Introduction
Bovine tuberculosis (BTB) is a chronic, infectious, and contagious illness of cattle and humans (zoonosis). It is caused by Mycobacterium bovis, a member of the M.tuberculosis complex (Desire et al., 2024). Infections can be spread to cattle from the environment (for example, by touching contaminated feces) to other cattle, animals, and people. The primary means of spreading within cattle is airborne, whereas humans typically acquire zoonotic diseases through close contact with infected animals, unpasteurized dairy products, or undercooked meat (Damene et al., 2023). BTB is defined by the advanced growth of nodular granulomas, also referred to as tubercles. These tubercles are frequently enclosed by connective tissue and typically contain yellowish core caseous necrosis, caseous-calcified, or calcified (de Kantor and Ritacco, 2006). Lesions may persist restricted or spread to adjacent tissues and organs via hematogenous or lymphatic dispersion of mycobacteria (Domingo et al., 2014).
Post-mortem hygienic investigation plays a crucial role in tuberculosis observation in endemic areas, as it can significantly lower prevalence when combined with eradication efforts (Silva et al., 2018). According to Pinto (2003), TB holds the highest economic and public health impact among foodborne zoonoses identified during meat inspection. The Mycobacterium spp. isolation, which is regarded as the gold-standard diagnostic test for tuberculosis, is a valuable instrument for an accurate assessment of mycobacteria, besides the post-mortem hygienic assessment. It is necessary to make the required efforts to prevent zoonotic diseases by regulating and handling infections caused by M. bovis in cattle (Devi et al., 2021). Early detection of BTB is the first step towards prevention (Filia et al., 2016). Good et al. (2018) have suggested that a tuberculin assessment on cattle can serve as a primary stage in the detection of BTB. It is recommended that the origin of cattle be investigated after the detection of BTB lesions in slaughterhouses or farms, in order to identify further cases (Here et al., 2022).
The polymerase chain reaction (PCR) is a quick and sensitive analytical technique that can identify the cause in clinical specimens; yet, the PCR’s effectiveness may be hampered by the presence of inhibitors in the samples (Elagdar et al., 2022). While the tuberculin test is beneficial for the early recognition of BTB, it is merely a measure of the existence of M. tuberculosis complex infection. Consequently, it is imperative to conduct an enzyme-linked immunosorbent assay (ELISA) or PCR examination to ascertain whether the infection is produced by M. bovis or another Mycobacterium species (Good et al., 2018). This investigation was intended to evaluate the risk factors that are linked to the occurrence of BTB in cattle in Upper Egypt through post-mortem, microbiological assays, and PCR techniques. This information will serve as a foundation for the early detection of BTB and the prediction of its effects on public and animal health.
Materials and Methods
The research was achieved in 2024 across several governorates in Upper Egypt (New Valley, Qena, and Aswan). The study population consisted of 600 cattle from Upper Egypt governorates (100 males and 100 females each from New Valley, Qena, and Aswan) districts; all were exposed to a single intradermal cervical tuberculin test (SICTT), and the positive animals were slaughtered in the central abattoirs of each governorate after approval from authorities. The preponderance of cattle was local breeds, Holstein Friesian, Simmental, and Brown. Animals less than 12 month and cows in late gestation (above 7 month) weren’t tested and not included in this study. A dentition pattern was used to estimate the animal’s age. Kellogg (2010) modified the five scales to classify the body condition score (BCS), which was then divided into three categories: poor, medium, and good.
The sample size was calculated using a 95% confidence interval (CI) and 5% absolute precision, according to Thrusfield et al. (2017). Thus, the expected occurrence of BTB lesions was estimated to be 3.7% (Hekal et al., 2022).
Where n=required sample size, Z=suitable rate for the standard average deviation for the desired confidence=1.96, Pexp= expected incidence, and d=desired absolute accuracy (usually 0.05).
Conversely, 600 samples were inspected for the occurrence of BTB lesions. Therefore increasing the sample size enhanced the probability of identifying positive cases.
Following the World Organization for Animal Health (WOAH, 2009), a SICTT was conducted. The injection site was located following a precise narrow haircut in the middle part of the neck, and the thickness of the skin was measured using a certified caliper. An intradermal injection of 0.1 mL of purified protein derivative (i.e. tuberculin) obtained from bovine tubercles (PPD-B) was administrated at the designated location. Skin thickness differences (mm) were measured 72±4 h post-injection to assess the swelling reaction. The Egyptian General Organization of Veterinary Services provided guidelines for interpreting the results, which stated that swelling <3 mm was negative while swelling of ≥4 mm was positive. Reactions of 3–4 mm were regarded as inconclusive.
The cattle involved in this research were assessed for both antemortem and post-mortem assessments for animals positive for the SICTT. Throughout the antemortem checkup, each animal’s information was collected (age, sex, and breed). The organs (lung, pleura, intestine, heart, kidneys, spleen, and liver) and lymph nodes (mediastinal, bronchial, hepatic, retropharyngeal, mesenteric, precrural, and prescapular) of each carcass were evaluated during post-mortem inspection, which was carried out by visual inspection, palpation, and incision for the recognition of suspected BTB lesions. Tissue samples were taken, placed into sterilized bags, and kept at –20°C until bacterial examination. Simultaneously, data concerning the morphological manifestations of lesions, including the kind of lesions (suppurative or caseous with or without mineralization), the anatomical site of infection, and the type of BTB (localized or generalized), were documented in the clinical file (Corner, 1994).
