Introduction
Beef has a rich composition of key nutrients, namely minerals (such as sodium, phosphorous, iron, & zinc), fatty acids, vitamin B groups, and high protein contents (Pereira and Vicente, 2013). However, beef has a limited shelf life from being prone to chemical and microbial changes attributed to its rich nutrients and appropriate environment for the growth of microorganisms including spoilage bacteria and food-borne pathogens (Conte-Junior et al., 2020). Food packaging protects the products against the external environment, serves a role as a marketing tool, and provides convenience to customer (Cheng et al., 2022).
For the maintenance of beef qualities, various packaging methods have been applied such as modified atmosphere packaging (MAP), vacuum packaging, and vacuum skin packaging. Among them, vacuum packaging depended on negative pressure to degas the ambient air while the vacuum state is maintained with the use of sealed pouches or roll-stock packaging (McMillin, 2017). Vacuum packaging has inhibitory effects on lipid oxidation, protein deterioration, and growth of aerobic microorganisms under low oxygen conditions. The maintenance of food shelf life in the vacuum-packed state differs depending on the properties of the vacuum film, such as the composition of layers, the structure of layer materials, the thickness of the film, and gas (O2 & H2O) permeability (Lee, 2010).
Among the various synthetic plastic materials for food vacuum packaging, polyvinylidene chloride (PVDC) and ethylene vinyl alcohol (EVOH) are broadly applied. PVDC is well-suited to apply in food packaging because gas barrier characteristics are not modified by water, oil, and grease, and have both a low oxygen and water permeability (Bauer et al., 2021; Mokwena and Tang, 2012). However, PVDC is faced with several concerns that involve processing problems and environmental issues. PVDC, an addition polymer of vinylidene chloride and vinyl chloride monomers, cannot be simply recycled into polymer streams because they are thoroughly cross-linked and contain chlorine which can lead to generating hazardous gas such as hydrogen chloride and dioxin during burning (Hui, 2006; Rodrigues et al., 2017).
EVOH, an addition polymer of ethylene and vinyl alcohol, is a typical non-PVDC film for foods. Much like PVDC, EVOH has low gas permeability, making it suitable for preserving the interior of the packaging against the external environment (López-de-Dicastillo et al., 2010). However, according to Mokwena and Tang (2012), EVOH is hydrophilic and increases O2 permeability by absorbing substantial amounts of moisture when directly exposed to high relative humidity. Therefore, the performance of EVOH as a barrier vacuum film for food packaging depends on the processing environments and storage conditions to which the packaging is exposed. These synthetic plastic resins for packaging are applied to food by compositing several layers to compensate for their limitations (Alias et al., 2022; Anukiruthika et al., 2020; Bauer et al., 2021).
The objective of this study was to investigate and compare the physicochemical and microbiological characteristics of Hanwoo (Korean native cattle) using different vacuum packaging materials, namely PVDC and EVOH, under refrigerated conditions.
Materials and Methods
Beef (round) were obtained from single Hanwoo carcasses at Minsok LPC Co., Ltd. (Gunwi, Korea). Following the removal of fat, connective tissue, and blood, beef samples were cut to around 2 kg and vacuum packaged with PVDC and EVOH films (ElSAKR, Seongnam, Korea). The physical specifications of experimented films were analyzed at the Korea Polymer Testing and Research Institute (KOPTRI, Seoul, Korea). Table 1 presents the specification of vacuum packaging films used in this study. The beef samples were sealed with PVDC and EVOH vacuum films, and the samples were placed in a chamber maintained at 74°C and heated for 4 s to deaerate. The packaged samples were immediately immersed in cold water for 4 s to cool off. The samples were transported to the Korea Food Research Institute (Wanju, Korea) under refrigerated conditions, and stored at 2±1°C and 85% of relative humidity.
| Parameter | Vacuum packaging films | |
|---|---|---|
| PVDC | EVOH | |
| Thickness (μm) | 56 | 133 |
| O2 transmission rate (cc/m2 · 24 h at 23°C) | 13.9 | 20.7 |
| H2O transmission rate (g/m2 · 24 h) | 9.66 | 22.4 |
The pH values of packaged beef samples were measured using a pH meter (Mettler-Toledo, Schwerzenbach, Switzerland). The pH was measured after homogenizing 5 g of each beef sample with 45 mL of distilled water, with all samples were being examined in triplicate.
