Introduction
The history of probiotics can probably be traced back to the first use of fermented food products, such as cheese and yogurt, which were recommended for daily consumption. Over time, numerous fermented foods with health-promoting properties based on the functional microbial strains involved in fermentation have entered the market. Meanwhile, traditional fermented foods such as kefir, kombucha, sauerkraut and kimchi have been shown to contain microbial strains with probiotic features (Marco et al., 2017). Kimchi is a Korean traditional fermented food prepared at low temperature by mixing vegetables such as radish, Chinese cabbage or other similar vegetables, cucumber, pepper, garlic, persimmon, and a low concentration of salt. In addition to beneficial lactic acid bacteria (LAB), it contains various minerals and vitamins. Taxonomic studies on the microbiota typical of kimchi fermentation have revealed a succession pattern typically initiated by Leuconostoc spp. and Weissella spp., and generally followed by Lactobacillus spp. (Rhee et al., 2011). There are Lactobacillus (11 strains), Lactococcus (1 strain), Enterococcus (2 strains), Streptococcus (1 strains), and Bifidoacterium (4 strains) among 19 Strains of probiotics authorized from Korean Food Standards Codex. Therefore, representative strains of Lactobacillus spp. are known to be the most promising probiotic candidates.
Recently, numerous studies aimed at identifying probiotic strains have shown that fermented dairy products can also be a good source of probiotics (Heller, 2001). Probiotics are defined as “living microorganisms which, when, administered in adequate quantities, confer a health benefit on the host” (FAO and WHO, 2002). Many cultivable and predominantly probiotic candidates in fermented dairy products have been widely isolated. The FAO/WHO guidelines on the development and application of probiotics constitute a set of parameters for strains to be called ‘probiotics’ and to prove health benefits for a particular condition or disease. The initial screening and selection of probiotics includes the inhibitory activities of lipase, α-amylase and α-glucosidase. In addition, selected probiotics should further be tested for their functional health characteristics. One method of investigating anti-adipogenicity in 3T3-L1 pre-adipocytes is a simple in vitro technique for selecting appropriate stains for in vivo studies conducted to support claims about probiotic. Likewise, each important strain property and its influence on health should ultimately be supported by clinical effects.
One assessment of safety and potential functionality of probiotics included antibiotic resistance testing (FEEDAP, 2012), bile salt and low pH tolerance, biogenic amine (BA) formation (BIOHAZ, 2011), enzymatic activity, and intestinal epithelial adhesion properties (Sanders et al., 2010) was used in the selection of an appropriate strain for an in vivo study in a diet induced murine model.
In this study, we isolated 167 different single strains from homemade kimchi, and carried out in vitro test and anti-adipogenic activity in 3T3-L1 cell to select functional strain. We investigated the physiological characteristics of selected strain and evaluated its potential as an anti-obesity probiotic in mice.
Materials and Methods
Two well-known and widely studied probiotic strains, Lactiplantibacillus plantarum 299V and Lactobacillus rhamnosus GG (LGG), have served as positive controls in various studies.
LAB were isolated from 40 kinds of homemade kimchi by using a modified man rogosa sharpe (MRS) medium (Lim et al., 2011). The strain was cultured for 18 h at 37°C in Lactobacillus MRS broth (BD Difco, Fraklin Lakes, NJ, USA) and stored at –80°C. Before use, the stock cultures were grown twice at 37°C for 18 h in MRS broth.
According to method described by Kim et al. (2018), lipase inhibitory activity, α-amylase inhibitory activity and α-glucosidase inhibitory activity was determined.
According to method described by Kim et al. (2018), 3T3-L1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM supplemented with 10% FBS and 1% P/S under 5% CO2 condition.
The strain was cultured in the MRS medium at 37°C for 18 h. After cultivation, all strains were harvested in a centrifuge at 1,500×g at 4°C for 15 min and washed three times with distilled water to remove the remaining MRS medium. The washed strain was lyophilized, re-suspended in distilled water at a concentration of 10 mg/mL, homogenized for 50 seconds using a sonicator (Branson 8800, Branson Ultrasonics, Danbury, CT, USA), and then rested for 3 minutes (repeated three times). The 3T3-L1 cells were treated with 100 μg/mL of strain (109 CFU/mL).
