Introduction
Meat is a good source of various macronutrients and micronutrients that are important for human health. However, it is perishable because of its high moisture content, the abundance of proteins, peptides, and amino acids, as well as its favorable pH for microbes (Holck et al., 2017). Therefore, strategies are required to ensure the safety, prevent spoilage, and extend the shelf life of meat. One of the conventional techniques for preserving meat is fermentation, which involves controlling the growth of beneficial microorganisms, lowering pH and water activity, and drying. The production of fermented sausages involves the addition of non-meat substances, such as sugars, salts, nitrites, and spices to a meat mixture made up of both fat and lean meat. The mixture is then placed in casings, allowed to ferment, and then dried (Feiner, 2006). In addition, fermented sausages are regarded as safe whole foods because they are fermented using starter cultures, which prevent the growth of unwanted bacteria (Xiao et al., 2020). Lactic acid bacteria (LAB) starter cultures are among the most crucial because of their hygiene benefits and ability to encourage proteolysis and the accumulation of bioactive peptides (Wang et al., 2017).
In addition, the flavor and biological activity of fermented sausages can be improved by the inclusion of food additives, such as exogenous enzymes. Exogenous enzymes such as papain, bromelain, and ficin facilitate the breakdown of proteins and enhance the texture and tenderness of products during meat processing (Sullivan and Calkins, 2010). Bromelain, a proteolytic enzyme in pineapple (Ananas comosus), supports digestion by breaking down dietary proteins (Zdrojewicz et al., 2018). The fig (Ficus carica), which has high nutritional value and is a rich source of ficin, is frequently used to tenderize meat (Kantale et al., 2019). As a result, natural proteases such as those found in pineapple and fig, may improve the quality of fermented sausages through proteolysis.
Moreover, fermentation-produced chemical compounds made from meat protein by exogenous enzymes contribute to the nutritional, sensory, and health benefits of the final product (Bourdichon et al., 2012). The fermentation-produced substances can be beneficial for consumers, who benefit from increased health-promoting properties. Aging-related changes in metabolism, anatomy, and sensory perception impact a person’s ability to chew and swallow, as well as their digestive system, appetite, perception of taste and smell, metabolism, and ability to absorb nutrients (Kremer et al., 2014). Fermentation offers an acidic environment that can lower the pH of meat, leading to an improvement in meat tenderness through proteolysis (Fadda et al., 2010), which can offer easier chewing and swallowing for consumers as well as the elderly. In addition, they contain essential amino acids required for various bodily functions, including tissue repair and immune function (Li et al., 2007). Therefore, researchers are actively working on developing functional food items by regulating various factors during the fermentation process to enhance the production of beneficial compounds.
Therefore, it is important to consider dietary modifications and strategies for consumers to ensure that they receive proper nutrition, such as texture-modified diets, frequent and smaller meals, and nutrient-dense foods. Furthermore, these foods must be nutritious and safe to use. Production of fermented sausages from beef and pork has the subject of the subject of numerous studies. Although goat meat provides consumers with vital amino acids and is a significant source of protein, there has been little research to make fermented sausage using goat meat. Therefore, the present research aims to develop fermented sausage with Korean native goat meat for health-conscious consumers and to determine the quality improvement of fermented sausages with the incorporation of pineapple and fig powders.
Materials and Methods
Muscles of the M. biceps femoris and M. semitendinosus from the meat of female goats were obtained from the goat farm in Gangjin-gun, Jeollanam-do, South Korea. The lean meat was kept for further use at 4°C after trimming all visible connective tissue and fat. The moisture, protein, fat, and pH of goat meat are 73.24%, 22.31%, 3.45% and 5.78 respectively. The curing solution was prepared using cold water (15%), sugar (2.5%), salt (1.6%), NaNO2 (0.005%), vitamin C (0.05%), glucose (5%), garlic powder (0.2%), and black pepper powder (0.3%), as shown in Table 1. Sausages were inoculated with approximately 107 CFU/g meat starter culture (0.05%) contains Lactobacillus sakei and Staphylococcus carnosus (Bactoferm F-RM-52 US). Seven sausage samples with different concentrations of pineapple and fig powders were prepared: control (without powder), P1 (0.1% pineapple), P2 (0.25% pineapple), P3 (0.5% pineapple), F1 (0.1% fig), F2 (0.25% fig), and F3 (0.5% fig). Pineapple powder and fig powder (100% pure) were obtained from Saemaeul, a food additive company based in Changnyeong, Korea. The carbohydrates, protein, and fat content of pineapple powder (79.9%, 2.95%, and 0.96%) whereas fig powder (81%, 4%, and 4%) respectively.
The lean meat (85%) was ground twice through plate holes with sizes of 0.5 mm and 0.3 mm. Subsequently, the ground meat was mixed with salt, pickling salt, and one-third cold water for 8 min and then mixed with fat (15%), other curing ingredients, starter culture, and the remaining water. After stuffing the mixtures with a collagen casing to a length of 52 mm, the sausages were sprayed with a meat surface mold solution (108 Log CFU/g), Penicillium nalgiovense. The sausages were transferred to a dough conditioner (Grand Woosung, Seoul, Korea) and the sausages were processed at 25°C with 90% relative humidity (RH) for 48 h, then 18°C with 80% RH for 7 d, and finally 12°C with 75% RH for 21 d. Samples were taken on the initial day, 2 days, 9 days, and 30 days of fermentation for analysis.
Each sausage sample was prepared and weighed on the initial day. The treated samples were weighed again after fermentation (day 2), dry-ripening (day 9), and drying (day 30). The percentage of weight loss was calculated using the formula below:
A: sample weight on the initial day; B: sample weight on each fermentation day.
The sausages’ moisture content was evaluated by calculating the weight loss after 24 h at 105°C in a drying oven (Helrich, 1990). A calibration plate was used to calibrate the colormeter (CR-410, Minolta, Osaka, Japan), which was used to estimate the color of the sample. The CIE L*, CIE a*, and CIE b* values of the surface of the sliced samples were calculated by averaging three repeated measurements. To estimate pH, 2 g of the sample was mixed with distilled water (18 mL) and homogenized for 30 s using a homogenizer (Polytron PT 10-39 GT, Kinematica AG, Luzern, Switzerland) at 2,000×g. Whatman No. 4 filter paper was then used to filter the homogenate, and a pH meter (Seven ExcellenceTM, Mettler-Toledo, Schwerzenbach, Switzerland) was used to measure the pH of the filtrate. Following the protocol of (Folch and Lees, 1951), the fat content of the sample (5 g) was ascertained by using a folch solution. In addition, an automatic Kjeldahi device (K-370, Buchi, Flawil, Switzerland) was used to calculate the crude protein content.
