Introduction
As populations have aged, osteoporosis has become a significant public health issue around the world. Osteoporosis is related with an imbalance in metabolism of bone whereby bone resorption is more than bone formation (Christiansen, 1992). Some treatments, including hormone replacement therapy, bisphosphonates, vitamin D and calcium supplementation, selective estrogen-receptor modulators, and teriparatide® have been displayed to effectively decrease the risk of bone fracture and loss in patients of osteoporotic (Collins et al., 2017; Kanis et al., 2013). Non-pharmacological therapies include a nutritional diet, exercise, and surgical treatment for fractures (Collins et al., 2017; Kanis et al., 2013). Some previous studies have reported potential benefits of probiotic supplementation on bone disease and some strains of probiotics have been shown to be effective treatments for osteoporosis in experimental animal models (Collins et al., 2017). The motivation behind this study was to evaluated the effects of Lactiplantibacillus plantarum MGE 3038, which was previously isolated from a natural cheese and might influence on bone health with other cheese components, supplementation on ovariectomized rats.
Materials and Methods
Twelve weeks old female Wistar rats (n=21; 250–300 g) were divided into 3 groups; ovariectomy (OVX) group, OVX and L. plantarum MGE 3038 (OVX/MGE 3038) group and Sham group (control). In these three groups; two went through respective OVX and one had daily MGE 3038 administration through oral gavage. This trial was permitted by the Animal Care and Use Committee of Asan Institute for Life Sciences, Seoul, Korea (2017-14-228).
OVX was achieved through a small skin incision in the back area under 5% isoflurane. We performed a peritoneal dissection to reach the ovary before cutting the uterine horn and Fallopian tube after vessel ligation. Finally, we removed the ovaries. After wound closure, an intramuscular antibiotic (cefazolin, 10 mg/kg) was injected for three days.
The MGE 3038 used in this study was previously isolated from a natural cheese and it’s partial 16S rRNA gene sequence was deposited in GenBank database (accession number OP269658) and has been stored at –80°C prior to use. Four weeks after OVX surgery, probiotic supplementation began. Over 16 weeks, OVX/MGE 3038 group rats were supplied once daily 400 μL of MGE 3038 treatment containing ca. 108 CFU. The OVX and Sham groups were administered with the same volume of sterile water.
We evaluated in-vivo CT (Skyscan NV, Kontich, Belgium) at 0, 5, 10, 15 weeks after OVX under inhalation anesthesia. Finally we extracted both tibiae and evaluated by high-resolution micro-CT (Skyscan NV) 16 weeks after OVX. The volume of interest was confined between a starting point 2.5 mm distal from the proximal growth plate of the tibia to the diaphysis; the volume was divided over 100 slices. An area of special interest was demarcated in the compartment of whole trabecular bone. The exposure time was 670 ms. Skyscan software (Skyscan NV) was used for image reconstruction and analysis. The trabecular bone volume (BV/TV; %), trabecular thickness (Tb.Th; mm), bone mineral density (BMD; mg/cm2), trabecular number (Tb.N; mm–1), and trabecular separation (Tb.Sp; mm) were evaluated.
For the assessment of hematoxylin- and eosin- staining (H&E), all pathological specimens were first decalcified. Afterward, specimens were cut in 3 μm thicknesses longitudinally. The samples were then deparaffinized and dehydrated. The sections were stained for 5 min with Hematoxylin-I (YD Diagnostics, Yongin, Korea) and Eosin Y (Sigma-Aldrich, St. Louis, MO, USA) for 2 min 30 s. For the Masson’s trichrome stain, we treated samples with a mordant solution (5 % iron alum) for 30 min in a 56°C dry oven. Following this, the sections were stained for 10 and 5 min using Weigert’s iron hematoxylin nuclear staining (Sigma-Aldrich) and Biebrich scarlet-acid fuchsin (Sigma-Aldrich) solutions, respectively. Then, the above stained sections were decolorized by treating for 5 min in a phosphotungstic-phosphomolybdic acid solution. Finally, samples were placed in solution of 1% glacial acetic acid for 3 min.
To collect the blood from heart, the heart was punctured, and enough blood was collected and subjected to centrifugation for serum separation at 1,100×g for 10 min at 4°C. Subsequently, type I collagen C-telopeptide (CTX), osteocalcin (OC), and the receptor activator of nuclear factor-ĸB ligand (RANKL) was examined using a Rat CTX ELISA kit (Cusabio, Houston, TX, USA), Rat-Mid OC ELISA kit (IDS, London, UK), and Rat RANK ELISA kit (Cusabio), respectively. The serum parameters were evaluated using a reader of microplate absorbance (Sunrise; TECAN, Nänikon, Switzerland).