Serum specimens were received from SICTT-positive animals. The serum was separated by centrifugation at 1,509×g for 10 min, transferred into dry, sterile, and labeled test tubes, and kept at –20°C (Al-Kasar et al., 2018). Following the slaughter of the animals, a post-mortem examination was conducted, and tissue samples (liver, spleen, and lung lymph nodes) and lymph nodes exhibiting tuberculous-like lesions were obtained. All samples were sent to the Meat Hygiene Laboratory at the Faculty of Veterinary Medicine at Aswan University for further analysis.
Sera from tuberculin-positive cattle were tested for the presence of anti-mycobacterium antibodies, using the antigen capture enzyme-linked immunosorbent assay (ELISA) of Aagaard et al. (2006). The Veterinary Serum Vaccine Research Institute (Abbassia, Cairo, Egypt) provided the coated antigens for the reagent bovine tuberculin PPD-B. The Tuberculosis Unit (Animals Health Research Institute, Dokki, Egypt) offered commercial polypeptide antigens (Prionics, Schlieren, Switzerland). Two different ELISAs were performed on each antigen, following diluting the tested antigen (1:1,000) in carbonate bicarbonate buffer (pH 9.6), 100 μL was added to each well in a 96-well plate, and the plate was incubated at 37°C for 12 h. After the plates were emptied, they were rinsed three times with ELISA wash (KPL) 20× concentrate and blocked with 100 μL/well BSA (KPL; 1:10), left to sit at 37°C for 1 h, and then cleaned three times with ELISA wash solution. The sample sera were diluted 1:20 in ELISA diluent (BSA 1:15), added to the coated plates (100 μL/well), and subsequently incubated at 37°C for 1 h. The plates were emptied and cleaned three times with ELISA wash. Each well received 100 μL of goat anti-bovine IgG-horseradish peroxidase conjugate (KPL, 1:1,000; Thermo Fischer Scientific, Waltham, MA, USA) and was incubated at 37°C for 1 h. The plates were cleaned three times with an ELISA wash. ABTS substrate (100 μL/well) was applied and incubated for 15 min. The Spectra III ELISA reader (Thermo Fischer Scientific) was employed to read the outcomes as optical density (OD) at 405 nm. The sample produced a mean OD of each group that was equal to or more than the cut-off value; the sample was considered to be positive. The cut-off value was determined using the method provided by Nassau et al. (1976), which was equal to the mean OD of negative serum plus two SD.
The procedure outlined by Roberts et al. (1991) was followed while processing the samples; however, PBS was added in place of HCl during the neutralization stage. Following tissue cutting, manual crushing, and homogenization with a pestle and mortar, the tissues were decontaminated for 15 min while being shaken regularly in an equal volume of 4% NaOH. Next, 50 mL of PBS buffer was mixed with the sample mixture, and the mixture was centrifuged at 1,750×g. The sediments were neutralized by adding PBS (50 mL) and centrifuging at 1,750×g for 15 min to concentrate them. Pellets were suspended in PBS (1 mL) and Löwenstein-Jensen (LJ) medium slants (3–5 drops), two of which were complemented with pyruvate and the other with glycerol were mixed into each pellet in triplicate. The slants were incubated at 37°C, with weekly inspections conducted to detect mycobacterial growth for 8 weeks. Cultures were considered negative if no observable growth appeared after 8 weeks of incubation. To detect acid-fast bacilli, cultures were analyzed under a microscope using the Ziehl-Neelsen staining procedure (WHO, 1998). Heat-killed cells of each isolate were developed by mixing colonies in 500 μL distilled H2O and incubating at 80°C for 1 h. Stocks of acid-fast positive cultures were kept at –80°C in Dubos Tween-albumin broth for later use. The acquired isolates were recognized using traditional methods (growth rate, colony morphology, pigmentation, and chemical characteristics) as described by Roberts et al. (1991).
Extraction of presumptive M. bovis DNA was performed using Quick-gDNA™ MiniPrep kit (Cat. No. D3024, Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions, PCR amplification was achieved in a total volume of 50 μL; 25 μL COSMO PCR REDMaster Mix (W1020300X, Willowfort, Nottingham, UK), 22 μL of Nuclease free water, 1 μL of each primer (20 μM), and 1 μL of DNA template (25 to 100 ng/ μL). A couple of SCAR (Sequenced Characterized Amplified Region Markers) were utilized to identify the occurrence of M. bovis in the selected tissues. Primer designations were JB21 (5’ TCGTCCGCTGATGCAAGTGC 3’) and JB22 (5’ CGTCCGCTGACCTCAAGAAG 3’), to amplify a specific 500 bp region (Rodríguez et al., 1999; Silva et al., 2018). The PCR condition involved amplification in a Thermal Cycler (T100, Bio-Rad Laboratories, Hercules, CA, USA), using one cycle (94°C for 5 min), 40 cycles (94°C for 1 min, 68°C for 1 min, and 72°C for 1 min), and, finally, one cycle (10 min at 72°C). The PCR yield was exposed to 1.5% agarose gel electrophoresis, marked with ethidium bromide (0.5 μg/mL), with a 100 pb Plus DNA Ladder® as standard molecular level. The gel was read under UV light (GEL DOC XR). A positive control from the Tuberculosis Unit (Animals Health Research Institute, Dokki, Egypt) was employed.