The antioxidant activity of fermented sausages was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity during processing (Blois, 1958). First, 2 g of each sample was homogenized in 18 mL distilled water. The mixture was filtered through a Whatman No. 4 filter paper, and 3 mL of filtrated solution was centrifuged at 1,500×g for 10 min. Following that, 0.4 mL of supernatant, 1.6 mL of distilled water, and 2 mL of DPPH solution (0.2 mM DPPH in methanol) were vortexed and incubated for 1 h in a dark room. The mixture was centrifuged again at 1,500×g for 10 min. A blank sample (2 mL of distilled water and 2 mL of methanol) and a control sample (2 mL of distilled water and 2 mL of DPPH solution) were prepared. Finally, the supernatant’s absorbance was measured at 517 nm and the DPPH radical scavenging activity was calculated with the following equation:
The sample (10 g) was mixed in a blender (LED Embossing Stomacher, BNF Kore, Gimpo, Korea) for 2 min after dilution with 90 mL of sterile saline solution (0.85% NaCl). Subsequently, serial decimal dilutions of the homogenate were prepared. A suitable dilution sample was promptly inoculated on the surface of a dry film culture medium (Aerobic Count Plate; 3M Petrifilm, 3M, St. Paul, MN, USA) and XLT4 (Merck, Darmstadt, Germany) to investigate the total plate count (TPC) and detect the presence of Salmonella spp. They were incubated at 37°C for 24 h after completely absorbing the sample on the surface. The total number of bacteria was calculated by multiplying the number of red colonies produced by the rate of dilution. According to Yang et al. (2009), the bacterium count is expressed as (Log CFU/g).
The process described by Olson et al. (1976) was followed in the production of the myofibrils using a buffer. The buffer was prepared with 0.1 M potassium chloride, 0.02 M potassium phosphate, 0.001 M ethylenediaminetetraacetic acid, 0.001 M magnesium chloride, and 0.001 M sodium azide. The sample, weighing 2 g, was mixed with 20 mL of ice-cold myofibrillar fragmentation index (MFI) buffer and subsequently homogenized at 3,800×g for 30 s to ensure thorough blending. The homogenate was centrifuged at 1,000×g for 15 min to separate the components, and the supernatant was carefully removed. The remaining residue was then re-mixed with 20 mL of ice-cold MFI buffer, and the centrifugation process was repeated. Following the repetition of this procedure with a fresh batch of residue dissolved in 10 mL of MFI buffer, the homogenate was vortexed and then filtered through an 18-mesh filter to eliminate connective tissue and fat. The liquid was collected, and the protein concentration at 540 nm was determined using the biuret method. According to Yu et al. (2009), MFI values were calculated as absorbance of units multiplied by 200 for myofibril protein concentrations of 0.5 mg/mL.
A texture profile analyzer (TA-XT2, Stable Micro System, Godalming, UK) was used to estimate the texture profile analysis of goat meat fermented sausages according to Bourne (1978) with a few modifications. The sample was chopped into pieces that were 15 mm long and 10 mm wide, and a double compression cycle test was conducted on the sample at a distance of 20 mm from the aluminium cylinder probe. The recording speed and crosshead speed were both 4 mm/s and the force-distance deformation curves were captured. Hardness (kgf) and springiness (%) were assessed and determined using the available software.
In accordance with Krzywicki (1979), 2 g of the sample was homogenized for 10 s using 10 mL of 0.04 M phosphate buffer (pH 6.8). The mixture was stored at 1°C for 24 h. After centrifuging the mixture for 30 min at 3,500×g, Whatman No. 1 filter paper was used to filter it. The filtrate’s absorbance was precisely measured at wavelengths 525, 572, and 700 nm with a spectrophotometer. The metmyoglobin content of the fermented sausages was determined using the formula below:
Where Aγ=Absorbance at γ nm.
The residual nitrite content of the fermented sausages was estimated as described by Shin et al. (2017). A 300 mL volumetric flask was filled with the sample (10 g), and 10 mL was set aside to create a blank sample. 150 mL of distilled water that had been heated to 80°C was added after the sample had been homogenized. The homogenate was then combined with 0.5 N NaOH solution (10 mL) and 12% ZnSO4 solution (10 mL), and it was incubated at 80°C for 20 min while being stirred every three minutes. The sample was cooled before being filled to 200 mL using 20 mL of CH3COONH4 (ammonium acetate) buffer (pH 9.0) and 10 mL of distilled water. After well mixing, the mixture was left to stand for 10 min at room temperature. After passing the mixture through a Whatman No. 1 filter paper twice, a clean filtrate was obtained. After filtration, 20 mL of the filtrate was placed in a 50 mL centrifuge tube for use as an experimental solution. One mL of sulfanilamide solution, 1 mL of N-(1-naphthyl) ethylenediamine dihydrochloride reagent, and 3 mL of distilled water were combined with the sample and allowed to sit at room temperature for twenty minutes in order to develop color. By measuring the absorbance at 540 nm, a standard curve made using a nitrite solution was used to calculate the residual nitrite content.
The fatty acid profile of the fermented sausages was determined according to the guidelines of O’fallon et al. (2007). To separate fatty acids methyl esters, a sample (1 g), 1 mL of the C13:0 internal standard (0.5 mg of C13:0/mL of methanol), 0.7 mL of 10 N KOH, and 6.3 mL of methanol were thoroughly mixed before being incubated at 55°C for 1.5 h. Every 30 min while heating, the samples were given a ferocious shake. The product was cooled in ice-cold water for 2 min before being added 0.58 mL of 24 N H2SO4 solution. The mixture was reheated for a second time at the same constant temperature and the same steps were followed. Subsequently, 3 mL of hexane was added to the mixture, which was shaken perfectly. The mixture was then centrifuged (HANIL Combi-514R, HANIL, Inchon, Korea) at 150×g for 5 min before being transferred to a vial using a Pasteur pipette. A gas chromatograph with a flame ionization detector (Agilent 7890 series, Agilent, Santa Clara, CA, USA) was used to analyze the fatty acids under the following settings. The column was an HP-88 column (60 m×250 μm×0.2 mm), the detector was a Flame Ionisation Detector, and the injector had a split ratio of 30:1. The temperature of the oven was 250°C. The carrier gas flow rates were 400, 40, and 40 for air, H2 (hydrogen), and He (helium), respectively. The fatty acid composition was expressed as a percentage.
