Introduction
Extracellular vesicles (EVs) are small membrane-enclosed vesicles released into the extracellular environment (Choi et al., 2023; Robbins and Morelli, 2014). EVs play an important role in cell-to-cell communication by delivering proteins, lipids, RNA, and DNA to recipient cells (Bok et al., 2024; Tkach and Thery, 2016). Since these characteristics, EVs are involved in various physiological processes and play a role in cancer and immune diseases (van Niel et al., 2018). EVs can be categorized into exosomes, microvesicles, and apoptotic bodies based on their origin, size, and mode of release (Zaborowski et al., 2015). In addition, they are increasingly being studied as biomarkers and therapeutics for diseases because of the specificity of their internal miRNAs (Chae et al., 2023; Lee et al., 2023a; McKelvey et al., 2015; Park and Kim, 2023; Zhang et al., 2024).
A growing body of evidence suggests that milk-derived EVs can benefit gut health by reshaping the gut microbiota (Lee et al., 2023b). Oral administration of milk-derived EVs to C57BL/six mice for 8 weeks increased the abundance of Ruminococcaceae and Lachnospiraceae in the large intestine (Tong et al., 2020). The same researchers reported that the oral administration of milk-derived EVs to a mouse model of colitis increased Akkermansia in the intestine (Tong et al., 2021). The oral administration of milk-derived EVs to mice with dextran sodium sulfate-induced chronic colitis increased Bifidobacterium, Lachnoclostridium, and Lachnospiraceae in the gut (Du et al., 2022). Furthermore, feeding milk-derived EVs to mice with osteoarthritis increased Ruminococcaceae and Akkermansiaceae and decreased Proteobacteria in the gut (Liu et al., 2023).
Akkermansia, specifically Akkermansiamuciniphila, is a bacterium that belongs to the next generation probiotics and is beneficial for gut health (Karamzin et al., 2021; Park et al., 2024). A. muciniphila degrades mucin, a major component of the mucus layer of the gut (Kim et al., 2023). This promotes mucin production by the goblet cells, which improves the integrity of the intestinal barrier (Kim et al., 2021; Kwak et al., 2024). Notably, A. muciniphila has been reported to be low in abundance in the gut of patients with inflammatory bowel disease or metabolic disorders, and may modulate anti-inflammatory activity (Derrien et al., 2017). However, little is known regarding the regulation of gut microbiota by colostrum-derived EVs and its mechanisms. Therefore, this study aimed to investigate whether colostrum-derived EVs could regulate the growth of commensal bacteria, specifically A. muciniphila.