To confirm the occurrence of M. bovis in the tuberculosis-like lesions, the same PCR protocol was employed for the bacterial colonies, but the reaction was achieved in a thermal cycler set for one cycle (95°C for 3 min), 45 cycles (94°C for 60 s, 60°C for 40 s, and 72°C for 1 min), and a final cycle of 10 min at 72°C (Silva et al., 2018).
Results
The occurrence of BTB in three Upper Egyptian Governorates (New Valley, Qena, and Aswan) is shown in Table 1 and Fig. 1. The finding reveals that 14 out of 600 (2.3%) showed a positive reaction on SICTT tests. Qena had the greatest rate of reactors (tuberculin-positive animals) and the largest proportion of isolation of actually diseased animals (3.5%), followed by Aswan (2.5%) and New Valley (1.5%).
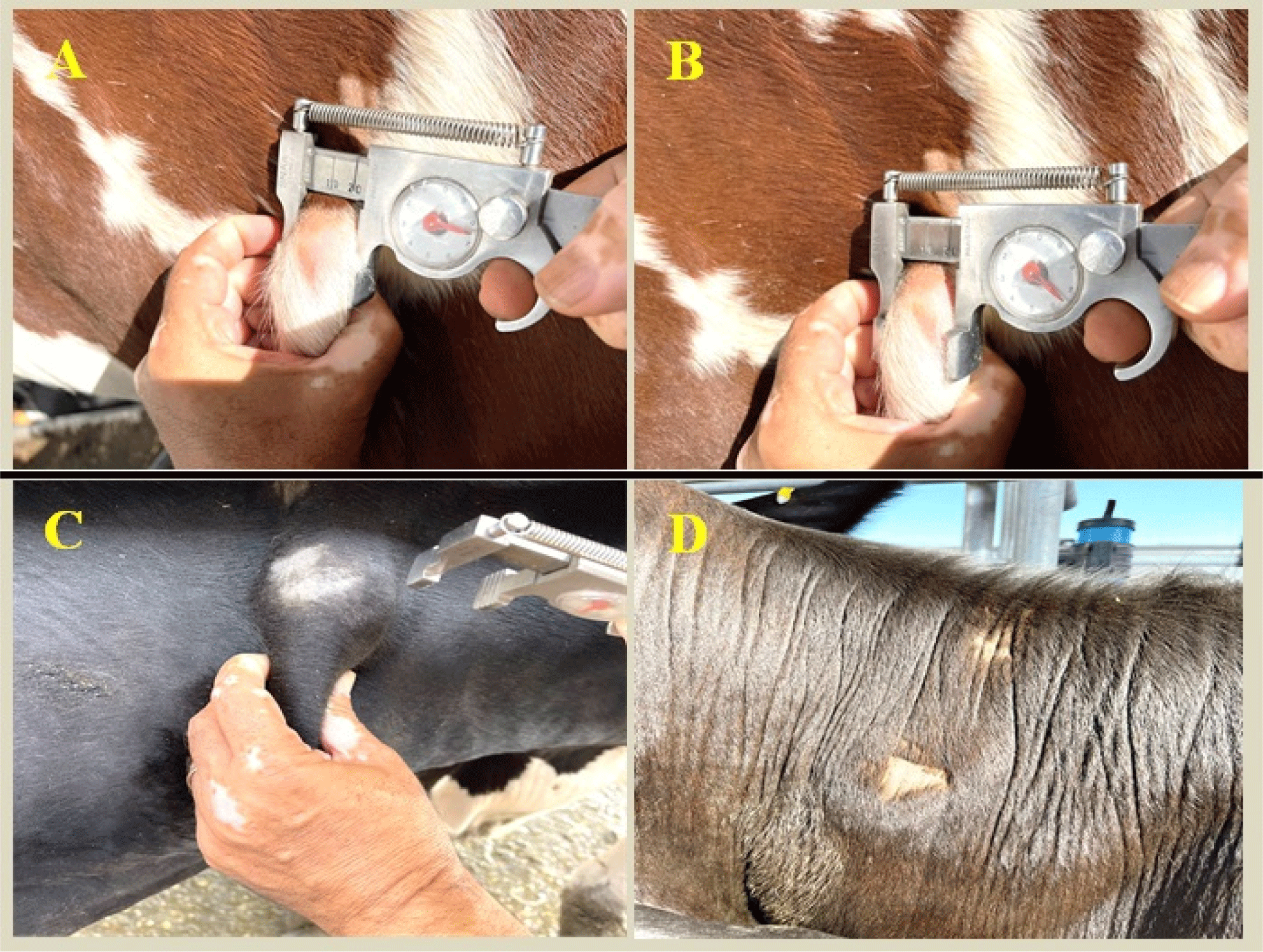
Table 2 lists the risk factors that were identified as being related to the occurrence of BTB, including location, mainly in Qena (CI. 95%: 1.26–6.74, p=0.0014), followed by Aswan (CI. 95%: 1.22–3.74, p=0.0163).