The amount of lipids accumulated in the cells was measured using Oil Red O (Sigma-Aldrich, St. Louis, MO, USA), which reacts specifically with intracellular lipids. 3T3-L1 adipocyte was measured according to method by Huang et al. (2021). The differentiated cells were washed three times with PBS and fixed with 10% formaldehyde, followed by oil red O solution (stock solution: 3.5 mg/mL in isopropanol; working solution: 60% oil red O stock solution and 40% distilled water) for 30 min at room temperature. After staining, the solution was removed and the sample washed three times with distilled water. The amount of lipid accumulation was determined by adding 2 mL of iso-propyl alcohol to the completely dried well, re-eluting the oil red O, and measuring the absorbance at 520 nm.
The isolated strain was identified by using the 16S rDNA sequencing method as described previously (Kim et al., 2018). Bacterial genomic DNA samples were extracted using the InstaGeneTM Matrix (Bio-Rad Laboratories, Hercules, CA, USA). The primers 27F (5’-AGA GTT TGA TCM TGG CTC AG-3’) and 1492R (5’-TAC GGY TAC CTT GTT ACG ACT T-3’) were used for the PCR.
The antibiotic susceptibility of L. plantarum KC3 was tested using the broth micro-dilution procedure according the method described by Phillips et al. (1991). The LAB susceptibility test medium with cysteine (LSM-C), which consists of a mixture of Iso-Sensitest broth (90%) and MRS broth (10%), supplemented with 0.3 g/L L-cysteine (Klare et al., 2007), was used as the medium. The enzyme activity of strain was determined using an API ZYM kit (bioMérieux, Lyon, France). Acid tolerance was measured according the method described by Clark et al. (1993). Bile tolerance was tested by the method of Gilliland and Walker (1990). The L. plantarum KC3 strain culture was inoculated into MRS broth containing 0.05% L-cysteine (Sigma-Aldrich) with or without 0.3% ox gall (Sigma-Aldrich). Antimicrobial activity was tested according to method of Gilliland and Speck (1977). Escherichia coli KCCM 11587 and Staphylococcus aureus KCCM 11335, antimicrobial indicator bacteria used in this study were purchased from the Korean Culture Center of Microorganisms (KCCM, Seoul, Korea), and Salmonella Typhimurium ATCC 14028 and Listeria monocytogenes ATCC 15313 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). BA formation was tested with LB agar (pH 5.0; BD Difco) containing 0.25% glycerol, 0.006% BCP, and 0.1% precursor amino acid, as described by Chang and Chang (2012). According to the method of Kim et al. (2018), the intestinal adhesion ability of the strain was performed using HT-29 cells.
The Committee on the Ethics of Animal Experiments of Handong Global University approved the animal experiments (20160615-002). Five-week-old C57BL/6J male mice were provided by Koatec (Gyeonggi, Korea) and housed in a controlled environment (at 23±1°C and 55±10% humidity, in a 12 h light/dark cycle) and given free access to filtered water and food. All of the mice were acclimated with normal diet during the first week. After this period, the mice were randomly assigned to groups (n=6/group) with different diets for 12 weeks. The customized (IF) high-fat diet was composed of 40% carbohydrate, 45% fat and 15% protein. The freeze-dried probiotic strains in the laboratory were incorporated into 3 grams of the IF diet to provide 5.0×109 CFU/mouse/day. The weight of each animal and its feed consumption was measured once a week. At the end of the experimental period, the animals were anesthetized by diethyl ether inhalation, samples collected, and their weight measured. Blood serum samples were extracted by centrifugation of the whole blood at 2,000×g for 20 min. Adipose tissues and serum samples were stored at –80°C without repeated freeze-and-thaw steps.
The statistical analysis was performed with a statistical analysis system (XLSTAT version 2015, Addinsoft, Paris, France). The significance of the differences was analyzed by conducting a one-way analysis of variance (ANOVA) using Duncan’s multiple range tests. Significance was considered to be p<0.05. Student’s t-test was performed with data from in probiotic characteristic test. In the animal study, the data were analyzed with ANOVA using Dunnett’s multiple range test compared to different groups. Significance was accepted at p<0.05. The statistical analysis was performed using a GraphPad Prism 7 Program (version 7.03, GraphPad Software, San Diego, CA, USA).