Materials and Methods
Colostrum samples from healthy Holstein cows were collected within 3 days after delivery. After milking, the milk samples were immediately refrigerated and transported to the laboratory for further analysis.
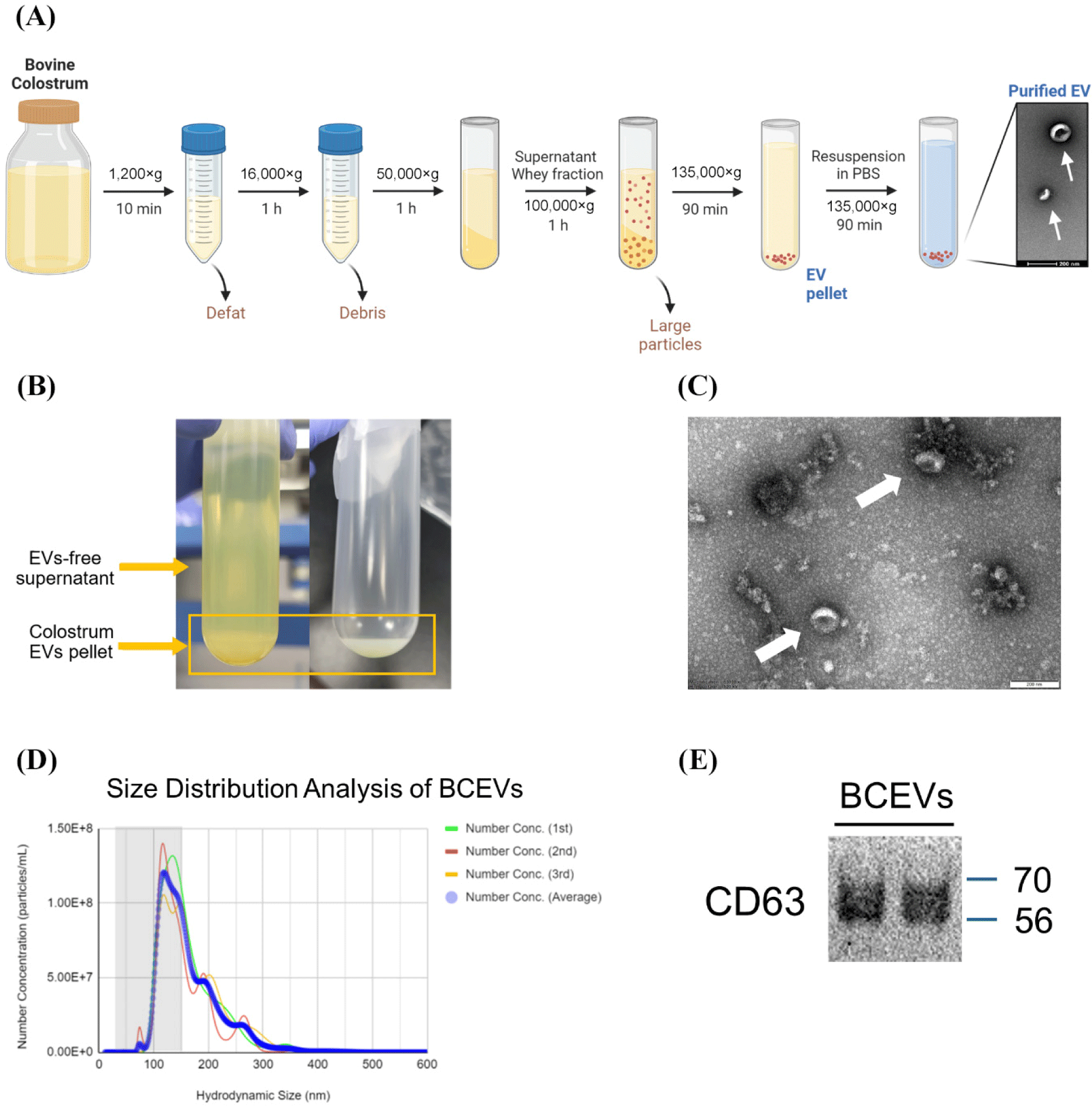
The colostrum was centrifuged at 1,200×g for 10 min for defatting, and then at 16,000×g for 1 h to pellet the cell debris. The supernatant was centrifuged at 50,000×g for 1 h to generate the whey fraction. The whey fraction was ultracentrifuged at 100,000×g to remove large particles. The supernatant was ultracentrifuged at 135,000×g for 90 min to pellet the EVs. The EVs were then resuspended in phosphate-buffered saline (PBS), washed under the same conditions, and frozen at –80°C until use (Fig. 1A). Transmission electron microscopy (TEM) was used to confirm the morphology of BCEVs, as previously described (Choi et al., 2023). The average size and particle concentration of the isolated EVs were measured using nanoparticle tracking analysis (NTA; Malvern NanoSight NS300, Malvern Technologies, Malvern, UK). For further characterization of EVs, the expression of CD63 was determined using a western blot assay, as previously described (Shimizu et al., 2021).
A. muciniphila strain KCTC 15667 was cultured in brain heart infusion (BHI) broth (Becton Dickinson, Franklin Lakes, NJ, USA) with 0.5% mucin type II (Sigma-Aldrich, St. Louis, MO, USA) and Bacteroides intestinalis KCTC 5441, Bacteroidesfragilis KCTC 5013, Phocaeicola massiliensis KCTC 5470 were cultured in BHI-supplemented (BHIS) medium (Bacic and Smith, 2008) in anaerobic chamber (COY Laboratories, Grass Lake, MI, USA). For bacterial inoculation, A. muciniphila was cultured for two days, whereas Bacteroides and Phocaeicola were cultured for 1 d before use in the experiment. To evaluate the growth curves of each bacterial strain supplemented with BCEVs, 1×109 or 1×1010 particles/mL of BCEVs or PBS were added to the BHI or BHIS broth, and then each bacterial strain was inoculated. The absorbance (OD600) of the bacterial cultures was measured every 2 h for 2 d for A. muciniphila and the number of viable cells was measured at the endpoint. Cultures of Bacteroides and Phocaeicola were measured every 2 h for 1 d.