A substantial relationship was detected in the incidence of suspicious BTB lesions regarding sex, age, breed, BCS, reproductive status for the pregnant group, and yard density (>20). Female cattle had significantly (3%) higher findings than males (1.7%). The greatest infection proportions were noticed in cattle aged >5 years (3.7%). Compared to local breeds (1.8%), Holstein Friesian (3.7%) and Simmental (2.3%) breeds were more severely impacted. As well as animals with poor BCS condition (3.42%), the yard with a density >40 (3.7%) has the highest infection rate.
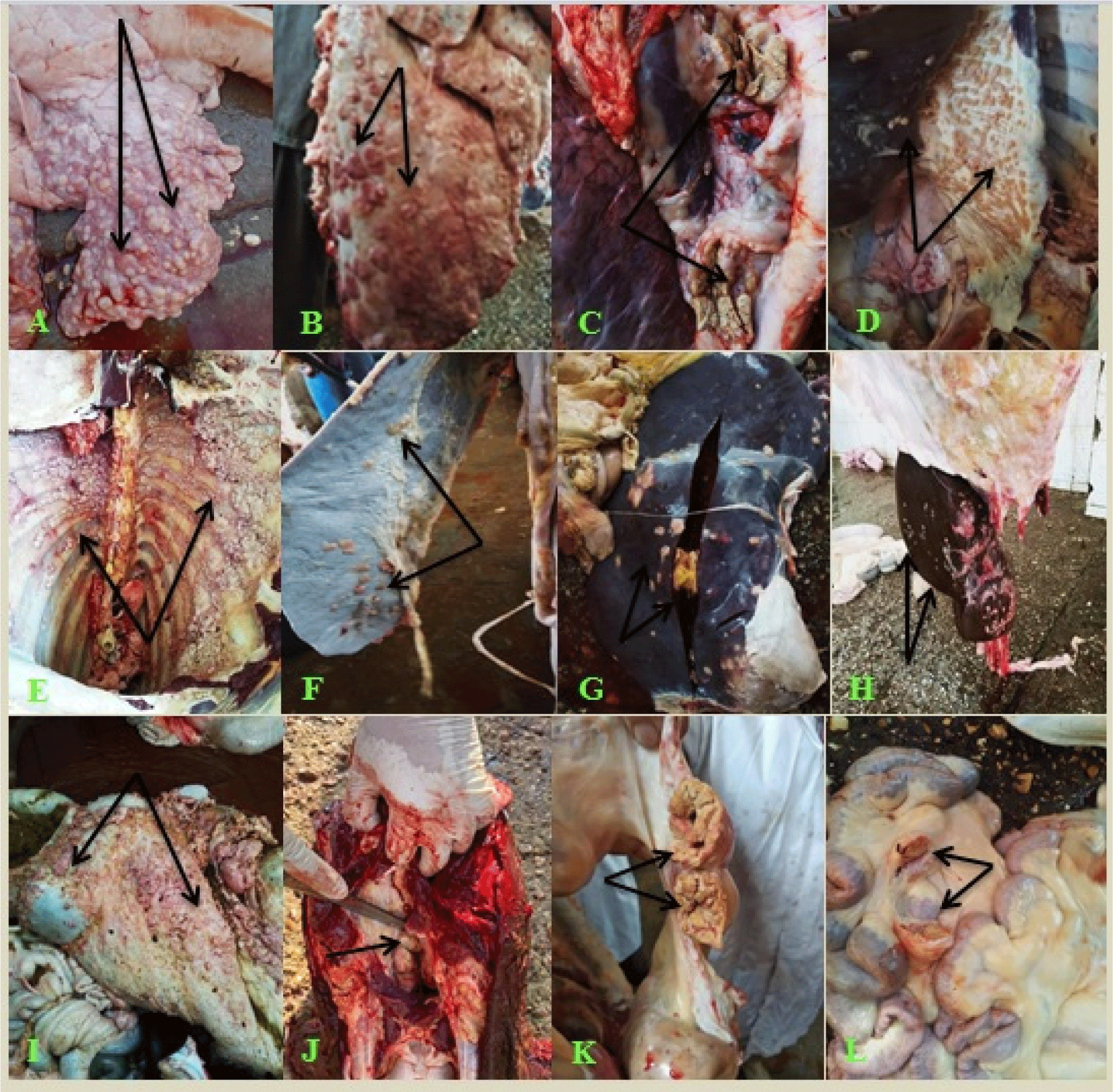
Based on the macroscopic characteristics, Tuberculin-positive slaughtered animals showed noticeable widespread lesions (28.6%) and distributed tubercles all over the carcass organs and lymph nodes, while 71.4% of the suspected lesions were confined to certain organs or lymph nodes. BTB-like lesions were mostly identified on the mediastinal, bronchial, and mesenteric lymph nodes; numerous lesions of the lung, intestine, diaphragm, and peritoneum showed caseous, calcified, and granulated necrotic areas with or without mineralization (Fig. 2 and Table 3).