Results and Discussion
Using the modified MRS medium, 167 single strains were isolated from 40 kinds of homemade Korean kimchi. Among the 167 single strains, 19 strains of L. plantarum were selected for their strong inhibitory activity against pancreatic lipase of over 80%, and were tested for their inhibitory activities against α-amylase and α-glucosidase. Six of these strains (KC3, K40, K42, K58, K112, and K134) showed strong inhibitory activity against α-amylase and α-glucosidase of over 90% (Table 1). Natural and synthetic pancreatic lipase inhibitors are effective in preventing obesity because they inhibit intestinal lipid absorption (Hirose et al., 2013). Since Asian diets generally contain considerably more carbohydrates than Western diets, a combined mechanism may be required to inhibit carbohydrate absorption and to improve obesity by inhibiting fat absorption (Jang and Jeong, 2010).
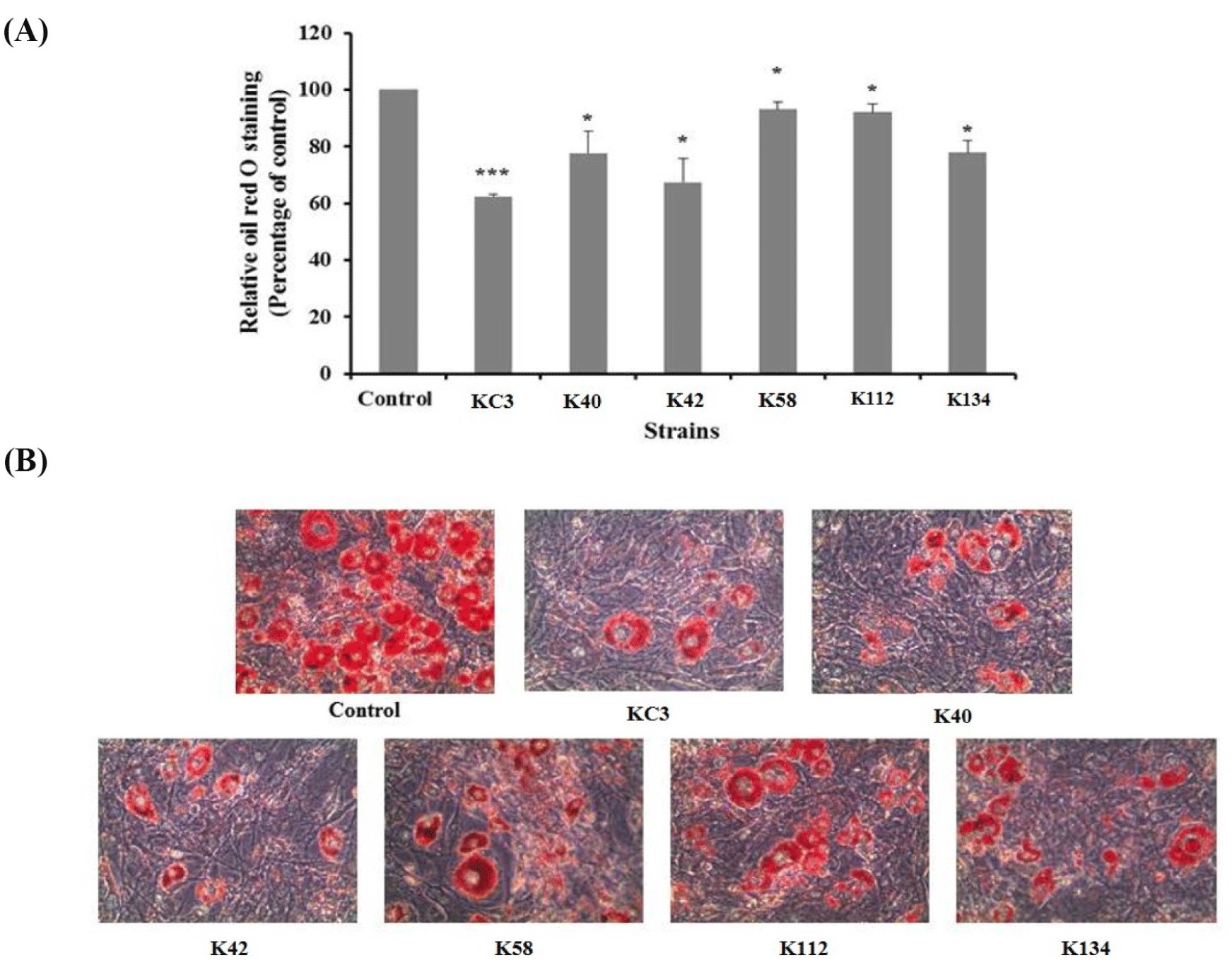
Obesity is also related to the degree of differentiation of pre-adipocytes into adipocytes, and to the enlargement of adipocytes in the adipose tissues (Wang and Jones, 2004). After the enzyme assay test of isolated strains, 6 single strains (KC3, K40, K42, K58, K112, and K134) were selected for the anti-adipogenic activity test. Fig. 1 shows the effect of these 6 single strains on 3T3-L1 adipocyte stained with Oil red O. The cells treated with KC3 resulted in a reduction of lipid accumulation of about 38%, compared with the untreated control (p<0.001, Fig. 1A). Among the strains, the greatest reduction in Oil red O staining was observed in KC3. As shown in Fig. 1B, KC3 also caused a greater reduction in lipid accumulation in rounded cells compared with the untreated control cells when visualized by staining. KC3 was then selected as the final experimental strain according to the results of the anti-adipogenesis test.