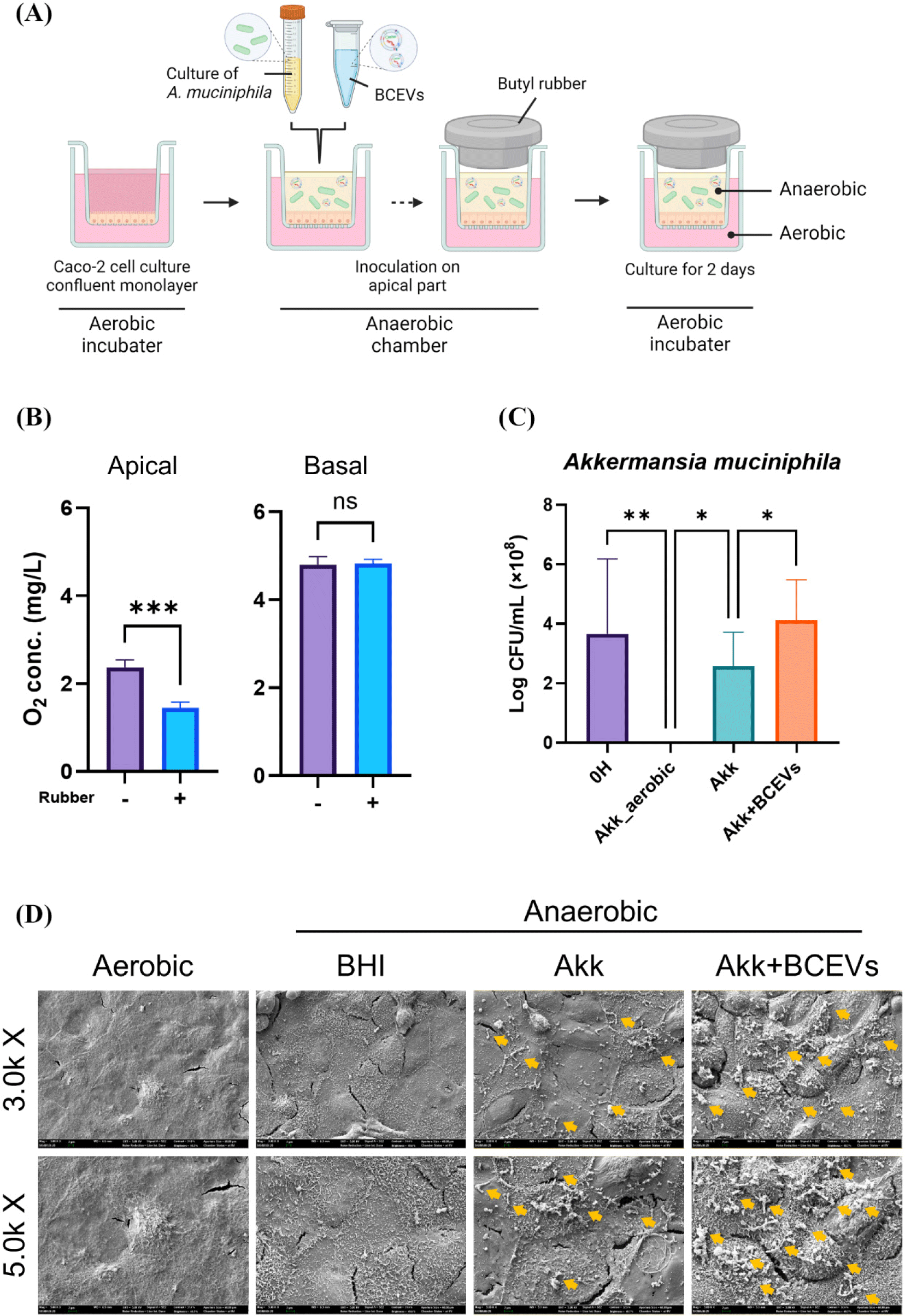
Caco-2 cells obtained from the American Type Culture Collection were grown in Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum. Inserts with 0.4 μm pore size (apical part) were placed in a 12-well plate (basal part) and Caco-2 cells were seeded in the apical part. After culturing Caco-2 cells in a conventional aerobic 5% CO2 incubator at 37°C for 3 weeks, the apical part was inoculated with A. muciniphila and BCEVs in an anaerobic chamber (COY Laboratories). Apical inserts were sealed with airtight butyl rubber to maintain the anaerobic conditions, then the plates were incubated again in an aerobic 5% CO2 at 37°C for 2 d, and the survival of A. muciniphila in the apical part was checked. Dissolved oxygen concentration was measured using a Milwaukee MW600 PRO (Milwaukee Instruments, Rocky Mount, NC, USA) according to the manufacturer's instructions.
