that inhibited L. casei. In contrast, the inhibitory hydrolysates in FPMP were further degraded by flavourzyme consisting of endoprotease and peptidase. As for the comparison of neutral proteases, flavourzyme might obtain more small peptide fragments. Enzymatic hydrolysis is a modest and efficient approach for obtaining soluble peptides from milk protein. Different enzyme types produce hydrolysates with different peptide profiles and have different stimulatory or inhibitory effects. Nevertheless, future studies must focus on screening suitable types of proteases, determining the molecular mass distribution of peptide fractions, analyzing the specificity of peptide transport systems and clarifying the stimulatory role of different peptide fractions in L. casei.
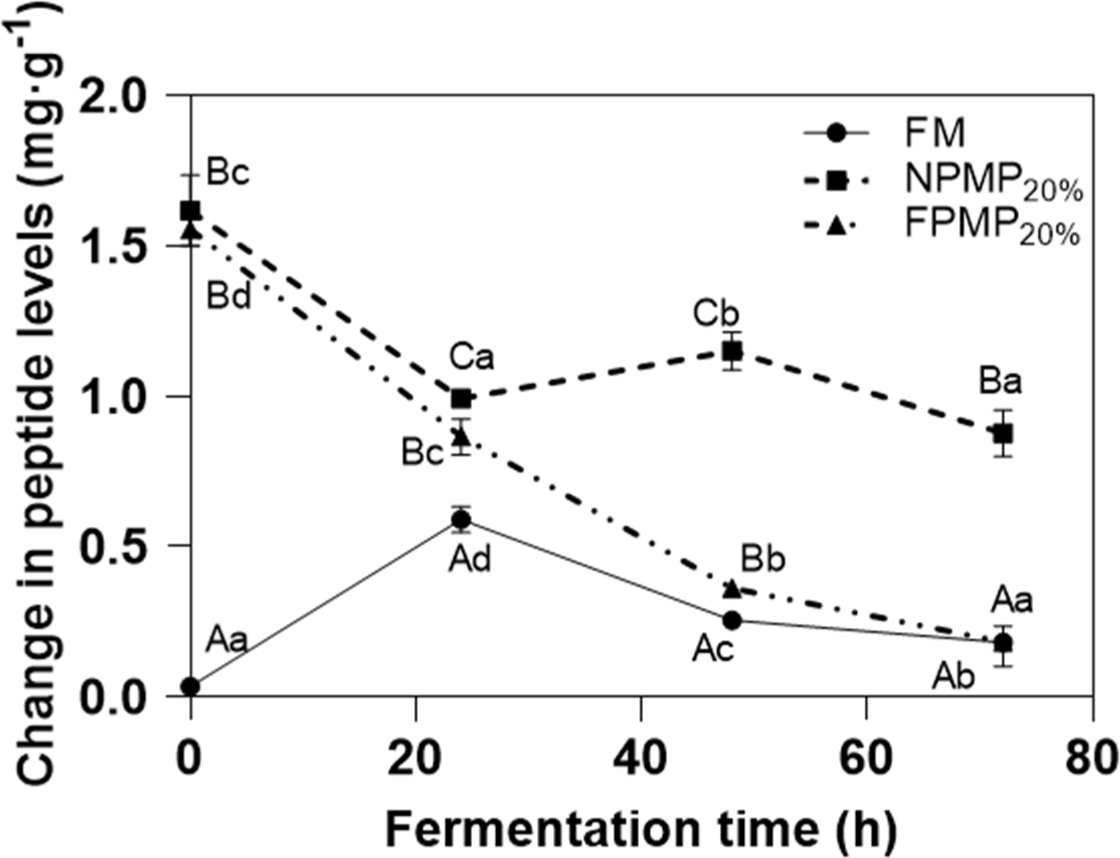
The peptide concentration in RSM was very low (0.036 mg/g, Fig. 3). In the control FM, the peptide concentrations increased as fermentation progressed from 0–24 h (Fig. 3), suggesting that the CEP of L. casei played a key role in hydrolyzing extracellular caseins to oligopeptides at this stage. As the peptides were transported and utilized intracellularly, the peptide concentrations in the milk gradually decreased from 24–72 h. In NPMP20% and FPMP20%-supplemented samples, the initial peptide concentrations were high at 1.687 mg/g and 1.556 mg/g, respectively. The peptide concentrations in FPMP20%-supplemented milk tended to decrease throughout the fermentation process (Fig. 3), indicating that FPMP could be well utilized by L. casei, and the peptide generation rate was lower than the rate of consumption. The peptide concentrations in NPMP20%-supplemented milk decreased from 0–24 h but increased slightly from 24–48 h. These results further suggested that some NPMP could be preferentially utilized by L. casei, while others were not utilized well. The peptide consumption rate in NPMP20%-supplemented milk was lower than the rate of generation, which was consistent with the finding that NPMP could not promote the propagation and survival of L. casei.