The color of each packaged beef sample was determined at three defined areas on the surface of the sample using a color meter (Minolta Chroma Meter CR-300, Konica Minolta, Osaka, Japan; illuminate C, calibrated with a white plate, CIE L*=+93.5, CIE a*=0.3114, CIE b*=0.319). The color of beef was assessed after blooming for 30 min. The result of color measurements is expressed as CIE L*, CIE a*, and CIE b*.
Lipid oxidation was evaluated by the previous method with modifications (Witte et al., 1970). The results are presented in milligrams of malondialdehyde (MA) per kilogram of beef sample. In brief, each 5 g-beef sample was blended with 50 mL of distilled water for 2 min using vortex mixer. A 5 mL-sample were mixed with 0.5 mL of BHT [7.2% (w/v) of butylated hydroxytoluence] and 2 mL of TBA/TCA solution [20 mM of thiobarbituric acid and 20% (w/v) of trichlroacetic acid], and heated in a water bath at 90°C for 15 min to develop color. Then it was cooled for 10 min with cold water and centrifuged at 1,800×g for 15 min. A 3 mL-supernatant was taken and absorbance was measured at 531 nm. TBARS values were calculated as follows.
VBN (mg%) was performed to estimate the extent of protein deterioration of vacuum-packaged beef during storage. VBN was measured according to modified methods of Pearson (1968). Briefly, a 10 g-beef sample was mixed with 90 mL of distilled water and filtered with Whatman No.1 paper (Whatman International, Maidstone, UK). On the inner section, 50 μL of indicator (methyl red & bromocresol green) and 1 mL of 0.01 N H3BO3 were set. On the outer section, 1 mL of filtered beef sample solution, and 50% K2CO3 solution were added. After incubation for 2 h at 37°C, the inner section solution was titrated with 0.02 N H2SO4.
where a indicates the volume (mL) of H2SO4 added to the sample for titration, b indicates the volume (mL) of H2SO4 added to the blank for titration, f represents factor of reagent (0.02 N H2SO4), N represents normality of titrated solution, and S represents the weight (g) of the beef sample.
A microbiological analysis of packaged beef was performed by determining the aerobic plate count (APC). A 10 gram-packaged beef sample was mixed with 90 mL of sterile saline solution (0.9% NaCl). Samples were homogenized using a stomacher for 5 min. The homogenates were diluted ten-fold with saline solution, and spread on plate count agar (PCA; Difco Laboratories, Detroit, MI, USA), while the plates were incubated at 37°C for 24 to 48 h. All experiments were conducted with triplicate and expressed as Log CFU/g.
In order to measure the change in the microbial diversity according to the vacuum packaging films for beef, a sampling was performed immediately after packaging as well as 4 and 8 weeks of refrigerated storage.
Metagenomic DNA was extracted from the PVDC and EVOH-packaged beef samples using the PowerMax Soil DNA Isolation Kit (MO BIO, Carlsbad, CA, USA) according to the manufacturer’s protocol. Bacterial metagenomic libraries were assembled using the Illumina 16S rRNA Metagenomic Sequencing Library protocols (Illumina, San Diego, CA, USA). A polymerase chain reaction (PCR) amplification of bacterial markers was performed using primer pairs containing the V3-V4 regions of 16S rRNA, while bacterial 16S rRNA genes were amplified by 16S V3-V4 primers using PCR (16S amplicon forward primer: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGGACAGCCTACGGGNGGCWGCAG-3′; 16S amplicon reverse primer: 5′-GGCTCGGATGTGTATAGAACAGGACTACHVGGGTATCTAATCC-3′). The final purified products were normalized PicoGreen kit and pooled, and the size of the metagenomic libraries was confirmed using the LabChip GX HT DNA high sensitivity kit (PerkinElmer, Waltham, MA, USA). Paired-end sequence processing was conducted using the Illumina MiSeq platform (Illumina), with sequence data being trimmed and analyzed using the FASTX tool (v 0.0.14) and QIIME 1.8.0, respectively (Caporaso et al., 2010). Sequences were clustered into operational taxonomic units (OTU) with the default UCLUST closed-reference OTU picking algorithm (pick_closed_referne_otus.py) against the curated sequences (Hong et al., 2019). OTU was given as >0.5% of the total sequences.