For scanning electron microscopy (SEM) analysis, the cultured cell culture inserts were fixed with Karnovsky's fixative overnight at 4°C and stained with 1% osmium tetroxide (Sigma-Aldrich) for 1 h at room temperature. The membrane part of the insert was then gradually dehydrated using ethanol. The membrane was thoroughly dried with hexamethyldisilazane, platinum coated, and imaged using field emission SEM (Sigma-Aldrich; Carl Zeiss, Cambridge, UK).
For transcriptome analysis, A. muciniphila cultured with BCEVs or PBS was grown to mid-log (OD600=0.35). The pellet was immediately harvested by centrifugation and RNA was isolated using TRIzol reagent (Invitrogen, Waltham, MA, USA) and an RNeasy Mini kit (Qiagen, Hilden, Germany). The quantity and quality of the extracted RNA were measured using a spectrophotometer (SpectraMax ABS Plus, Molecular Devices, San Jose, CA, USA). Library construction and sequencing were performed by Macrogen (Seoul, Korea). Libraries were prepared using the NEBNext rRNA Depletion (Bacteria) and TruSeq Stranded Total RNA Library Prep Gold Kit and sequenced on an Illumina NovaSeq platform. Preprocessed trimmed reads were mapped to known reference genomes using the Bowtie software. After read mapping, the HTSeq program was used to extract gene-specific read counts for each sample based on the gene annotation of the species. EdgeR was used to perform differentially expressed genes (DEGs) analysis to select DEGs between the two groups, and genes that met the conditions of |fold change (fc)|≥1.5 and exactTest raw p-value<0.05 were extracted. If known gene information was available, functional annotation and gene-set enrichment analysis based on the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases were performed on the DEGs (Munyaneza et al., 2024).
Comparisons between the two groups were performed using Student’s t-test, and one-way analysis of variance was used to compare different treatment groups. Each group was compared using Tukey’s test to assess any differences from the control and between the treatments, unless otherwise indicated. Growth rates were analyzed using the Growthcurver R package. Statistical analyses and visualizations were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
Results
Milk-derived EVs play a role in modulating the gut microbiota, particularly by increasing the abundance of Akkermansia, which has recently been considered a next-generation probiotic (Hänninen et al., 2018; Tong et al., 2021). Therefore, this study aimed to determine how BCEVs interact with gut microbiota, specifically with A. muciniphila. We isolated and identified BCEVs from colostrum by ultracentrifugation (Fig. 1), following the guidelines of the International Society for Extracellular Vesicles (Théry et al., 2018). TEM imaging confirmed their morphology (Fig. 1C), NTA determined their size and concentration (Fig. 1D), and western blotting assays identified CD63 as a protein marker for EVs, confirming the successful isolation of BCEVs (Fig. 1E). The average size of the BCEVs was 147.6 nm.