Nutritional quality indices of the fermented sausages were evaluated using fatty acid profiles. The Ulbricht and Southgate (1991) method was used to calculate the thrombogenicity index (TI) and atherogenicity index (AI), while the Santos-Silva et al. (2002) method was used to estimate the hypocholesterolemic/hypercholesterolemic (HH) index. The following equations were used to compute the AI, TI, and HH index. Additional measures of nutritional quality, including the ratio of n6/n3 and polyunsaturated fatty acid (PUFA)/saturated fatty acid (SFA), were also determined.
Ten panelists who had attended eight orientation sessions to become familiar with the standards and scales to be used assessed sensory analysis. After removing the fermented sausage casing, the sausages were sliced into pieces approximately 2 mm in thickness. The samples were graded on a scale of 1 to 9 points, with the control group receiving 5 points. The panelists rate attractiveness, taste preference, goaty notes, tenderness, juiciness, and overall acceptability on a scale from 1 to 9. It is an analysis of senses, from visual appeal to the final verdict of acceptability.
Experimental data with three replicates were analyzed with the R statistics software (version 4.2.3). The number of replicates in an experiment is crucial for assessing the statistical power of the results. Statistical power is the chance of the experiment properly rejecting the incorrect null hypothesis. A one-way analysis of variance (ANOVA) was used to determine any statistically significant differences between the means of treatments with a 95% confidence level. After determining a significant overall difference using ANOVA, Tukey’s multiple range test was used to perform pairwise comparisons between group means.
Results and Discussion
The final product of the fermented sausage loses weight depending on factors, such as temperature, degree of meat mixture comminution, casing width, and amount of fat in the sausage (Bloukas et al., 1997). In the present study, no difference in weight loss was observed on day 2; however, differences were observed after day 9 (Fig. 1). Among the final products, F3 and P3 showed the lowest weight loss, whereas the control showed the highest weight loss. This is possible because of the differences in water-binding capacity. According to current investigations, there is a correlation between the weight loss and fat content of the final products. However, there were no significant differences among P1, P2, F1, and F2. Humectants and tenderizers such as pineapple, and figs play a significant role in enhancing water absorption and retention in meat products, which can lead to lower weight loss during processing, cooking, and storage (Aung et al., 2024; Kim et al., 2021). This is particularly beneficial in developing functional meat products that are easier to chew, more palatable, and retain more nutrients. Therefore, the pineapple and fig powders (0.5%) had a positive impact on weight loss during processing.
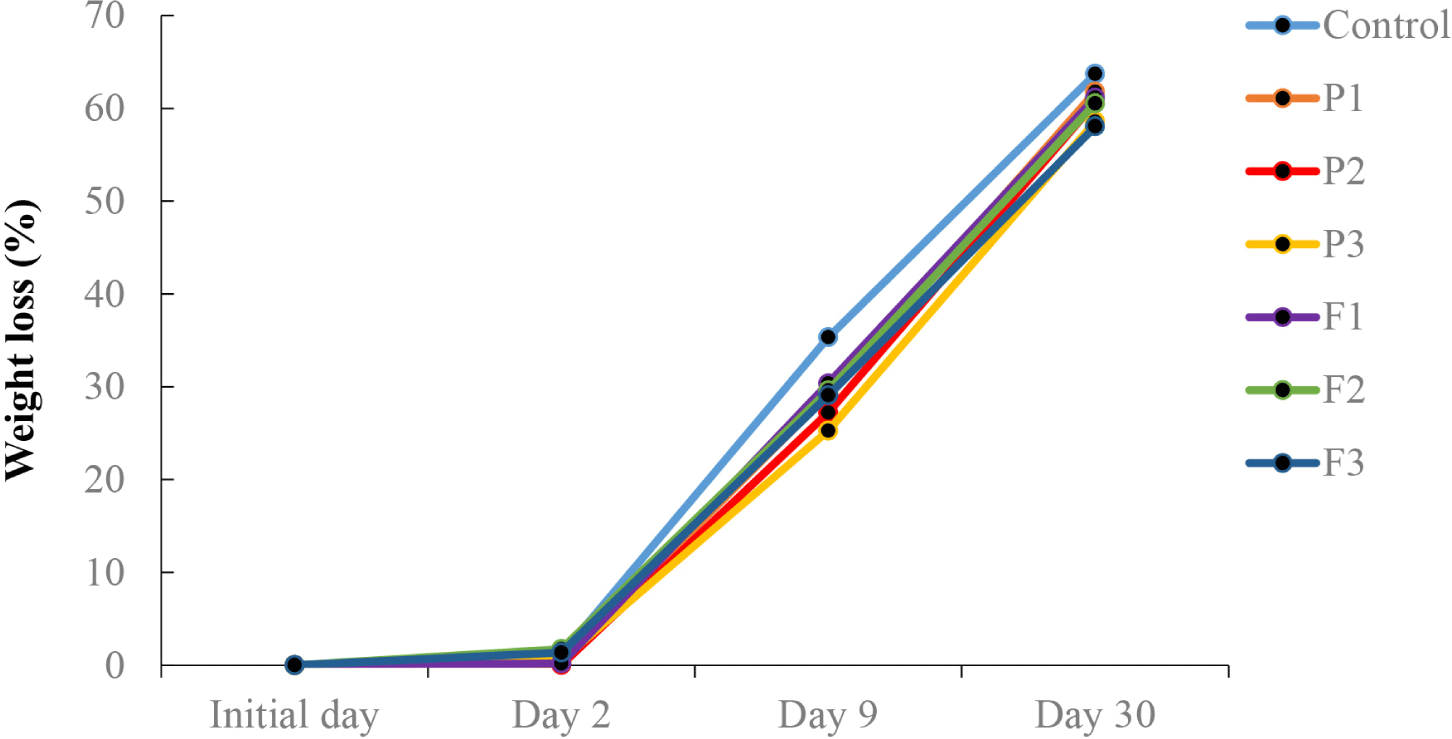
Table 2 demonstrates that processing time had a substantial impact on the amount of moisture in fermented sausages. Moisture content remained consistent across all treatments, showing no significant differences between them or between the initial day and day 2 (p>0.05). After day 9 of processing, all of the treatments significantly decreased (p<0.05). The study found that the control group had the lowest moisture content (p<0.05), although there was no significant difference (p>0.05) between the other treatments. The drying rate does affect the fermented sausage’s moisture content. During fermentation and drying, moisture is removed from the sausage through evaporation. On day 30, the moisture content significantly decreased again (26.83%–31.50%; p<0.05). Whereas the control group displayed the lowest value (p<0.05) and F3 had the highest value (p<0.05). According to the current results, the inclusion of pineapple and fig powder increased, and moisture content increased due to their moisture retention and water binding capacity. Aung et al. (2024) suggested that pineapple powder has the ability to retain water during meat processing. In addition, Integrating pineapple peel and fig powder into meat products can enhance water retention and improve texture (Kantale et al., 2019). According to Konieczny et al. (2007), meat products with a higher moisture content offer the desired tenderness for consumers; nevertheless, this moisture content also causes a quicker deterioration of the product because of increased microbial proliferation.