The total nucleotide sequence of 1508 bp was determined from the 16S rDNA gene of KC3. After PCR amplification using universal primers targeting 16S rDNA, and the subsequent sequence analysis, the alignment of this sequence showed a strong similarity (of around 99%) with the L. plantarum type strain. Based on the nucleotide sequence of the 16S rDNA gene, it was confirmed that it was identical to L. plantarum, and it was named L. plantarum KC3.
The tolerance of the L. plantarum KC3 to 16 types of antibiotics is shown in Table 2. According to Klarin et al. (2019), the ampicillin minimum inhibitory concentration (MIC) values of L. plantarum 299v and L. plantarum 299 were both 0.094 μg/mL. The MIC of L. plantarum KC3 showed a high resistance to ampicillin (MIC>256 μg/mL); while its resistance to penicillin, vancomycin, gentamicin, streptomycin, erythromycin, clindamycin, and chloramphenicol was found to be similar to the 46 L. plantarum strains reported by Klare et al. (2007). KC3 was more sensitive to clindamycin and erythromycin than to other antibiotics, but showed the highest resistance to ampicillin and vancomycin. The resistance of KC3 to kanamycin, streptomycin, clindamycin, rifampicin, and chloramphenicol was within the range accepted by the European Food Safety Authority (EFSA, 2008) and the Scientific Committee for Animal Nutrition (European Commission, 2001). However, KC3 was resistant to gentamycin, ampicillin, ciprofloxacin, tetracycline, and vancomycin, and had an equal or higher MICs according to the European Food Safety Authority (EFSA, 2008) and the Scientific Committee for Animal Nutrition (European Commission, 2001).
1) A value ranging from 0 to 5 is assigned to the standard color: Zero represents a negative; 5 represents a reaction of maximum intensity. Values 1 to 5 represent intermediate reactions depending on the level of intensity. The approximate activity may be estimated from the color strength: 1 Corresponds to the liberation of 5 nanomoles, 2 to 10 nanomoles, 3 to 20 nanomoles, 4 to 30 nanomoles, and 5 to 40 nanomoles or more.
Unlike Bacillus spp. and fungi, Lactobacillus is known for producing intracellular enzymes (Jeon, 1998). The results of the enzyme activity of L. plantarum KC3 are shown in Table 2. L. plantarum KC3 produced enzymes such as esterase, lipase, leucine arylamidase, valine arylamidase, cystimearylamidase, acid phosphatase, naphtol-AS-BI-phosphohydrolase, β-galactosidase, α-glucosidase, β-glucosidase, and N-acetyl-β-glucosaminidase. In particular, it produced large amounts of such enzymes as leucine arylamidase, valine arylamidase, β-galactosidase, and β-glucosidase. However, no activity associated with β-glucuronidase, a pro-carcinogenic enzyme that converts benzopyrene to a carcinogenic substance (Rhee et al., 1998) was detected in this particular strain.
Enzymes secreted by probiotics can improve the utilization of nutrients such as starch, protein and fat when consumed by humans or animals, thereby increasing the energy value of food or feed (Walsh et al., 1993). In particular, β-galactosidase enzymes could alleviate the symptoms of lactose intolerance by converting lactose into galactose and glucose in milk (de Vrese et al., 2001). The β-galactosidase activity in L. plantarum KC3 was determined at 5 degree.
After oral ingestion, bacteria encounter several hurdles erected by the human defense system, such as mucins in the gut, gastric acid and bile acid. The bile acid secreted into the duodenum destroys the membrane of bacterial cells and inhibits their growth. Therefore, in order to function as probiotics, resistance to physiological (and at least 0.3%) bile concentration is essential (Saarela et al., 2000).