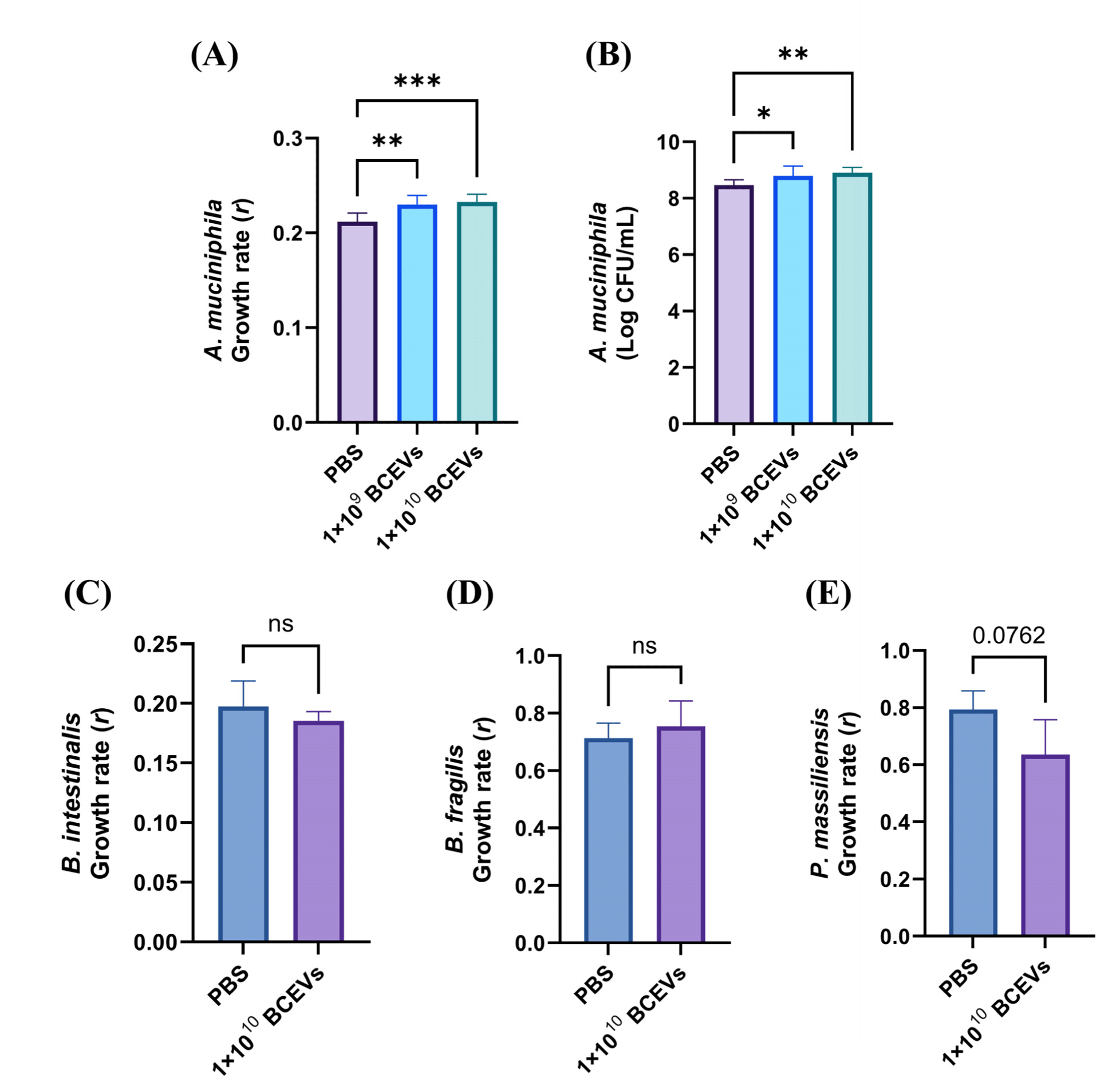
To assess the effect of BCEVs on the growth rate of A. muciniphila, two concentrations of BCEVs were co-cultured with A. muciniphila and the growth rates were measured (Fig. 2A). Both concentrations significantly increased the growth rate of A. muciniphila. To verify the direct effects on the growth of A. muciniphila, cell viability was measured at the endpoint. Both concentrations significantly increased cell viability compared to the control, which was consistent with previous results (Fig. 2B). In contrast, co-culture of BCEVs did not affect the growth rate of the gut commensals B. intestinalis and B. fragilis and tended to decrease the growth rate of P. massiliensis (Figs. 2C, D, and E). These results suggest that BCEVs can regulate the growth rate differently depending on the species and that they increase the growth rate of A. muciniphila.