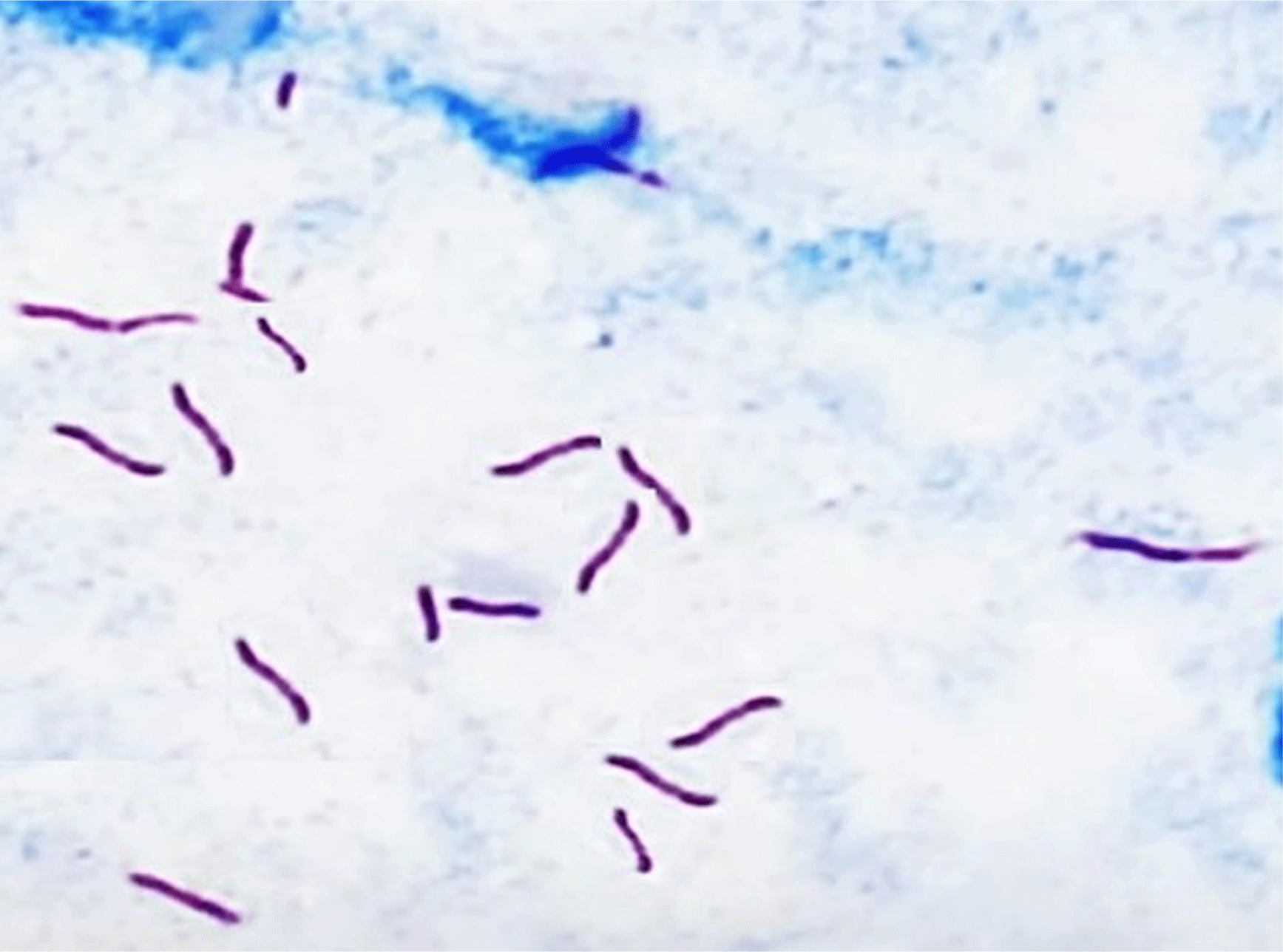
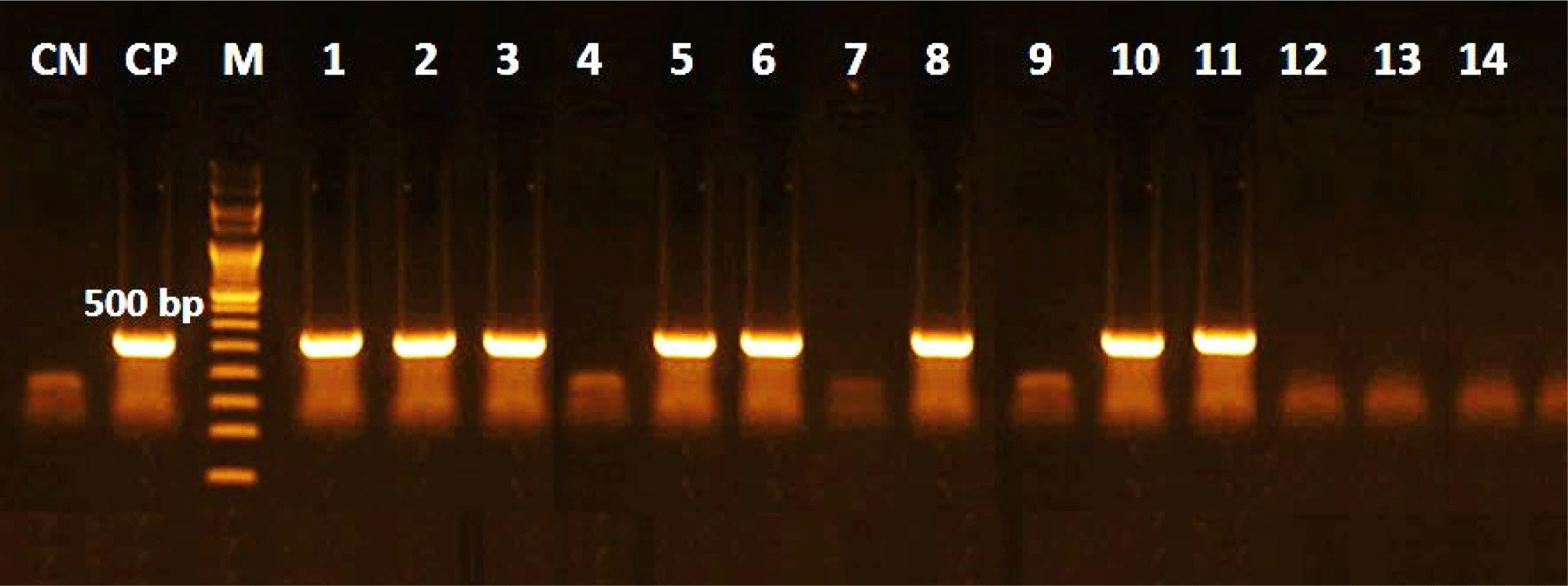
The traditional culture procedure comprises plating-ready samples collected from tuberculin-positive animals on L-J media, all tuberculin-positive reactors, and mycobacterium isolates (100%). The microscopic inspection was directed at all isolated strains by ZN stain, and only 85.7% were identified as acid-fast bacilli (Table 4 and Figs. 3 and 4). In the current research, ELISA using PPD antigen exhibited that 11 serum samples were positive (78.6%), whereas commercial polypeptide antigen indicated that 9 serum specimens were positive (71.4%), and the highest outcome was established in the sera of animals that had generalized lesions (Table 4). Furthermore, there was no significant variation between the methods of M. bovis isolation by PCR using tissue samples or isolates. The quality and validity of M. bovis DNA were applied to each of the 14 tissue samples, and M. bovis isolates, and the results revealed the occurrence of M. bovis DNA in 8 of each examined category (Fig. 5).

Discussion
One of the most significant illnesses facing Egypt’s farming community, cattle owners, government, abattoir employees, and veterinary specialists is BTB caused by M. bovis (Hamed et al., 2021). Consistent with Awah-Ndukum et al. (2016), the intradermal tuberculin test is a low-cost technique for evaluating latent and active tuberculin infections in