All analyses were achieved using R (version 3.3.2; The R Foundation for Statistical Computing, Vienna, Austria). Two-way ANOVA with Bonferroni post-test correction and Mann-Whitney U test for body weight and micro-CT measurements were conducted to define whether the parameters differed significantly. All data are presented as mean±SD or mean±SEM. p-value of 0.05 or less was considered statistically significant.
Results
After OVX, it was found that the mean body weight increased over the twenty-weeks in all tested groups. A significant increase in the weight of OVX and OVX/MGE 3038 treated groups were observed when compared with the control group. However, the mean body weight of OVX group was higher than the OVX/MGE 3038 group and the difference between the two groups increased over time (data not shown).
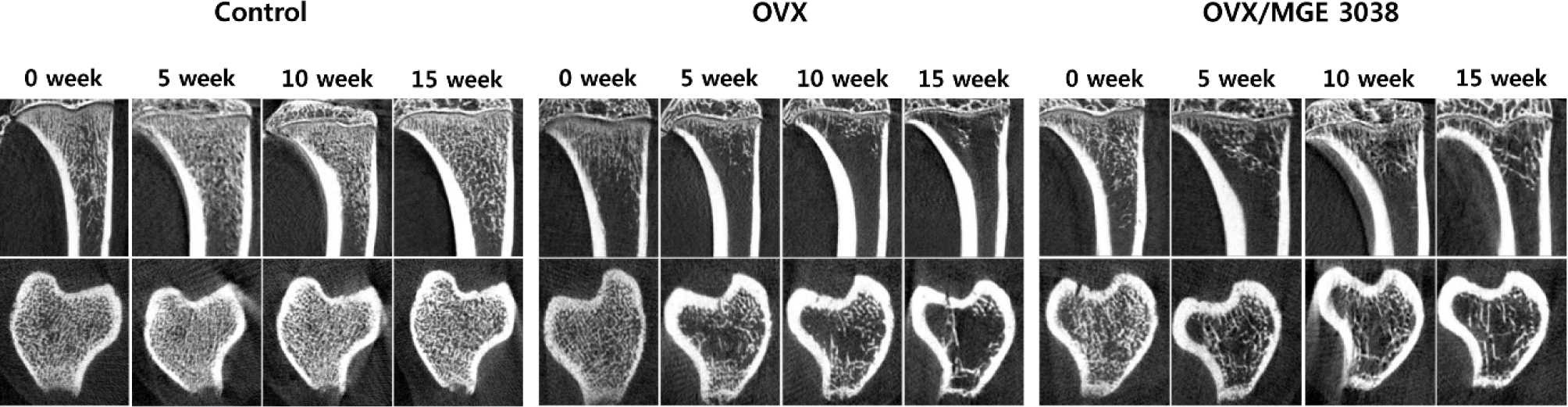
In micro-CT images, changes in osteoporosis were observed in OVX and OVX/MGE 3038 group. The preservation of trabecular structure was more prominent in the OVX/MGE 3038 than in OVX (Fig. 1). The maximum values of BMD, BV/TV, Tb.N, and Tb.Th, and the lowest value of Tb.Sp were observed in control group, while the Tb.Th value difference between the control group and OVX/MGE 