To assess whether BCEVs exerted a similar influence on the growth of A. muciniphila in the presence of intestinal epithelial cells, a co-culture system of anaerobic bacteria and Caco-2 cells was established (Fig. 3A). The amount of dissolved oxygen was significantly lower in the apical portion of the wells with rubber than in those without rubber, with no significant difference in the basal portion (Fig. 3B). In addition, the number of colonies of A. muciniphila was measured, and it was found that A. muciniphila could not survive in the absence of rubber; the number of colonies was significantly higher when co-inoculated with A. muciniphila and BCEVs than when inoculated with A. muciniphila alone (Fig. 3C). SEM images also showed that more A. muciniphila was observed when co-cultured with BCEVs, which is consistent with previous results (Fig. 3D). These results confirmed the establishment of a co-culture system of anaerobic bacteria and cells and showed that treatment with BCEVs enhanced the viability of A. muciniphila even in the presence of intestinal epithelial cells.
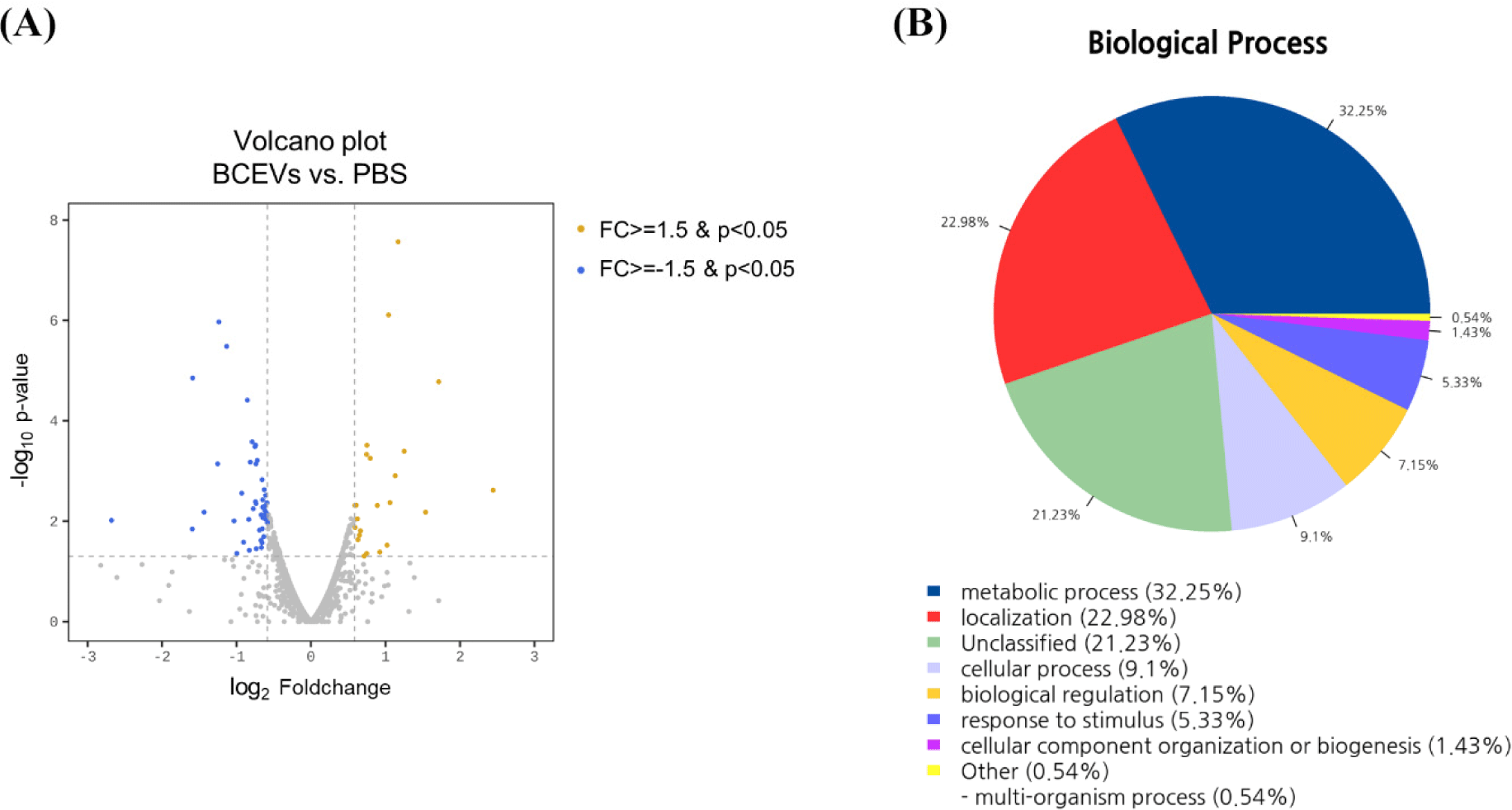
After confirming that BCEVs promoted the growth of A. muciniphila, transcriptomic analysis was performed by extracting RNA from A. muciniphila cells co-incubated with PBS or BCEVs to identify the mechanism of increased growth. Of the 2,201 observed genes, 70 DEGs with |fc|≥1.5 & raw p<0.05 were identified, and 23 and 47 genes were up- and downregulated, respectively, in the BCEVs-treated group compared to the PBS-treated group (Fig. 4A and Table 1). GO terms and KEGG pathway enrichment analyses were performed to predict the function of the identified DEGs. Among the 70 DEGs, 35 genes had no hits in GO analysis, 12 genes were involved in metabolic processes, and 10 genes were involved in cellular and biological processes (Fig. 4B). KEGG pathway analysis showed that the two-component system and nitrogen metabolism-related pathway was significantly increased when co-cultured with BCEVs, and protein export was decreased (Table 2). In particular, Amuc_1252 and Amuc_1253, genes related to glutamine synthetase, were upregulated following BCEVs treatment. Thus, it was predicted that BCEVs might promote the growth of A. muciniphila by regulating nitrogen metabolism, particularly of glutamine synthetase.