The processing time significantly affected the pH (Table 1). The initial pH ranged from 5.70 to 5.64. There were no significant differences among the other treatments, although F2 had the highest pH (p<0.05). On day 2, the fermented sausages’ pH decreased significantly (p<0.05). This is because the LAB (starter culture) convert sugar in the meat mixture into organic acids, mainly lactic acid, which can lower the pH of sausages during fermentation. Control, P1, P2, and F1 showed the highest pH values with no difference between them (p>0.05), whereas F3 and P3 showed the lowest pH values (4.31 and 4.33), followed by F2 (p<0.05). In all treatments, the pH significantly increased (p<0.05) on day 9. One possible explanation for this phenomenon could stem from enzymatic activity leading to the production of ammonia and biogenic amines (Lücke, 1998). Furthermore, this can result from the fermentation process’s hydrolysis with proteinases, which produce alkaline components such as peptides and amines (Lakshman et al., 2010). On day 30, the other treatments showed a substantial increase (p<0.05), but the control, P3, and F3 treatments did not change significantly from day 9. The final product of P3 had the lowest pH (4.90), whereas the control had the highest pH (5.21; p<0.05). Yoo et al. (2016) reported that a large and rapid decrease was observed in the sausages containing pineapple. Notably, the addition of pineapple dietary fibers caused a reduction in the pH value of species sausage (Henning et al., 2016). Yoo et al. (2016) reported that the incorporation of pineapple decreased the pH of the sausage. In the current study, the pH values of fermented sausages treated with pineapple and fig powders were lower than those of the control, possibly because of their acidic nature. Pineapple and fig powders affect the pH value of fermented goat meat sausages during processing. In particular, the current results suggest that the incorporation of pineapple powder (0.5%) positively affects safety by inhibiting the growth of undesirable bacteria.
A high-fat content (40%–50%) is necessary for the organoleptic qualities (tenderness, juiciness, and flavor) of fermented sausage (Lazic et al., 2019). However, consumers are becoming increasingly concerned about the potential health effects of excessive fat consumption. Low-fat sausages become rigid as a result of rapid weight reduction and have an unappealing appearance owing to wrinkled surfaces and casing hardening (Muguerza et al., 2002). Therefore, an appropriate amount of fat is required to resolve this issue. Table 2 illustrates how the processing time affected the amount of fat in the fermented sausages made from goat meat. Throughout the 30 days of fermentation, the fat content increased dramatically across all treatments, from 8.23%–9.68% to 19.83%–21.87%. This was possibly due to the moisture loss from the sausages, leading to a concentration effect on the remaining components, including fat. On the initial day, P2, P3, and F2 had significantly higher fat content (p<0.05), while no significant differences were observed between the other treatments (p>0.05). The differences in how goat meat is chopped may be the cause of these variations (Olivares et al., 2010). In addition, one of the factors is different fat concentrations of powders in treatments that can result in varying initial fat contents. On day 2, the fat content of all treatments decreased with P3 showing the highest fat content (p<0.05); however, the remaining treatments did not show significant differences. This decrease can be attributed to lipolysis and oxidation. On day 9, the control, P1, and P2 showed the highest value (p<0.05), whereas P3 and F3 showed the lowest value (p<0.05). F1 and F2 were significantly lower than those of control, P1, and P2, but significantly higher than those of F1 and F2 (p<0.05). After 30 days of fermentation, the control, P1, P2, and F1 had the highest fat content (p<0.05), whereas F3 had the lowest value (p<0.05). P3 and F2 were significantly higher than F3 but significantly lower than the other treatments (p<0.05). The fat content of the fermented sausages decreased as the amounts of pineapple and fig increased. In the present study, there was a correlation among weight loss, moisture content, and fat content into fermented sausages.
The antioxidant activity of the fermented sausages was assessed throughout the processing based on their ability to scavenge DPPH radicals (Table 2). The DPPH radical scavenging activity increased considerably across all treatments, rising from 53.24%–69.15% to 75.01%–90.56% after 30 days of fermentation. An increase in DPPH scavenging activity during the fermentation of fermented sausages suggests an increase in antioxidant activity. This could be due to microbial activity (Kim et al., 2004), the release of bioactive compounds, or the activation of antioxidant enzymes (Broncano et al., 2012; Li et al., 2008). On the initial day, F3 showed the highest value (p<0.05), whereas the control showed the lowest value (p<0.05). On day 2 of fermentation, all treatments decreased compared to the initial day, while the control and F3 treatments showed significant decreases (p<0.05). The influence of lipid oxidation before the activation of antioxidant compounds may be the cause of the diminishing DPPH scavenging activity. The values of all treatments were considerably higher than those of the control (p<0.05), whereas F1 was lower than P1, P2, P3, F2, and F3 (p<0.05). It is assumed that this could be due to the rich antioxidant compounds of pineapple and fig powders. Pineapple and fig are rich in phenolic compounds and flavonoids (Bachir bey et al., 2014; Lasunon et al., 2022). After nine days of fermentation, the values for each treatment increased; P2, P3, F1, F2, and F3 increased considerably from day 2. F3 demonstrated the highest DPPH radical scavenging activity, followed by P3, F2, P2, F1, P1, and the control (p<0.05). In the final product (30 days of fermentation), P3 had the highest activity (p<0.05), whereas the control and F1 showed the lowest DPPH scavenging activity (p<0.05). There was no significant difference between P2, F2, and F3 (p>0.05), but it was higher than that of P1 (p<0.05). In the present study, the DPPH scavenging activity of the samples increased as the concentration of pineapple and fig powder increased. This is possible due to the different antioxidant concentrations (Kumar et al., 2021) and the release of antioxidant compounds by the protein degradation activity of proteolytic enzymes. Therefore, the incorporation of pineapple and fig powders into sausage can neutralize free radicals, reducing oxidative stress and the risk of chronic diseases such as heart disease and cancer in consumers.