All experimental data for packaged beef are expressed as means±SD, and analyzed using SPSS version 23 (IBM, Armonk, NY, USA). A one-way analysis of variance (ANOVA) and Duncan’s multiple range tests were performed to determine significant differences among groups. For the significance test between the two groups, an independent sample t-test was performed (p<0.05).
Results and Discussion
The pH values and blooming color of packaged beef during refrigerated storage are presented in Table 2. The pH values of all packaged beef decreased during storage (p<0.05). For PVDC, the pH values decreased from 5.46 to 5.00 during 12 weeks of storage, and decreased from 5.46 to 4.91 for EVOH. The decreases of vacuum-packaged beef in pH as storage time increased were consistent with the results of previous studies (Gedarawatte et al., 2020; Kim et al., 2019; Yu et al., 2020). During storage, EVOH-packaged beef tended to show a lower pH than PVDC-packaged beef. EVOH exhibited higher oxygen and water permeability than PVDC, likely due to the relative increase in metabolites of microorganisms such as lactic acid bacteria (Egan, 1983; Gill, 1996).
The PVDC-packaged beef samples were able to maintain CIE L* similar to the initial storage values. However, the EVOH-packaged beef samples tended to decrease in CIE L* during storage, with the CIE L* showing a significantly higher trend after 5 weeks of storage in the PVDC-packaged beef than EVOH-packaged beef (p<0.05; Table 2). For the CIE a*, the PVDC-packaged beef maintained stable values during storage, but EVOH-packaged beef did not show a constant trend, decreasing sharply upon 12 weeks of storage. The beef sample packed with PVDC maintained a significantly higher CIE a* level than that of the beef sample packed with EVOH after 3 weeks of storage (p<0.05). The CIE b* of packaged beef during refrigerated storage presented a similar tendency to the result of CIE a*.
The vacuum packaging materials used in this study were reported to maintain beef color. According to Yang et al. (2022), the CIE L* and CIE a* of beef strip loin steak are maintained continuously for 21 days using PVDC vacuum films. Rodrigues et al. (2017) reported that PVDC-packaged beef showed a higher color stability in sensory analysis than EVOH-packaged beef. These previous studies were consistent with our result that PVDC had better meat color retention than EVOH. In the case of beef, the CIE L* and CIE a* could influence consumer acceptance (Font-i-Furnols and Guerrero, 2014). With the color of beef well-known to be greatly affected by oxygen, vacuum packaging is a packaging method that improves the storability of food by degassing the inside using an oxygen and moisture-impermeable film. Therefore, the myoglobin of beef represents a dark red color in a vacuum state. The dark color of vacuum-packaged meat is not considered as a substantial disadvantage since oxygen becomes accessible on the meat surface when opening the packaging and the dark red color returns to its bright red color (Narasimha Rao and Sachindra, 2002). As mentioned above, the lower CIE L* and CIE a* of EVOH-packaged beef than PVDC-packaged beef seems to be due to the reduced barrier effect of EVOH at low temperature and high relative humidity.
Lipid rancidity is one of chemical changes that occur in meat, and has negative effects on the sensory properties such as an off-putting flavor, taste, and discoloration (Shin et al., 2022). The TBARS value is a measure of the formation of secondary oxidation products, including MA, alkadienals, and alkenals (Shahidi et al., 2003). Fig. 1A illustrates the changes in the TBARS value for PVDC- and EVOH-packaged beef during refrigerated storage. For all storage periods except 8, 9, and 12 weeks of storage, the TBARS values of EVOH-packaged beef were significantly lower than those of PVDC-packaged beef (p<0.05). However, the TBARS values in this experiment were relatively lower compared to other studies (Choi et al., 2011; Yu et al., 2018). If the TBARS value is higher than 0.5 mg MA/kg, a rancid odor could be detected (Choi et al., 2011). Therefore, both vacuum films are considered to be appropriate in preventing lipid oxidation in beef.