Discussion
This study confirmed the effect of BCEVs on A. muciniphila growth. Co-culture with BCEVs increased the growth rate and viability of A. muciniphila. Consistent with this, increased viability of A. muciniphila was observed when it was co-cultured with Caco-2 cells. Transcriptomic analysis revealed an increase in nitrogen metabolism-related genes in BCEV-treated A. muciniphila. These results suggest that treatment with BCEVs modulates the expression of genes in A. muciniphila, specifically by regulating energy metabolism, resulting in increased growth.
A. muciniphila has been found to be beneficial for gut health in several recent studies (Choi et al., 2024; Karamzin et al., 2021). Increased colonization by A. muciniphila enhances the expression of cyclic AMP-responsive element-binding protein H in the gut, which alleviates endoplasmic reticulum stress, intestinal barrier leakage, and endotoxemia in the blood caused by inflammatory stress (Wade et al., 2023). A. muciniphila ameliorates transmural colonic wall defects by promoting interleukin (IL)-22 secretion via myeloid differentiation primary response 88 (Bachmann et al., 2022). A. muciniphila also has a major effect on intestinal homeostasis, mediated by alpha kinase 1 in enterocytes in vitro (Martin-Gallausiaux et al., 2022). The outer membrane protein of A. muciniphila has been shown to exert a proinflammatory function by inducing the secretion of IL-10 (Ottman et al., 2017). Considering this, it is possible that BCEVs increase the growth of A. muciniphila, which may enhance the anti-inflammatory responses in the gut. This growth promotion may also strengthen the role of A. muciniphila in maintaining gut homeostasis and supporting the gut barrier. Further research is needed to investigate how BCEVs influence A. muciniphila within the broader gut microbiome and their potential therapeutic applications.