Instrumental color is a common indicator for assessing the sensory quality of meat products, and it can influence customer preferences, purchase decisions, and satisfaction. Table 3 shows the color characteristics of sausages during fermentation. The CIE L* values of P2, P3, and F1 did not significantly change during the 9-day processing time, whereas those of the control, P1, F2, and F3 significantly changed (p<0.05). After 30 days of fermentation, the CIE L* values of all treatments decreased significantly (p<0.05). F3, P3, and F2 showed the highest CIE L* values and were significantly higher than those of the control (p<0.05), where P2, F1, and P1 were not significantly different among all treatments (p>0.05). Marušić et al. (2011) noted that meat products with higher moisture content have a lighter appearance owing to the presence of an aqueous layer on their surface, which increases light scattering and the CIE L* value. This can be explained by variations in the red pigments produced by fermented minced meat (Yu et al., 2015).
On the initial day, the CIE a* value of the cured meat ranged from 7.02 to 8.32. The control and P1 groups showed the highest CIE a* values (p<0.05). No significant differences were observed among P2, P3, F2, and F3 (p>0.05), and F1 was not significantly different among treatments. After 2 and 9 days of fermentation, the CIE a* value significantly increased in all treatments (p<0.05), and no significant differences between them. This could be related to how nitrite affects meat products because the creation of a distinct color in cured meat is a common function of nitrite. It has been established that nitrite is the cause of the red color in meat products (Lehmann, 1899). Nitrite decomposes into nitric oxide, which reacts with myoglobin to develop the red color of cured meat upon the application of heat. After 30 days of fermentation, the CIE a* of all the treatments significantly decreased again (p<0.05), but this is significantly higher compared with the initial day (15.73–14.01). The decrease in CIE a* color is possibly due to the protein breakdown and Maillard reaction. Proteolytic enzymes produced during fermentation can break down proteins, leading to the formation of peptides and amino acids. Melanoidins are produced from breakdown products and reducing sugars, which can alter the overall color and induce browning (Starowicz and Zieliński, 2019). The CIE a* values of the final products of P1 and F1 were higher compared to the other treatments (p<0.05), whereas there was no considerable difference between the remaining treatments. According to the current results, the pineapple and fig powders increased and the CIE a* value decreased. This could be due to differences in carotenoid content, which may reduce the CIE a* and increase the CIE b* of products (Bae et al., 2023; Jeong et al., 2020). The concentration of metmyoglobin may be the reason for the control group’s lower CIE a* value.
The CIE b* values of all treatments were significantly increased after 2 and 9 days of fermentation (p<0.05) and significantly decreased again on day 30 (p<0.05). According to the current investigation, pineapple and fig powder increased, and the CIE b* value increased in the final products owing to differences in carotenoid concentration. In addition, this may be owing to the antibacterial chemicals created by fermented sausages treated with pineapple and fig powders, which suppress the growth of microorganisms that cause spoiling (Yu et al., 2015).
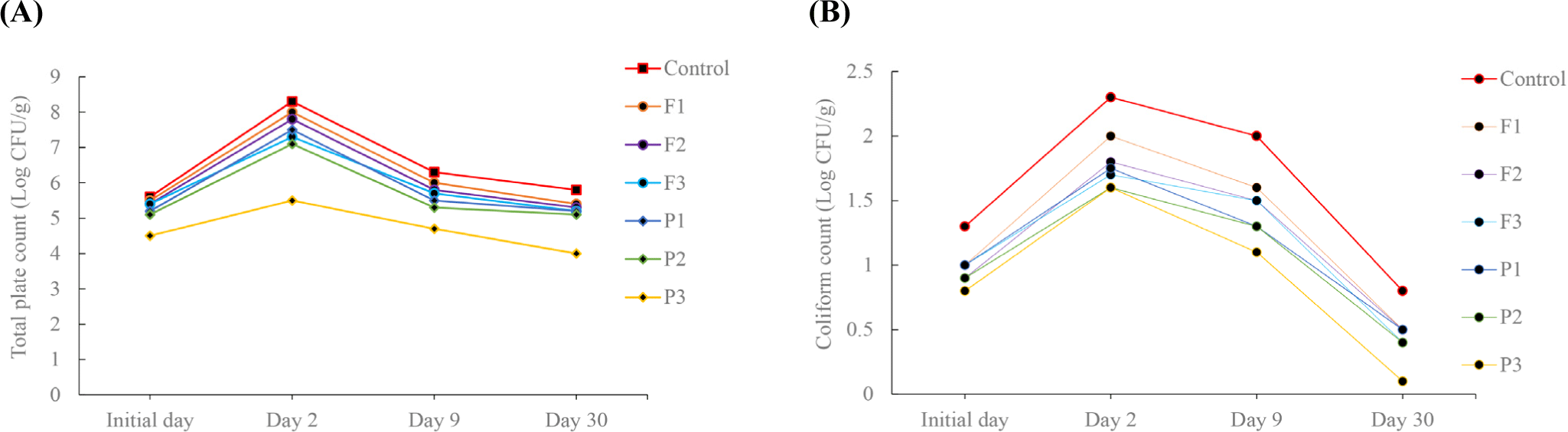
Microbial analysis of fermented sausages is essential for ensuring product safety and quality. The fermentation process involves the activity of beneficial microorganisms that contribute to flavor development, preservation, and texture (Sharma et al., 2020). It is critical to monitor and control microbial populations to prevent the spread of harmful pathogens. The first day’s TPC varied from 5.6 to 4.5 Log CFU/g and the coliform counts from 1.3 to 0.8 Log CFU/g, with P3 having the lowest and control having the highest count (Figs. 2A and B). On day 2, the TPC and coliform counts increased dramatically with pH reduction. Yoo et al. (2016) noted that lowering pH is crucial during the early stages of fermentation because it inhibits undesirable microorganisms. There was a gradual decline in both TPC and coliform count during the later stages of processing. This could be due to increasing salt concentrations and decreasing water activity. There was a significant reduction in TPC and coliform counts in the pineapple and fig powder-treated finished products. Among them, P3 had significantly lower TPC and coliform count (4 and 0.1 Log CFU/g), respectively. Obinna-Echem (2015) reported that a combination of low pH and high acidity could inhibit the survival of Escherichia coli. According to the current investigations, pineapple powder (0.5%) had a strong effect on the reduction of TPC and coliform counts.