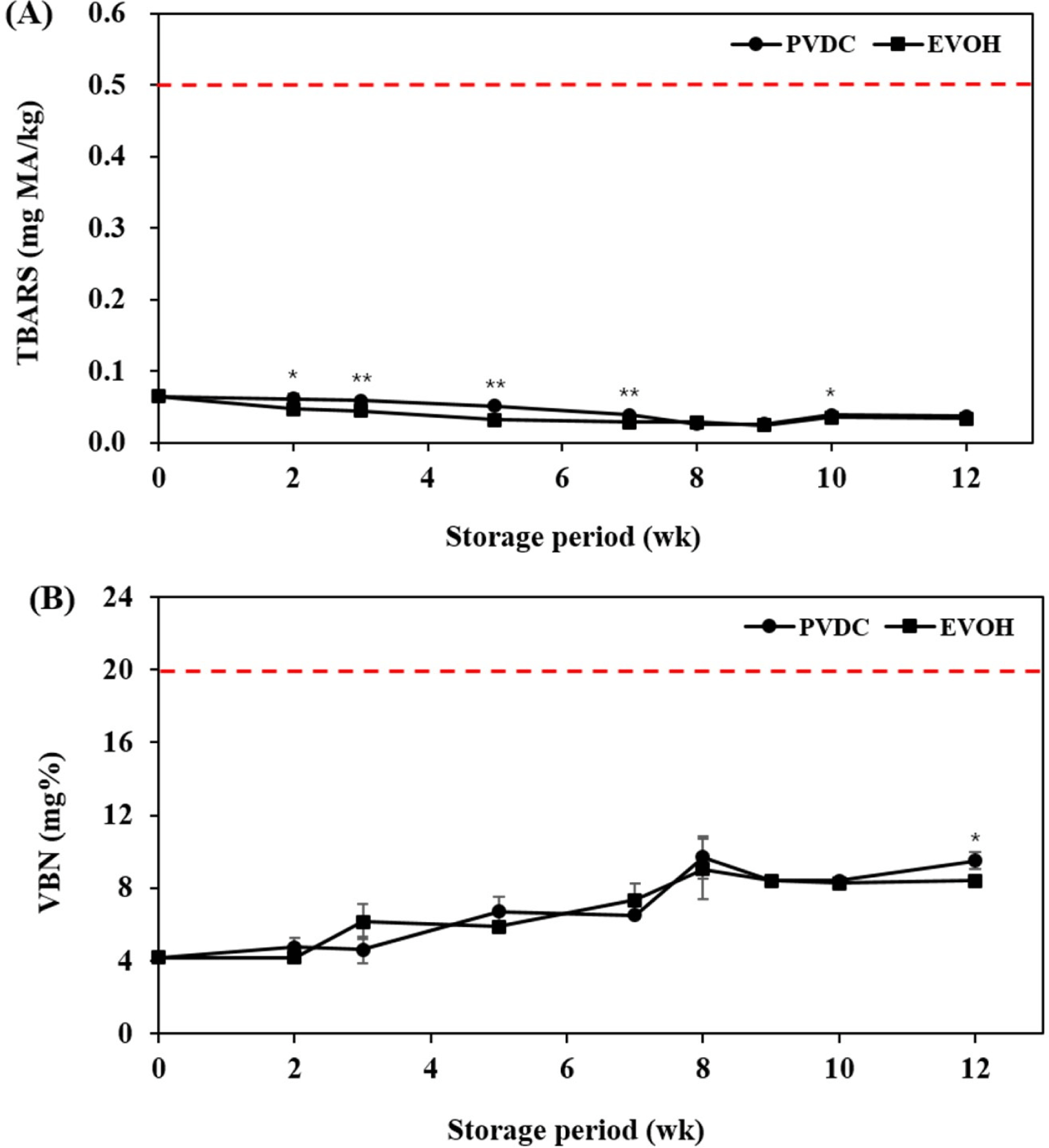
Degradation of protein and other nitrogen-containing components resulting from microbial activities brings the increase of organic amines that are generally recognized as VBN (Tahir et al., 2022). These compounds are harmful and induce substantial changes of sensory properties (color and flavor) that affect the overall acceptability of meat and meat products (Bekhit et al., 2021). Fig. 1B presents the changes in VBN values for PVDC- and EVOH-packaged beef during refrigerated storage, with increasing VBN values of experimented beef samples exhibited during storage. There was no significant difference in VBN values until 10 weeks of storage between the experimental groups. The PVDC-packaged beef showed a significantly higher value than EVOH-packed beef at 12 weeks (p<0.05). In Korea, The Ministry of Food and Drug Safety (2014) presented that beef is considered to be spoiled if the VBN value exceeds 20 mg%. Both experimental groups did not exceed the protein deterioration level standard, and it seems that two vacuum films could delay the protein deterioration of refrigerated beef.