3038 group was not significant. However, increased values in BMD, BV/TV, Tb.N, and Tb.Th, and a decrease in Tb.Sp were noticed in the OVX/MGE 3038 group as compared to the OVX group (data not shown).
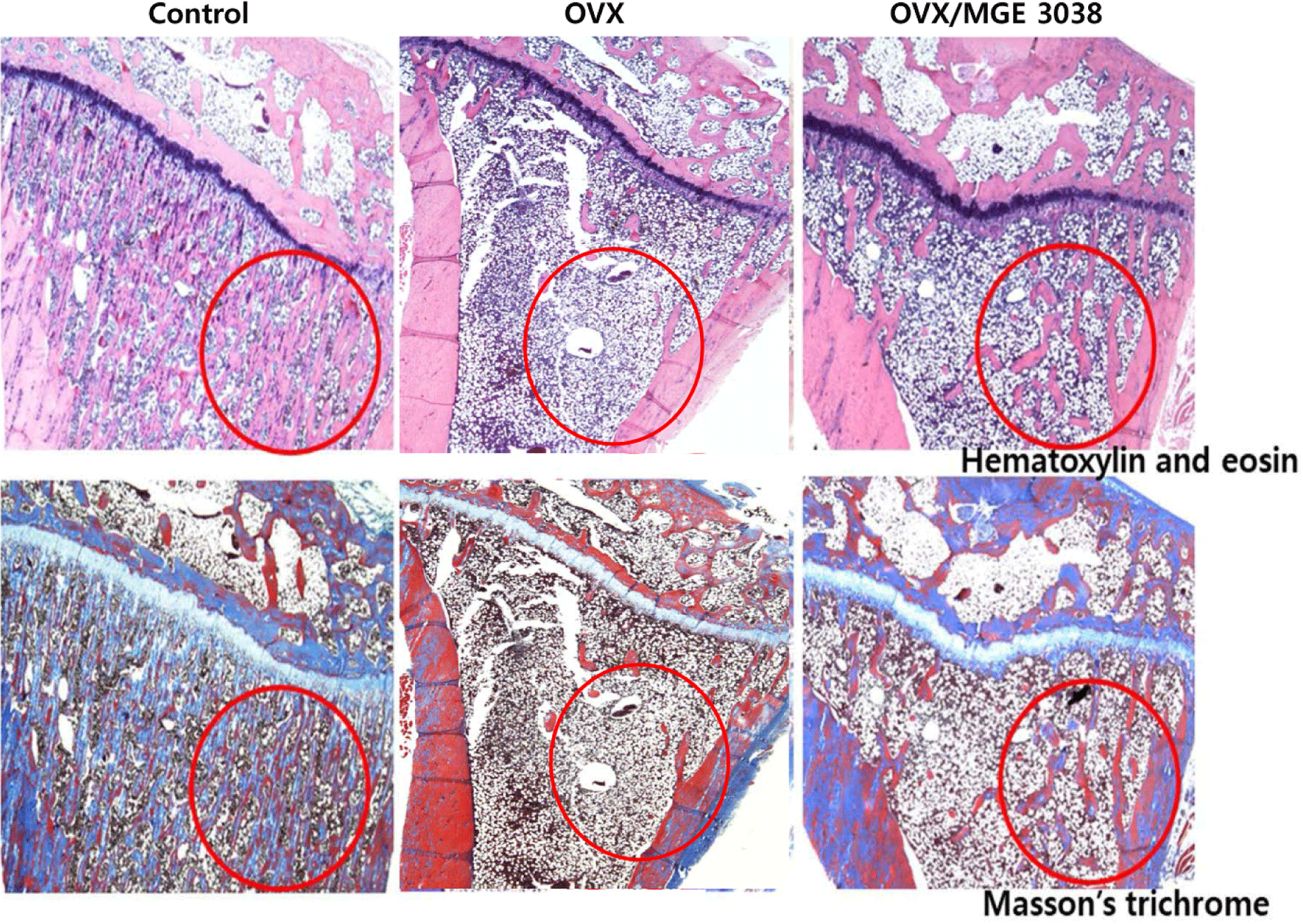
In H&E and Masson’s trichrome-stained sections, decreased bony trabeculae and increased adipose tissue infiltration of the bone marrow were exhibited in the OVX and OVX/MGE 3038 groups compared to control group. The maintenance of trabecular bone structure in the OVX/MGE 3038 group was improved in comparison with the OVX group (Fig. 2).
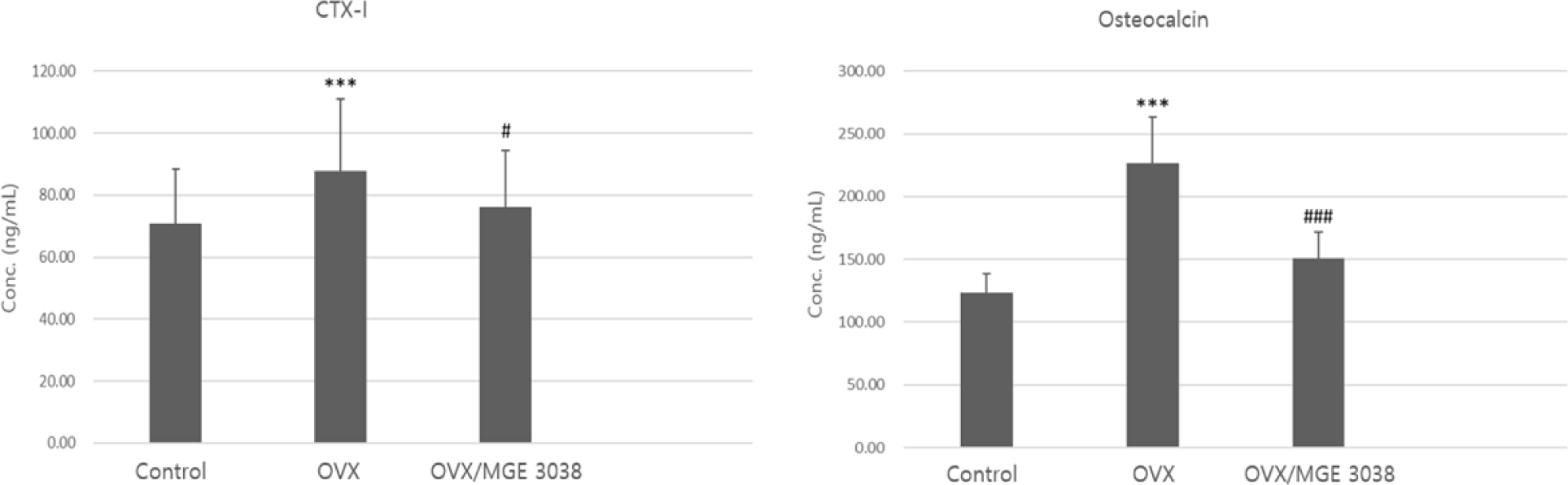
Serum levels of CTX and OC between the control group and OVX group were significantly different (p<0.001). However, no meaningful differences were observed in between the control group and OVX/MGE 3038 group (p>0.05). While, between the OVX and OVX/MGE 3038 group, serum levels of CTX and OC were also different significantly (p<0.05 and p<0.001, respectively; Fig. 3). Additionally the RANKL was the highest in the OVX group, with significant difference between OVX (113.54±37.99 ng/mL) and control group (80.821±11.64). However, there was no difference between control and OVX/MGE 3038 group (92.63±11.11; p>0.05).