cattle. However, the approach is restricted in sensitivity and specificity and is influenced by a variety of immunological response variables (Ortega et al., 2021). Tuberculin potency is subject to variation among samples and has a substantial impact on the quantity of revealed reactors (Duignan et al., 2019). Based on the SICTT assessment, the individual animal-level frequency of BTB was 2.3%. These outcomes agreed with those described by other investigators in Egypt; Algammal et al. (2019), Hamed et al. (2021), Hekal et al. (2022), Moussa et al. (2011), and Nasr et al. (2016) reported 1.8%, 2.4%, 1.67%, 3.7%, and 1.6%, respectively. In contrast, some researchers have verified either low occurrence rates of 0.13% (Liu et al., 2019), and 0.30% (Rocha et al., 2016) or high values of 11.3% (Habitu et al., 2019), and 4.3% (Ghebremariam et al., 2016). The diversity in BTB occurrence could be credited to differences in geography, cattle handling procedures, species of cattle used in the research, BTB history in that region, and the buying of a diseased animal, all of which can impact disease epidemiology. The movement of infected cattle from areas where the disease is endemic to regions relatively free of BTB has been cited as possible reasons (Mishra et al., 2005). The implications of this in terms of habitual or organized or disorganized cattle migrations in developing countries are significant to the spread of M. bovis.