The two types of vacuum films would be capable of minimizing the physicochemical changes (TBARS & VBN) of beef during refrigerated storage. These trends seem to minimize the physicochemical changes of beef by storing at a low temperature of 2°C or less and vacuum packaging. In conclusion, there was no substantial difference between the vacuum films of lipid rancidity and protein deterioration under the experimental conditions that we performed.
A microbiological analysis of vacuum-packaged beef is summarized in Table 3. Previous studies suggested that an APC of less than 7 Log CFU/g as fresh beef (Holman et al., 2021; Yu et al., 2018). The initial APC was 1.20 Log CFU/g for all packaged beef samples. It continued to increase until 7 weeks and subsequently decreased until 12 weeks in all experimental samples. The APC of EVOH-packaged beef was significantly lower than that of PVDC-packaged beef at 2 weeks of storage (p<0.05). As the storage period increased, the APC values of EVOH-packaged samples tended to be higher than those of PVDC. These results are likely attributed to a modification in physical properties such as oxygen and moisture permeability of the EVOH film in high relative humidity and refrigerated conditions (Mokwena and Tang, 2012). The APC did not exceed 7 Log CFU/g during all experimental periods. Therefore, the beef packaged with both vacuum films maintained its freshness during refrigerated storage.
Fig. 2 presents the results of metagenomic analysis for PVDC- and EVOH-packaged beef samples. Fig. 2A shows taxonomic composition for the phylum level of beef samples according to vacuum film types. Immediately after packaging (Control), the microbial distribution consisted of 13 phyla. Firmicutes accounted for 42.2% of the total, followed by Proteobacteria at 30.8% and Deinococcus at 18.23%. After refrigerated storage at 4 and 8-week intervals, the distribution of microorganisms in all samples confirmed by Firmicutes attained over 99.9% of the total. After a 4-week interval, four phyla were observed in the beef samples packaged with PVDC and EVOH films. After 8 weeks of refrigerated storage, three phyla were detected in beef packaged with PVDC film, while four phyla were detected in beef packaged with EVOH film. The detailed differences in the microbial diversity based on the vacuum packaging film did not change notably at the class (Bacilli) and order (Lactobacillales) levels, and showed a similar trend to the phylum level (data not shown). In a previous study, Firmicutes gradually became the dominant phylum in vacuum-packaged beef at a super-chilled temperature (Chen et al., 2019). Wen et al. (2022) reported that Firmicutes became the dominant phylum in vacuum-packaged lamb by 28 days of storage. This was a different pattern from the dominant phylum being as Proteobacteria in aerobic packaged lamb.
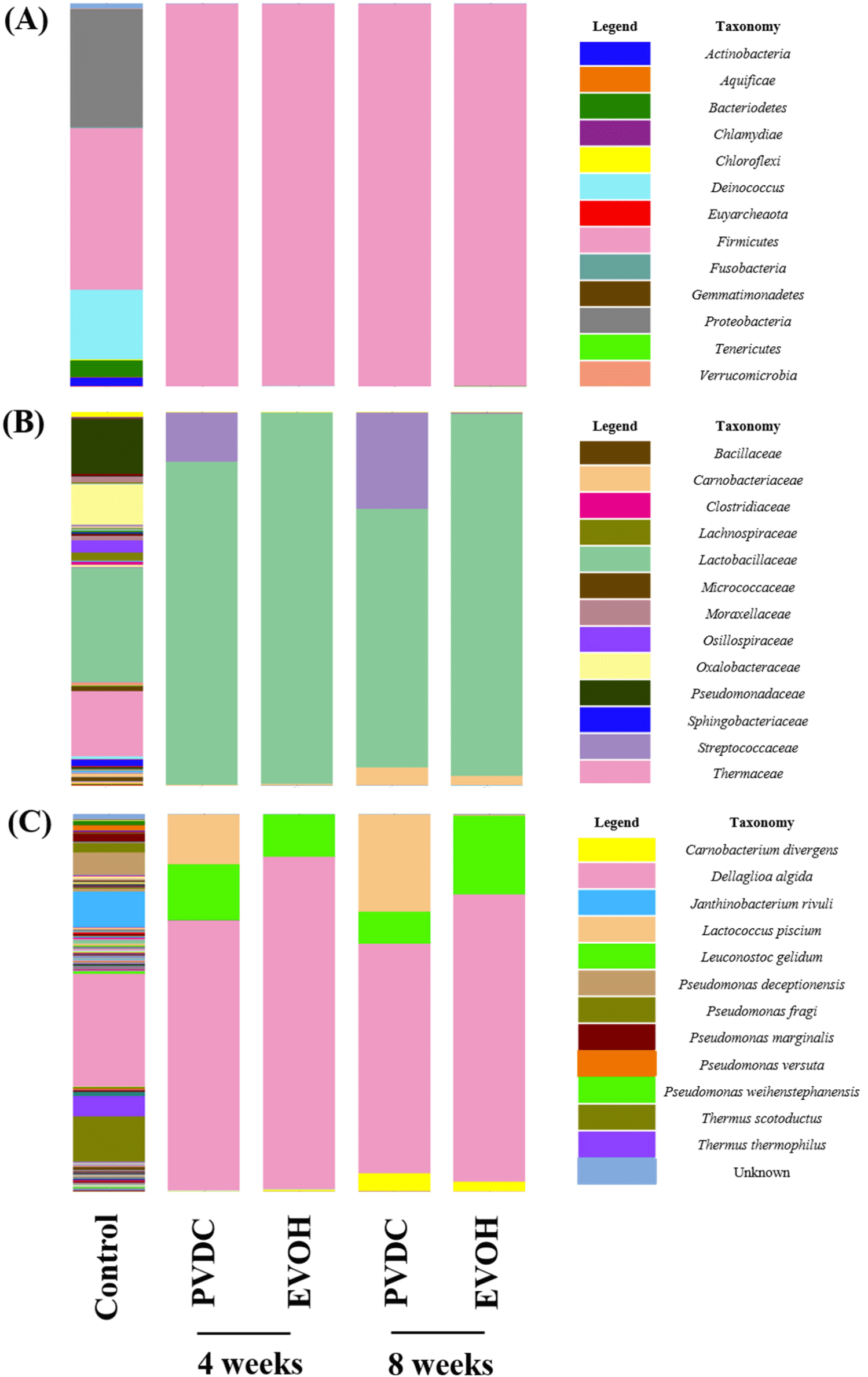