The highest protein level was observed in the control (p<0.05), whereas the lowest levels were observed in the P3 and F3 groups (p<0.05), as shown in Table 4. There were no significant differences between P1, P2, and F1 (p>0.05), or between P2 and F2 (p>0.05). F2 showed significantly lower than control, P1, and F1, but higher than P3 and F3 (p<0.05). Protein levels decreased as the amount of fig and pineapple powder increased in the fermented sausage formulation. One reason for this could be the reduction in moisture during processing. As the amounts of tomato and flaxseed powders in the sausage recipe increased, the moisture content of the samples decreased, and their protein content increased (Ghafouri-Oskuei et al., 2020). The decrease in moisture content during ripening leads to an increase in protein and fat content (Olivares et al., 2010). Fig and pineapple powders contain components that can retain water, such as fibers and sugars (Santos et al., 2021). This can lead to lower protein content in the sausage.
Myofibril fragmentation is measured using the MFI, which can also evaluate the texture and structure of meat products. The MFI is dependent on pH (Purchas, 1990), which can influence the activity of proteolytic enzymes (Singh et al., 2019) and the growth and activity of microorganisms. Higher MFI was observed in fermented sausage with higher concentrations of pineapple and fig powders (Table 4). The highest MFI value was observed in P3 (p<0.05), whereas the lowest value was in control (p<0.05). There were no significant differences between P1 and F1 or between P2, F2, and F3 (p>0.05). According to Yoo et al. (2016), pineapple juice causes protein breakdown, resulting in an increase in most amino acids in the fermented sausage. Aung et al. (2024) also reported that the MFI value increased in jerky using pineapple powder, which impacted tenderness. This could be due to the acidic environment of pineapple and fig powders, which can influence the proteolytic enzyme activity, affecting the breakdown of myofibrils and contributing to changes in MFI. According to the current findings, P3 provided the more acidic environment, followed by F2, F3, and P2. Therefore, the inclusion of pineapple and fig powders in sausages could provide bioactive peptides for consumers.
Meat tenderness significantly influences overall preference and is often considered a key factor in consumer satisfaction. The Korean Industrial Standards (KS) use three grades: lower than 0.2 kgf, 0.2 kgf–0.5 kgf, and 0.5 kgf–5 kgf to assess the tenderness level needed for senior-friendly food. Table 4 shows that the hardness value was highest (6.00 kgf) in the control group and lowest (1.80 kgf and 1.44 kgf, respectively) in the F2 and F3 groups (p<0.05). P1 and F1, as well as P2 and P3, did not show significant differences (p>0.05); however, there were significant differences between them (p<0.05). However, pineapple and fig powder-treated fermented sausages had grade 3 KS standards and were suitable for the elderly regarding tenderness. Gregg et al. (1993) discovered a strong association (r=0.86) between hardness and fat content of Bologna sausages. Furthermore, Grigelmo-Miguel et al. (1997) reported there is a negative correlation between fruit fiber content and hardness. In addition, there is a curvilinear relationship between pH and tenderness, with a minimum of 5.8 to 6.2 for tenderness (Jeremiah et al., 1991; Purchas and Aungsupakorn, 1993). Current investigations support these observations, and moisture also influences the texture of fermented sausages. The springiness of the control and P1 treatments was higher than that of the other treatments (p<0.05), whereas there was no significant difference among the remaining treatments. Hardness and springiness were not significantly related (Di Monaco et al., 2008).
A chemical reaction between myoglobin and oxygen transforms an iron atom from its usual ferrous (Fe2+) state to a ferric (Fe3+) state, resulting in metmyoglobin production (Bekhit and Faustman, 2005). After 30 days of fermentation, P3 had the lowest metmyoglobin level (p<0.05), followed by F3 (p<0.05), while the control displayed the highest content (p<0.05; Fig 3A). P1, P2, F1, and F2 did not differ significantly (p>0.05). Increasing the concentration of fig and pineapple powders resulted in a reduction of metmyoglobin content in the final products. This could be due to the metmyoglobin-reductive activity of pineapple and fig powders. Figs are high in polyphenolic components, such as flavonoids and phenolic acids (Veberic et al., 2008), and pineapple is a strong source of antioxidants because of its high phenolic content (Ali et al., 2020). Metmyoglobin is in an undesirable physiological state that provides a brownish color to meat and meat products (Suman and Joseph, 2013). In the current study, probably protein degradation and the Maillard reaction likely influenced the color of the fermented sausages (P1, P2, P3, F1, F2, and F3) more than metmyoglobin.