Subsequent to the antibiotic susceptibility test, bile acid and acid tolerance were tested againt L. plantarum KC3. The growth curves of the KC3 strain in MRS broth and in MRS broth with 0.3% ox gall are shown Fig. 2A. After incubation for 7 h, the number of viable cells was counted in both the MRS broth and the MRS broth with ox gall. Compared to other strains, L. plantarum KC3 was not affected by the addition of 0.3% ox gall up to 5 h, after which a slight decrease was detected. After incubation for 7 h, the number of the viable bacteria was 9.37 Log CFU/mL without ox gall (bile acid) and 8.96 Log CFU/mL with ox gall. Strain KC3 showed a high survival rate of 95.62% in the MRS broth with 0.3% ox gall, compared to the control without bile acid.
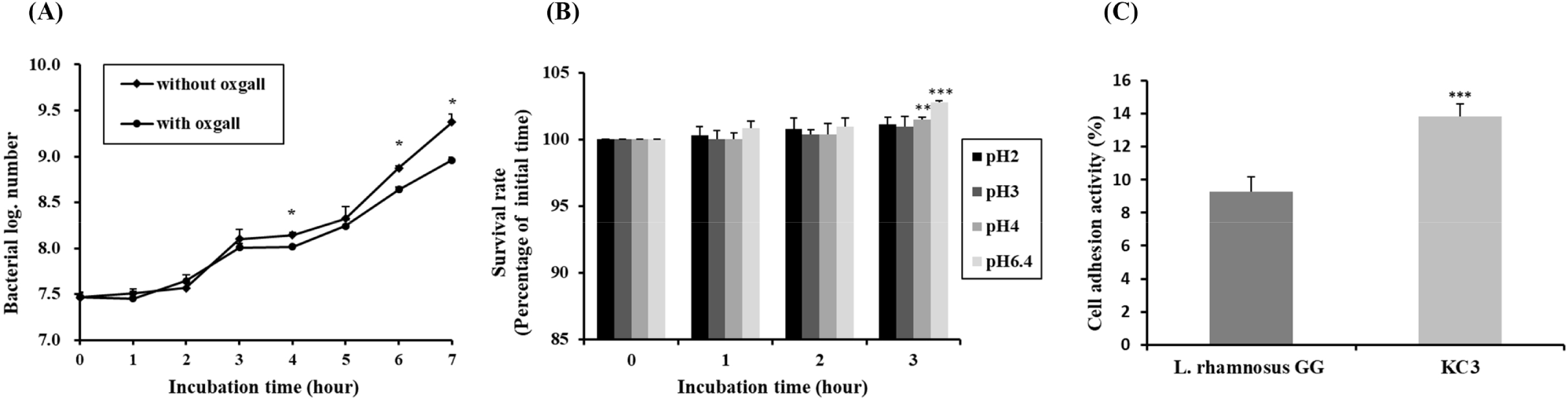
To function effectively as a probiotic, survival at a pH value of pH 3 or lower would be necessary to survive passage through the upper gastrointestinal tract. The pH of gastric juice is pH 0.9, but when food is ingested its pH rises to pH 3 (Erkkilä and Petäjä, 2000). High acid tolerance was detected in strain KC3. The results of pH tolerance of L. plantarum KC3 and other strains are shown in Fig. 2B, indicating that, in comparison to 6.4, the growth of the strain was not significantly influenced by the pH values 2, 3, and 4. Based on its survival rates, L. plantarum KC3 showed the highest bile and acid tolerance among the tested strains. Because a comparatively high percentage of the strain survived under bile acid and acidic conditions, L. plantarum KC3 has probiotic potential under in vivo conditions.
Table 3 shows the antimicrobial activity of L. plantarum KC3 against various pathogenic strains. L. plantarum KC3 showed resistance to E. coli, S. Typhimurium, L. monocytogenes and S. aureus at rates of 53.78%, 76.80%, 26.27%, and 34.61%, respectively. After incubation for 6 h, the pH value of the pathogens was around 5.98–6.10, while that of the mixed culture of L. plantarum KC3 and pathogens was around pH 4.98–5.54, which indicates that even though the lactic acid produced during incubation had an effect on antimicrobial activity it was not large.
The antimicrobial effects of LAB in the GIT are related to inhibition during pH reduction, the competition for consumption between nutrients and pathogens, a reduction of redox potential, the production of hydrogen peroxide under aerobic conditions, and the secretion of antimicrobial active substances such as bacteriocin (Havenaar et al., 1992). Some strains of LAB produce different antimicrobial compounds which can prevent the growth of pathogenic and spoilage bacteria (Ahmadova et al., 2013). The antimicrobial activity of a strain varies depending on the pathogen, even if it is a strain of the same species (Jacobsen et al., 1999).