Fig. 2B presents a taxonomic composition for the family level of beef samples according to vacuum film types. In control groups, it was confirmed to contain a total of 93 families including four unknown families, with Lactobacillaceae family accounting for 30.55% of the total, Thermaceae family 17.53%, Pseudomonadaceae family 14.7%, and Oxalobacteraceae 10.75%. At a 4-week interval, the Lactobacillaceae family accounted for 86.58%, an increase of about 56% compared to the control, and the Streptococcaceae family accounted for 13.2%, an increase of about 12.9% from the initial 0.23% in PVDC-packaged beef. On the other hand, in EVOH-packaged beef, Lactobacillaceae increased by 68.8% compared to the initial sample to reach 99.36%, which was confirmed as the major flora. In the case of PVDC-packaged beef during 8 weeks of storage, it was observed that the Lactobacillaceae family decreased by about 17.3% to 69.27% at a 4-week interval, while the Streptococcaceae family increased by about 12.6% to 25.82% at a 4-week interval. Moreover, it was confirmed that the Carnobacteriaceae, Gram-positive lactic acid bacteria, accounted for 4.86% in PVDC-packaged beef and 2.5% in EVOH-packaged beef, respectively. For EVOH-packaged beef, Lactobacillaceae was clearly the dominant family by 8 weeks of storage, but for PVDC-packaged beef, the ratio of Streptococccaceae and Carnobacteraceae increased considerably during storage.
Fig. 2C presents a taxonomic composition for the species level of beef samples according to vacuum film types. Immediately after packaging, 246 species, including Dellaglioa algida (29.89%), Thermus scotoductus (11.94%), Janthinobacterium rivuli (9.4%), Pseudomonas deceptionensis (6.06%), Thermus thermophilus (5.37%) and unknown microorganisms (1.37%), were present in the beef samples. After 4 weeks of refrigeration, eight species were identified in beef packaged with PVDC film, while 14 species were identified in beef packaged with EVOH film. After 8 weeks, seven species were identified in beef packaged with PVDC film, while 31 species were identified in beef packaged with EVOH film. D. algida (formerly Lactobacillus algida) was the dominant species in all experimental samples during the entire storage periods. D. algida is known as facultatively anaerobic, psychrotrophic lactic acid bacteria, and is one of the representative microorganisms that cause spoilage in refrigerated meat (Zheng et al., 2020). The cell-free supernatant of D. algida was reported to have a strong antibacterial effect against Pseudomonas fluorescens and P. fragi (Sun et al., 2022). This is one of the suggestions that D. algida was the dominant species in vacuum-packaged beef, and Lactococcus spp. and Leuconostoc spp. were part of the microbial diversity of vacuum-packaged beef because of belonging to the same group of lactic acid bacteria that are less susceptible to organic acids and antibacterial substances secreted by D. algida.