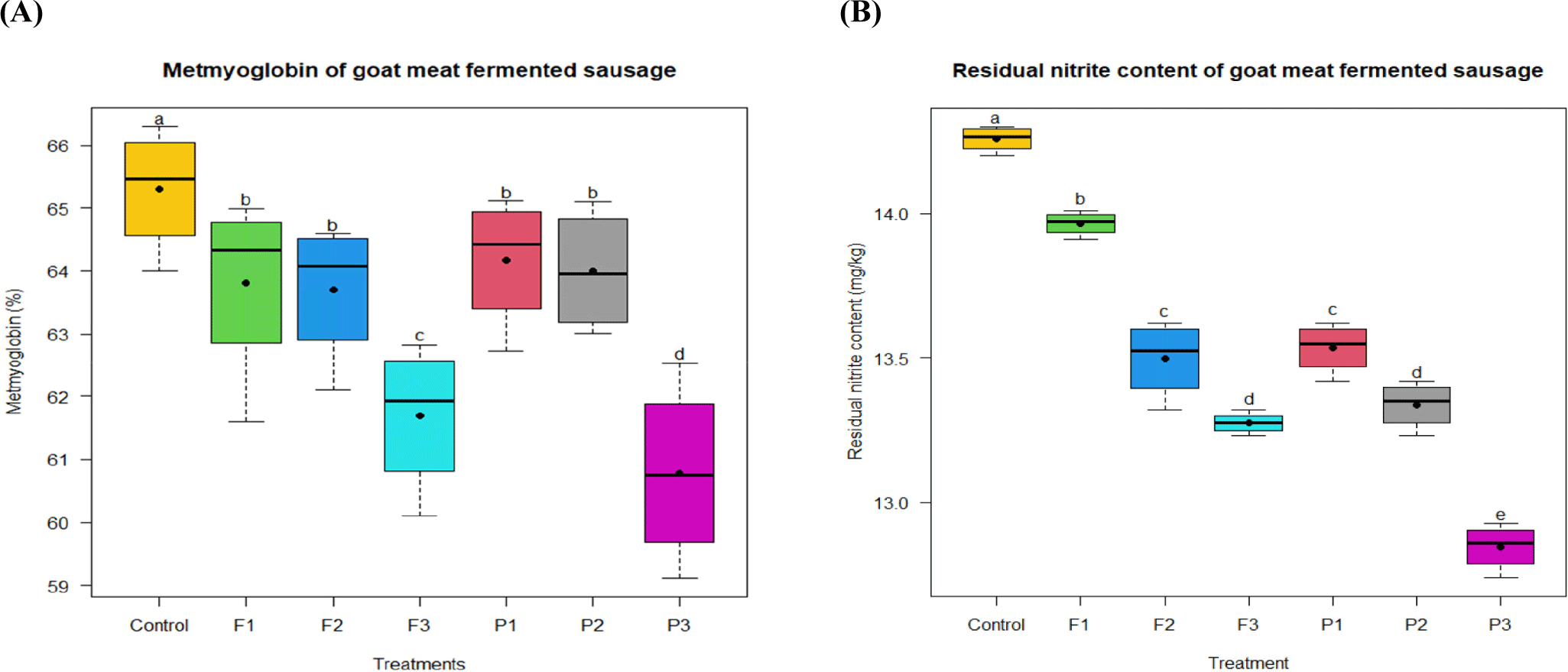
During fermentation, the amount of residual nitrite in fermented sausages decreased, accounting for approximately 19% of the original nitrite applied in each treatment. The residual nitrite level was the highest (14.25 mg/kg) in the control group and lowest (12.84 mg/kg) in P3 (p<0.05; Fig 3B). The residual nitrite content in samples treated with pineapple and fig powders decreased significantly as the powder concentration increased (p<0.05). It is assumed that dietary fiber can potentially influence the degradation or reactivity of nitrites. It was also noted by Aung et al. (2024) and Fernández-López et al. (2007) that the powders’ dietary fiber reduced the amounts of residual nitrite. Furthermore, lower pH may contribute to reduced residual nitrite levels by increasing the reactivity of the nitrite system (Pegg and Shahidi, 2008). In the current study, the addition of a higher concentration of powder resulted in a lower pH, which increased the rate of NO production and, lowered the level of residual nitrite. Excessive nitrite consumption can contribute to oxidative stress and inflammation, which are risk factors for many chronic diseases, including cardiovascular diseases (Karwowska and Kononiuk, 2020). According to this finding, the incorporation of pineapple and fig powder in fermented sausage can reduce the risk of chronic diseases for consumers.
In fermented sausages, the production of fatty acids during lipolysis is primarily responsible for flavor formation (Gandemer, 2002). The fatty acids in the sausages after 30 days of fermentation were analyzed (Table 5). Of the fatty acids, P1, F1, and F2 had considerably greater levels of monounsaturated fatty acids (MUFAs), while P2, P3, and F3 had higher levels of SFAs, and P1 and F2 had higher levels of PUFAs (p<0.05). Traditional meat products contain SFAs that are harmful to human health, particularly in patients with coronary heart disease (Muguerza et al., 2004).
SFAs, such as lauric acid (12:0) and myristic acid (14:0), were highest in the control (p<0.05), while palmitic acid (16:0) and stearic acid (18:0) were the highest in P2, P3, and F3 (p<0.05). Fermented sausages with pineapple contain higher quantities of palmitic acid during the early stages of fermentation (Yoo et al., 2016). Oleic acid (18:1) was the most prevalent MUFA, and its levels were highest in P1 and F2 (p<0.05). The primary flavor difference is attributed to oleic acid, which has several positive health effects, including lowered blood pressure and altered insulin resistance (Benet et al., 2015). The consumption of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) has been shown to have positive physiological effects on heart rate and blood pressure, as well as possible inflammation. In the current study, EPA and DHA are not significantly different between treatments (p<0.05).
The index of nutritional qualities for fermented sausages is shown in Table 4. Diets rich in n-3 fatty acids lower plasma levels of triglyceride levels, inhibit platelet aggregation, have antiarrhythmic effects, and improve endothelial dysfunction (von Schacky, 2000). In this study, P1 and F2 had the highest n-3 levels (p<0.05), whereas F3 had the lowest (p<0.05). If n-3 consumption is insufficient to offset excessive n-6 fatty acid intake, it can result in unfavorable ratios (n-6/n-3). Several diseases can be prevented by consuming foods with low n-6/n-3 ratios (Muguerza et al., 2004). The lowest ratio of n-6/n-3 was observed in P1 and F2 (p<0.05). In order to minimize the risk of numerous chronic diseases in consumers, a lower ratio of omega-6 to omega-3 fatty acids is preferable (Simopoulos, 2008). There was no significant difference in the PUFA/SFA ratios among the treatments (p>0.05), but the ratios were lower than the recommended levels. The UK Department of Health (Group GBCR, 1994) states that the PUFA/SFA ratio should not be lower than 0.4 because it has the potential to raise blood cholesterol levels. However, P1 and F2 showed decreased AI and TI (p<0.05). Higher AI and TI can lead to the collapse of lipids and plaque development, which can weaken blood vessel endothelial strength (Cebulska et al., 2018). In addition, P1 and F2 had significantly higher HH indices, which measure the ratio of hypo-to-hypercholesterolemic fatty acids. A higher HH ratio indicates a greater proportion of beneficial than detrimental fatty acids, suggesting a potential cholesterol-lowering effect (Ojha et al., 2017). According to current fatty acid profile investigations, P1 and F2 indicate a good nutritional quality index that can fulfil the requirements of consumers.