As research on the potential of various dietary EVs to modulate the gut microbiome grows, the impact of EVs on bacterial growth and the mechanisms involved are increasingly being investigated. A previous study showed that milk-derived EVs promoted the growth of Escherichia coli MG1655 and Lactobacillus plantarum WCFS1, which was attributed to the bacterial gene regulation properties of milk-derived EVs (Yu et al., 2019). Milk-derived EVs are taken up by bifidobacteria and promote the growth of bifidobacteria by promoting carbohydrate metabolism (Luo et al., 2023). Human urine-derived exosomes inhibit the growth of E. coli associated with urinary tract infections (Hiemstra et al., 2014). Furthermore, miRNAs have been shown to enter bacteria, regulate bacterial genes, and affect bacterial growth (Liu et al., 2016). In this study, treatment with BCEVs had a minimal impact on the growth of other commensal bacteria but significantly increased the growth rate and viability of A. muciniphila. These findings demonstrate that BCEVs can influence the growth rate and survival of gut commensal bacteria, particularly in a bacteria-specific manner. Further research involving a broader range of microbial species is necessary to elucidate the interactions between colostrum-derived EVs and microorganisms.
With the development of culturomic techniques and the growing importance of characterizing individual bacteria, the need to understand anaerobic bacterial–host interactions has increased (Na and Guan, 2022). Therefore, attempts have been made to develop a human gut mimetic system that can be co-cultivated with anaerobic bacteria (Kim, 2023; Moossavi et al., 2022; Sasaki et al., 2020). In a previous study, a system for co-culturing human organoids and anaerobic bacteria using a Transwell cell culture insert and butyl rubber was devised, demonstrating that commensal gut microbiota such as A. muciniphila, Bifidobacterium adventitiae, B. fragilis, and Clostridium butyricum could survive in this system and influence gene expression in human cells (Sasaki et al., 2020). Umehara and Aoyagi (2023) developed a system that allowed the co-culture of aerobic cells and anaerobic bacteria using a cell culture insert and liquid paraffin, and reported that Bifidobacterium bifidum had a higher growth rate in the upper section containing mineral oil (Umehara and Aoyagi, 2023). In the present study, to verify whether the growth-promoting effects of BCEVs on A. muciniphila persist in the presence of intestinal epithelial cells, we developed a simple anaerobic culture system using a Transwell cell culture insert to co-culture Caco-2 cells and anaerobic bacteria. The system effectively maintained a significantly reduced oxygen concentration in the apical insert sealed with butyl rubber, confirming the viability of A. muciniphila under these conditions. Additionally, it was observed that A. muciniphila viability increased when co-cultured with BCEVs in this system. These findings suggest that BCEVs could enhance the growth of A. muciniphila even in the presence of intestinal epithelial cells. Further analysis is required to assess the impact of increased A. muciniphila on Caco-2 cells following BCEV treatment.
Transcriptomic analysis confirmed that glutamate metabolism in A. muciniphila was regulated by BCEVs. Glutamate is considered highly important in bacterial metabolism and is known for its role in linking nitrogen and carbon metabolism (Commichau et al., 2006; Commichau et al., 2008). Additionally, it is known to influence pathways, such as protein synthesis and the TCA cycle (Feehily and Karatzas, 2013), allowing us to predict that BCEV-treated A. muciniphila may have adapted to its environment and increased its energy metabolism, which increased its growth. However, the transcriptomic results in this study showed that the differences in gene expression were slight, and only a few genes were identified in the predicted pathways. Therefore, further analysis is needed to identify more precise mechanisms.
Conclusion
This study demonstrates that BCEVs significantly enhance the growth rate and viability of A. muciniphila, in the presence and absence of host cells. This effect appears to be partially mediated by the modulation of genes involved in nitrogen metabolism. To the best of our knowledge, this is the first transcriptomic analysis exploring the impact of colostrum-derived EVs on the growth of A. muciniphila. Considering our initial findings, further studies are needed to elucidate the specific roles of colostrum-derived EVs and microRNAs in bacterial growth dynamics.