In addition, Leuconostoc gelidum, which had a low position (0.57%) in the initial packaging, was found to be one of the predominant species presented in PVDC- and EVOH-packaged samples during refrigerated storage, but it did not show a large portion until 4 weeks of storage. After 8 weeks of storage, Carnobacterium divergens accounted for 4.86% for PVDC-packaged beef and 2.50% for EVOH-packaged beef. Interestingly, the principal difference between the two different vacuum films-packaged samples was the existence of Lactococcus piscium as the predominant species. In PVDC-packaged beef, L. piscium increased by 13.19% at a 4-week interval and 25.82% at an 8-week interval. However, in EVOH-packaged beef, L. piscium did not occupy a large portion of the microflora during storage. In previous studies, psychrotrophic lactic acid bacteria are reported as the dominant spoilage organisms in vacuum packaged-meat products during refrigerated storage (Juszczuk-Kubiak et al., 2021; Wang et al., 2019). L. piscium and L. gelidum are psychrotrophic lactic acid bacteria related to spoilage of meat using vacuum packaging and MAP (Pothakos et al., 2014b). These two lactic acid bacteria were closely linked with metabolites such as ethanol, ethyl acetate, 1-propanol, 3-methyl-1-butanol as well as 2-butanone, and were found to be one of the causes of an off-flavor in meat products (Luong et al., 2021). Pothakos et al. (2014a) experimented that L. gelidum subsp. gasicomitatum and L. piscium were stored at 4°C for 20 days under four different packaging types (vacuum & 3 types of MAP). As a results, both species maintained high bacterial counts of 8 Log CFU/g or more in vacuum packaging, but the growth of L. piscium was slower in MAP composed of 21% O2 and 79% N2 than vacuum packaging, and L. piscium decreased rapidly under MAP composed of 50% O2 and 50% CO2. Based on this study, it could be inferred that L. piscium is a facultative anaerobic bacterium, but its growth may be negatively affected by the presence of oxygen. Therefore, combined with our results, PVDC films had low oxygen permeability, so L. piscium was more likely to exist than other species. On the other hand, in EVOH-packaged beef, oxygen permeability was increased due to film denaturation in an environment with a low temperature and high relative humidity. This could explain one of the reasons why L. piscium is only found in PVDC-packaged beef.
Lactic acid bacteria are regarded as inhibiting the growth of other bacteria by producing various metabolites such as hydrogen peroxide, lactic acid, and bacteriocin (Stoops et al., 2015). These characteristics was likely associated with the ability of lactic acid bacteria to grow in vacuum packaging as a facultative anaerobic bacterium, making it the dominant microorganism in beef vacuum packaging. The differences of the two vacuum packaging films are considered to be attributed to different characteristics of each vacuum film in terms of O2 permeability and H2O permeability between them. Therefore, depending on the characteristics of each vacuum film, it could affect the microbial diversity during refrigerated storage.
Conclusion
The physicochemical and microbiological characteristics of Hanwoo packaged with different vacuum packaging films, namely PVDC and EVOH, during refrigerated storage were performed. In conclusion, there were various physicochemical and microbiological differences between PVDC-packaged beef and EVOH-packaged beef due to the physical properties of the vacuum film, which were noted in terms of surface color and microbial diversity. The pH values and color stability of EVOH-packaged beef tended to be lower than those of PVDC-packaged beef, while the VBN and TBARS values maintained acceptable levels in both experimental groups. For metagenomics analysis, Firmicutes in phylum groups, Lactobacillaceae in family groups, and D. algida were dominant species over 4 and 8 week intervals in both vacuum films. The difference between the two vacuum films was the existence of L. piscium as the predominant species.
Our results could be applicable to the selection of vacuum film materials for long-term refrigerated storage of beef. Although EVOH is a better eco-friendly packaging material than PVDC, it has a negative effect, notably color stability, for beef at a low temperature and high relative humidity. In this respect, it is considered that PVDC film is more advantageous than EVOH for long-term refrigerated storage under our limited experimental conditions.