Risk variables for the existence of BTB have been observed to include location, with the greatest incidence in Qena (CI. 95%: 1.26–6.74, p=0.0014), and Aswan (CI. 95%: 1.22–3.74, p=0.0163). A substantial association was found between the occurrence of animals and the various agricultural locations. The high incidence of BTB in Qena Governorate may be due to the high animal trade exchange, climatic conditions in the farms, stress on cattle when held under inadequate control, and an overfull environment (Kemal et al., 2019). The occurrence of BTB in the current research was higher in cows than bulls. It was noticed that adult cattle (>5 years old) had the highest incidence of BTB compared to animals <5 years old. Suggesting the fact that farmers keep the cows for breeding purposes for longer than they do the males, and since tuberculosis is a chronic illness, adult cattle have been shown to have a higher incidence of the disease than younger animals. The results corroborated earlier findings that TB was more prevalent in aged and female cattle (Jajere et al., 2018; Lawan et al., 2020; Mekonnen et al., 2019). Additionally, pregnant cows were found to be more susceptible to infection, which may be attributed to the fact that females are required for breeding and milk production. Additionally, the stress of lactation and gestation can render females more susceptible to disease (Habarugira et al., 2014).
Holstein Friesian cattle displayed a higher frequency than other breeds. These findings matched some earlier investigations (Hamed et al., 2021; Tuncay and Hatipoğlu, 2018). According to previous studies (Hamed et al., 2021; Reilly and Courtenay, 2007), there was an increase in tuberculin-positive cattle housed in high-density yards (>40 animals) than in low-density yards. This observation may be because of close proximity of animal and inadequate ventilation, which can lead to cattle-to-cattle spreading. The conclusions of this investigation demonstrated that cattle with poor physical conditions had an elevated incidence of BTB. Additionally, it indicates that BTB is a long-lasting disease that causes gradual emaciation in infected cattle (Fentahun and Luke, 2012; Lawan et al., 2020). Conversely, the discovery that reproductive status and body condition were not risk factors for BTB was recorded in prior research (Demelash et al., 2009; Hamed et al., 2021).
The meat screening system involves an inspection of the divided carcasses, organs, and lymph nodes. The purpose of inspecting meat from slaughterhouses is to guarantee that the animals are healthy and suitable for human consumption. Additionally, significant epidemiological data on animal and zoonotic illnesses, like BTB, in various regions of the world have been made available by abattoir meat inspection (Adesokan et al., 2019; Lawan et al., 2020). Mycobacterium spp. isolation, which is recognized as the gold standard assessment for analysis, is a valuable instrument for the diagnosis of tuberculosis, as it enables the precise identification of the mycobacteria besides the post-mortem hygienic assessment (Silva et al., 2018). According to the present findings, the lung and its lymph nodes, especially the bronchial and mediastinal lymph nodes, have a high rate of BTB. The outcomes aligned with earlier research conducted by Damene et al. (2023), Elagdar et al. (2022), Hamed et al. (2021), and Lawan et al. (2020). Suggesting that the site of BTB lesions is determined by the spread route and that the infection is primarily diffused through a respiratory pathway (Damene et al., 2023).
In this investigation, post-mortem analyses allow us to evaluate the progression of BTB lesions as related to morphological features. The highest occurrence of presumptive BTB lesions was described by caseous necrosis, with or without mineralization, and calcified and granulated tubercle lesions. Many researchers have described the majority of caseous necrosis and calcification lesions that come across as natural illnesses (Damene et al., 2023; Elagdar et al., 2022; Elnaker et al., 2018; Hamed et al., 2021; Mecherouk et al., 2023), suggesting the chronic nature of the infection (clarifying the diffusion of TB from the lungs to other organs) has been credited to the long incubation time of the disease, and development of parental immunity (Mecherouk et al., 2023).
While every lesion exhibited typical features of tuberculosis, not all lesions could be cultured due to various restrictions, such as low levels of acid-fast bacilli in the specimens or challenges posed by high levels of natural contamination. Z-N stain was employed to conduct microscopic inspections of all isolates, and of the 14 cultures tested, 12 were positive. Results are similar to other reports by Hekal et al. (2022), Kanyala et al. (2022), Lawan et al. (2020), Mecherouk et al. (2023), and Proaño-Pérez et al. (2011). The traditional M. bovis culture technique is still thought to be the best way to diagnose BTB, but it is less accurate than other approaches and takes a long time, up to 12 weeks to grow (Hekal et al., 2022). Additionally, a consistently dispersed bacterial load greater than 104 bacilli/mL is necessary for the microscopic inspection (Campelo et al., 2021). Other than the high rate of false-negative results, the procedure necessitates stringent safety measures to avoid contamination by related species (Lekko et al., 2020).
The evolution of an efficient serological approach for identifying BTB is one of the most pressing issues in the veterinary medical field (Hekal et al., 2022). The humoral immune response, which is defined by the generation of antibodies, is the focus of antibody-based diagnostic techniques like ELISA (Thomas and Chambers, 2021). Antibodies are typically undetectable in the early stages of BTB and are only generated during the disease’s severe phases (Ortega et al., 2021). Low detection sensitivity is another benefit of single antigen testing (Sun et al., 2021). On the other hand, ELISA can be used to verify the findings of skin tests and track the advancement of infection (Hekal et al., 2022). It has been used as a sensitive approach for detecting antibodies in the serum of positive animals. However, as compared to conservative culture approaches and based on the antigens utilized, the ELISA approach produces varying sensitivity and specificity.