FAA analysis is a promising approach to evaluate the protein hydrolysis activity in fermented dairy products based on the FAA contents (Garavand et al., 2023). After fermentation, there was a notable rise in the FAA concentrations in all three milk samples (Table 2). The increase in the total FAA in the FPMP20% samples was much greater than in the FM at the end of fermentation (341.06 mg/kg for FPMP20% and 54.93 mg/kg for FM). It was worth noting that both before and after fermentation, some essential amino acids such as leucine, valine, methionine and isoleucine in FPMP20% samples were much higher than in the FM and NPMP20% samples (Table 2).
Casein can be degraded to amino acids by LAB only when milk proteins are hydrolyzed to oligopeptides. Herein, FPMP was easily utilized and the FAA concentrations in the FPMP20% samples were the highest. Flavourzyme degradation obtained the highest FAA contents during the hydrolysis of lentil protein concentrates due to its exopeptidase activity (Vogelsang-O’Dwyer et al., 2023). Although the initial peptide concentrations in the NPMP20% and NPMP20% samples were equivalent (1.687 mg/g for NPMP20% vs. 1.556 mg/g for FPMP20%) with no significant difference (p>0.05), the total FAA levels in the NPMP20% samples were much lower than in the FPMP20% samples (409.96 mg/kg for FPMP20% vs. 226.34 mg/kg for NPMP20%). It was possible that some NPMPs could not be effectively utilized and degraded to FAA, which was consistent with the smaller decrease in peptide concentrations in the NPMP20% samples.
Table 3 displays the volatile flavour compounds detected in three fermented milk samples with an end-point pH 3.7. L. casei produced heptanone and nonanone as the primary volatile aroma compounds in all three samples, followed by nonanol, pentanone, acetic acid, and undecanone. The amounts of these volatile compounds were the highest in the FPMP20% samples. The amount of only a few aldehydes and carboxylic acids (for example, butanal, octanal, nonanal, and butanoic acid) were slightly lower in FPMP20% than in FM or NPMP20%. The flavor is crucial for the food senses. Heptanone was believed to give fermented milk a fruity and cinnamon aroma; nonanone was believed to give fermented milk a green and floral aroma (Shi et al., 2024). The volatile aromatic compounds in fermented products are largely derived from peptides and FAA. These ingredients are transformed into flavour compounds through milk protein degradation and metabolic pathways of branched amino acids such as leucine, isoleucine and valine (Tian et al., 2023; Zhang et al., 2023b). FPMP supplementation significantly enhanced the initial peptide and FAA contents in the fermented milk. Therefore, the characteristic volatile flavour compounds in L. casei-fermented milk were enriched, and the enrichment improved when the milk protein was partially hydrolyzed in the initial stage of fermentation. Further, as these hydrolyzed peptides are derived from milk, the “off-flavour” caused by the additions of exogenous ingredients, such as yeast powder, soybean peptone, and tea, can be eliminated. Therefore, pre-treatment of milk using flavourzyme could be considered for large-scale fermentation, which provides an optimized strategy for improving probiotic fermented dairy products.
Conclusion
In this study, the supplementation of neutral protease or flavourzyme significantly increased the peptide contents in milk. The results showed that the peptides could be efficiently utilized by L. casei and the peptide contents in the FPMP-supplemented fermented milk tended to decrease throughout the fermentation process. The peptide contents in the NPMP-supplemented fermented milk decreased in the early stage of fermentation but slightly increased in the middle stage of fermentation, indicating some NPMP were not utilized efficiently by L. casei. NPMP promoted the acidification of L. casei while impairing the propagation and survival of L. casei. FPMP supplementation significantly stimulated the growth and propagation of probiotic L. casei during milk fermentation. Moreover, FPMP20% supplementation significantly increased the FAA levels and the contents of critical flavour components in FPMP20% fermented milk were significantly higher than in FM and NPMP20% fermented milk. Furthermore, higher cell numbers, reduced fermentation time, and increased abundance of flavour components were achieved in FPMP-supplemented fermentation with L. casei. Overall, this approach could be an economically effective method for large-scale fermentation.