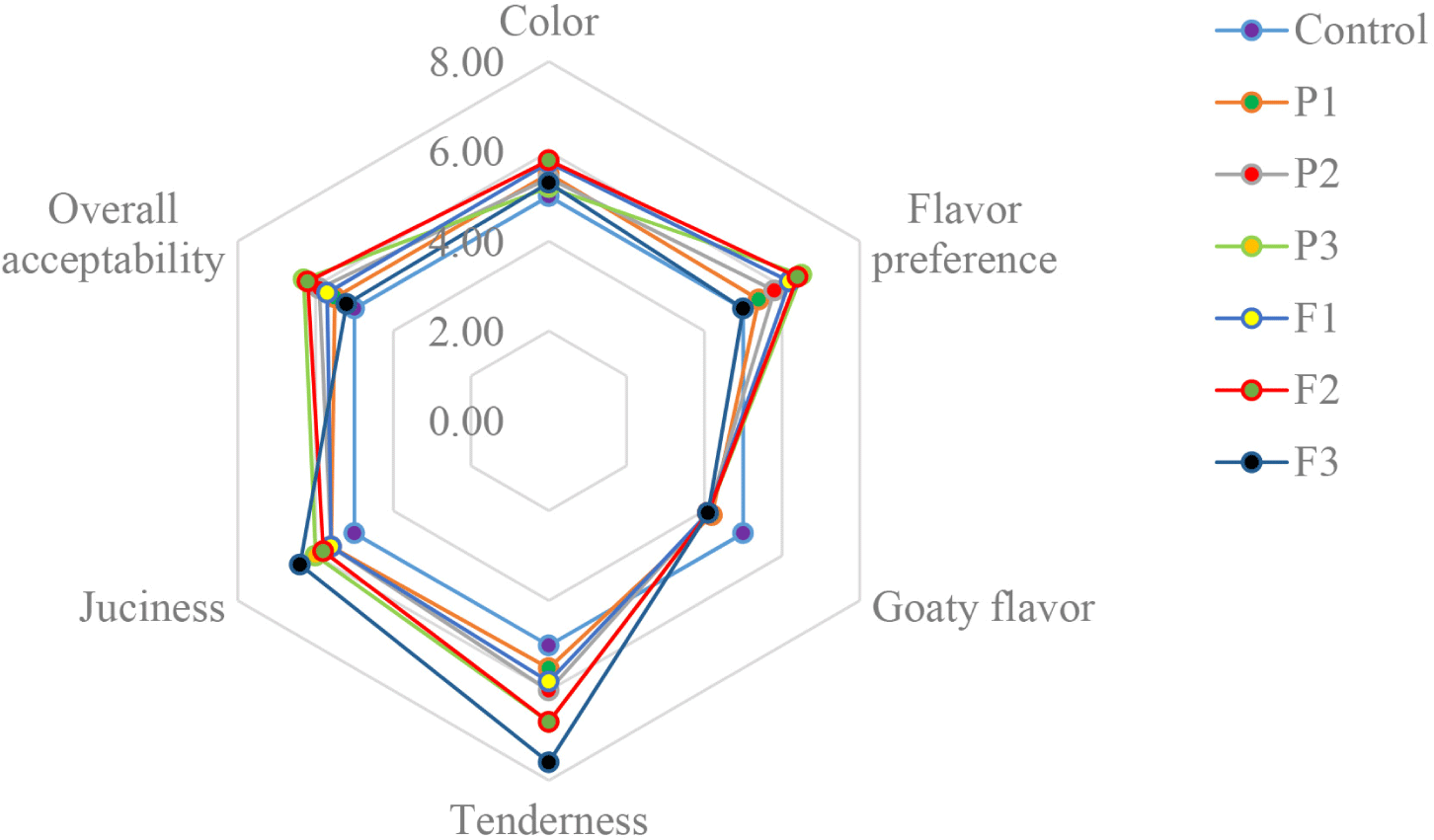
Fig. 4 shows the assessment of sensory panels and how they influenced the color, tenderness, juiciness, appearance, flavor preference, and overall acceptability of fermented sausages with the inclusion of pineapple and fig powders. Color, flavor, and tenderness are the three primary sensory attributes that change depending on the basic ingredients and processing methods (Albright et al., 2000). Of all treatments, F1 and F2 had the highest color score (p<0.05). The P3 and F2 flavors were more favored by the panelists than the other treatments (p<0.05). Free amino acids are released during protein degradation and are the building blocks of volatile chemicals that contribute to a pleasant aroma (Toldrá and Flores, 1998). In the current study, P3 and F2 showed the highest myofibrillar protein degradation, which could provide a flavor preference. F3 had the highest tenderness and juiciness scores (p<0.05), followed by P3 and F2. Although F3 had the highest tenderness and juiciness, it also had a soft and mushy texture, causing the sausage to lose its desired firmness and structure. This could be due to the ability of moisture retention to affect the texture and juiciness of sausages. In addition, pineapple and fig powder had a positive effect on the reduction of the goaty flavor of fermented goat meat sausage. The current results indicate that P3 (0.5% pineapple powder) and F2 (0.25% fig powder) had a positive effect on the overall acceptance of the sensory panelists. According to Zhao et al. (2017), there is a correlation between higher amounts of MUFAs and large quantities of odor-active aroma compounds resulting from Maillard reactions. These compounds are associated with a pleasant roasted flavor and increased sensory appeal. In addition, the high oleic acid (18:1) content in meat products meets the high preferences of panelists (Navarro et al., 2021). In the present study, P1 and F2 exhibited higher oleic acid and MUFA levels. However, P3 and F2 received the highest overall acceptance from the panelists, possibly because of their higher protein degradation, desirable texture, and nutritional fatty acid profiles.
Conclusion
Fermented sausages were prepared from Korean native goat meat and modified with different levels of pineapple and fig powders (0.1%, 0.25%, and 0.5%). The results showed that adding pineapple and fig powders to the fermented goat meat sausage improved its moisture content, DPPH radical scavenging activity, CIE L*, MFI, and tenderness. However, when the levels of pineapple and fig powders increased, the pH, fat, CIE a*, protein, TPC, coliform count, metmyoglobin, and residual nitrite decreased in fermented sausages. In particular, P3 (0.5% pineapple) had a strong effect on the pH, MFI, microbial analysis, metmyoglobin, and residual nitrite content. F3 (0.5% fig) had a strong effect on the fat content and hardness. However, the addition of 0.5% fig powder resulted in a mushy texture in the sausages, which can negatively affect consumer appetite and acceptance. It is crucial to address this issue to ensure that the final product is both nutritious and appealing. In addition, P1 (0.1% pineapple) and F2 (0.25% fig) showed a good nutritional quality index in the fatty acid profile. However, achieving a desirable n6/n3 fatty acid ratio may require careful consideration to avoid the overuse of these additives, which can negatively impact texture and consumer acceptance. Finally, the inclusion of 0.5% pineapple and 0.25% fig powders in the sausage formulation resulted in the highest sensory acceptance owing to protein degradation, desirable texture, and nutritional fatty acid profiles. Future research can optimize the use of pineapple and fig powder by addressing current limitations and exploring new methods. This will enhance meat quality and provide significant health benefits, particularly for consumers.