In this investigation, ELISA utilizing PPD antigen revealed that 11 serums were positive (78.6%), while ELISA utilizing PAg revealed that 9 serums were positive (71.4%). These findings were consistent with a previous study conducted in Egypt utilizing B-PPD and PAg, which revealed 87.03% and 89.81% positivity among tuberculin-positive reactors, respectively (Hekal et al., 2022). Out of 77 specimens analyzed using ELISA, 40.29% of the serum samples exhibited positive results in another study conducted in Egypt (Hamed et al., 2021). These outcomes propose that for the diagnosis of BTB, ELISA employing commercial polypeptide antigen is more sensitive than ELISA utilizing conventional PPD antigen. A combination of carefully chosen antigens could show promise as a unique diagnostic tool.
The results of the ELISA test in the current research were lower than those of the tuberculin test and bacteriological assessment, which may be due to the humoral immune response being more prevalent in the later stages of infection, but the cell-mediated immune reaction, as in the result of the tuberculin skin test, can show up as soon as three weeks post-infection (Hamed et al., 2021). Accordingly, the main techniques for diagnosing BTB in live animals are the evaluation of cell-mediated immune reactions (de la Rua-Domenech et al., 2006) and antibody responses, which are only observed in animals that are severely infected or in anergic states (da Silva et al., 2011). Therefore, ELISA serves as a supplement to the tuberculin test rather than as a stand-alone BTB diagnostic.
The most dependable method for the quick and specific discovery of M. bovis is PCR, as it minimizes the lack of specificity in other conventional laboratory procedures and enables the recognition of M. bovis from culture isolates or genomic DNA taken out from clinical specimens (Algammal et al., 2019). The most persuasive substitute method for the quick and correct finding of tuberculosis is the PCR assay (Desire et al., 2024). The method is capable of discovering the tiniest amount of genome in a sample, which confirms being subjected to the bacteria. It does not necessitate the isolation of the microorganism and can recognize DNA from both living and non-living organisms (Sonekar et al., 2021). Additionally, the DNA of the M. bovis bacterium can be identified in a PCR investigation, even though the bacteria died (Desire et al., 2024).
The current study found no significant relationship between molecular recognition using tissue samples and isolates, as the stability and purity of bacterial DNA were checked in each of the 14 tissue specimens and M. bovis isolates, with the results revealing an appearance of M. bovis DNA in 8 of each investigated category. PCR can detect live or dead mycobacteria at all stages of infection. Therefore, the PCR is not influenced by the presence or absence of lesions on animal carcasses (Singhla and Boonyayatra, 2022). Standardization would be beneficial for PCR examinations, which typically show low bacterial quantities. According to Silva et al. (2018), the procedure facilitates a quicker and more accurate assessment, which aids in public health and animal health surveillance initiatives. Furlanetto et al. (2012) found that 7% (6/198) of tissue specimens had detectable bacterial DNA. Cardoso et al. (2009), alternatively, presented records that differed from these; in 54.5% (18/33) of the samples, M. bovis DNA was found. Conversely, Silva et al. (2018) reported that M. bovis DNA was found in 100% (28/28) of the 28 strains, while only 20% (10/50) of the 50 tissue specimens that had been submitted to PCR were positive.
The varying results of PCR technique performance are mainly because of technical differences in the setting up of assays, particularly during DNA extraction from lesions, and their sensitivity is conditional on sensitivity of necropsy and volume of DNA. Further, contamination of the PCR reaction and the presence of environmental bacteria can prompt false positives and cause insufficient specificity. Differences of the PCR primers used and the presence of inhibitory substances in samples or reagents can also cause reduced sensitivity (Borham et al., 2022).The molecular assay employed in this investigation has been demonstrated to be more accurate for BTB, unlike culture, the PCR technique does not distinguish between live and dead mycobacteria, and as a result, it can be used to complement current strategies for managing and avoiding the disease to protect the health of both humans and animals. This will facilitate the acquisition of dependable data and enhanced epidemiological surveillance of the disease. It is also crucial to conduct additional research on the species that are implicated in the BTB-suggestive lesions in Egypt.
Conclusion
The current investigation has underscored the overall incidence of BTB in cattle in the provinces of Upper Egypt, with a particularly high percentage in the Qena province. This work tackles the One Health concept and emphasizes the utility of molecular technologies for BTB screening. Using PCR to recognize DNA from lesions resembling tuberculosis is a quicker and more effective way of discovering BTB. The results of the molecular screening show that it is more sensitive than both SICTT and ELISA, enabling a more precise evaluation that may aid in the epidemiological investigation of tuberculosis in cattle as well as the discovery of outbreaks in Egypt. We find that no single technique may recognize all BTB-positive cases; thus, at least two tests are needed to achieve maximum specificity and sensitivity. These results underscore the significance of active surveillance, enhancing current control techniques, or establishing new regulations to cut off the transmission of BTB between animals and humans.