The ability to adhere to the intestinal epithelium is one of the key criteria when selecting probiotic strains (Sanders et al., 2010). In a recent study, HT-29 cells were used as an in vitro model of epithelial cell adherence (Lee et al., 2011). The ability of L. plantarum KC3 to adhere to the human intestinal cell line HT-29 is shown in Fig. 2C. L. plantarum KC3 (13.85%) adhered to the HT-20 cells, thus showing greater adhesiveness than L. rhamnosus GG (with only 9.26%), the positive control. Although L. rhamnosus GG’s strong ability to adhere to HT-29 cells has been reported in several previous studies, our data were similar to those of Verdenelli et al. (2009).
Some LAB strains may form BA by amino acid decarboxylation. BA is an alkaline organic substance with biological activity that is commonly found in fermented foods or fermented beans, and is formed mainly by the enzymes of food or by decarboxylation of the amino acids in microorganisms (Silla Santos, 1996). BAs are found in various foods such as non-fermented foods (fish, fruits, vegetables, and meat), dairy products, fermented fish/meat products, soybean fermented products, and alcoholic drinks such as wine and beer (Silla Santos, 1996). BAs have diverse biological activities, including negative effects such as toxicity and causing allergenic responses. Especially histamine and tyramine can cause migraines, flushing, nervous disorders, headaches, vomiting, nausea, heart palpitations, respiratory distress, hypertension, and blood pressure instability (BIOHAZ, 2011). L. plantarum KC3 did not show any BA formation from the precursor amino acids used in this study (data not shown), namely tyrosine, histidine, ornithine, and lysine.
To evaluate the anti-obesity effect of L. plantarum KC3 on the aberrant host conditions, lyophilized probiotic strains were incorporated with the IF diet at a level of 5.0×109 CFU/mouse/day. LGG and 299V were used in the studies as reference probiotic strains, and Xenical (Xen) was used as the positive chemical control for anti-obesity. Each animal group that received the LGG strain, Xen and KC3 showed a significantly lower bodyweight and total weight gain compared to the high-fat diet group (Table 4). Moreover, the weight of the liver and other adipose tissues of the LGG and KC3 groups, but not that of the 299v group, showed a significant reduction. The concentrations of other parameters such as the glucose/lipid metabolism-related biomarkers, total cholesterol, triacylglycerol (TG) and low-density lipoprotein cholesterol, were alleviated in the LGG and KC3 groups, indicating an amelioration of the biomarkers of metabolic disease induced by the IF diet (Table 4).
The whole bodyweight, liver and adipose tissues were measured after 12 weeks from the initial point (n=6–9). The indicators for the serum analysis were measured by the blood analyzer. The data are represented as the mean±SD and analyzed in a comparison with the HFD group.
ND, control group with a normal chow diet; HFD, positive control group with a high-fat diet; Xen, negative control group treated with Xenical; LGG, receiving Lactobacillus rhamnosus strain GG mixed with a high-fat diet; 299v, receiving Lactiplantibacillus plantarum strain 299v mixed with a high-fat diet; KC3, receiving L. plantarum strain KC3 mixed with a high-fat diet; EAT, epidydimal adipose tissue; MAT, mesenteric adipose tissue; SAT, subcutaneous adipose tissue; TG, triacyl-glyceride; LDLC, low density lipoprotein cholesterol.
Bile acids play an essential role in maintaining TG and cholesterol homeostasis (Li et al., 2013). According to Kwon et al. (2020), it was reported that the expression of genes involved in bile acid synthesis was significantly increased in the liver of L. plantarum treated mice. These assessments have shown that L. plantarum KC3 is adequate for use in anti-obesity investigations in an in vivo study. Freeze-dried L. plantarum KC3 was incorporated into the IF diet and administered during an abnormal host status of diet- induced obesity. As a result, L. plantarum KC3 showed an ability to reduce fat accumulation. Based on these results, it is necessary to confirm the change in the intestinal microbiota during administration of L. plantarum KC3. The regulation of gene expression related to lipid metabolism in the adipose tissue has probably been altered. As such, it would be necessary to adopt a mechanistic approach in order to determine the exact way in which L. plantarum KC3 modulated this interactive cascade.