Discussion
Probiotics known as good or beneficial live microorganisms, have proved to have positive effects on host health when administered in a sufficient amount (Seddik et al., 2017). Among the lactic acid bacterial species, L. plantarum is a well-known probiotic bacterium that can be largely found in fermented food and nutritional products (Seddik et al., 2017), vegetables (Cherdyntseva et al., 2016), beef (Schillinger and Lücke, 1989), herb (Seddik et al., 2017), wine (Berbegal et al., 2016), as well as the gastrointestinal, urogenital, and vaginal tracts (Al Kassaa et al., 2014; Jose et al., 2015). The impressive ability of this bacterium to exist across a large range of environments derives from a high degree of metabolic pathway diversities (Fiocco et al., 2010). The MGE 3038 used in this study was previously isolated from a natural cheese.
For postmenopausal osteoporosis examination, a number of studies have been conducted using different animal models (Kalu, 1991; Turner, 2001). Among these animal models, the OVX rat model is mostly used to study postmenopausal osteoporosis, as this rat model is easy to handle and cheaper (Jin et al., 2019). This model shows cancellous bone change including postmenopausal bone loss (Kalu, 1991; Turner, 2001) and is also used as an obesity model (Jeong et al., 2015). The mean body weight in all groups after OVX increased over the time. However, the body weight of rats in the OVX group was higher than the rats of OVX/MGE 3038 group (data not shown). In the micro-CT findings, it was observed that the trabecular structure of rats in the OVX/MGE 3038 group is well maintained in comparison with OVX group (Fig. 1). The trabecular formation was also better maintained in the rats of OVX/MGE 3038 group than in the rats of OVX group as shown in the pathological findings (Fig. 2). These data allow us to support the hypothesis that MGE 3038 is effective for preventing osteoporotic changes in ovariectomized rats.
Osteoporosis is caused by an age-related decrease of osteoblast progenitor cells in the bone marrow and reduction in estrogen levels as well (Friedenstein, 1976). Some probiotics have shown the potential for bone structure protection in osteoporosis by reducing aging-induced bone loss in senescence-enhanced mice (Kimoto-Nira et al., 2007) while others enhanced bone thickness (McCabe et al., 2013) or improved mineral contents in the cortical bone of chicken (Mutuş et al., 2006). While, the principle mechanism and underlying effects of MGE 3038’s treatment have not been fully clarified and are still being investigated. Some hypotheses have proposed that enhanced antimicrobial peptide secretion, host immune system regulation by altering the intestinal microflora, or increased mucus production to regulate luminal pH in the gut thereby improving the absorption of calcium may be responsible for the effects of MGE 3038 supplementation on osteoporotic bone. Kim et al. (2019) presented that L. plantarum NK3 and Bifidobacterium longum NK39 might alleviate osteoporosis in a ovariectomized mouse model by controlling NF-κ-B-linked TNF-α expression via gut microbiota modulation. In another previous study, Lactobacillus paracasei strain alone or mixed with L. plantarum strains showed protection effects for OVX-induced cortical bone loss and resorption via regulation of inflammatory cytokines and inhibitor of osteoclastogenesis and influenced on frequency of regulatory T cells in bone marrow in the ovariectomized mouse model (Ohlsson et al., 2014). Recently gut microbiome has been highlighted since it is systematically linked to human physiology such as brain function, immune system, skin health, etc. In particular, osteoporosis could be influenced by gut microbes which modulates nutrient absorption, immune cell balance, and neurotrasmitters via gut-brain axis (Ding et al., 2020). Even though more sophisticated researches on the relationship between gut microbiome and bone metabolism should be done under interdisciplinary approach which includes experts including microbiologist, immunologist, bone pathologist, etc., gut microbiome diversity seems to be one of important factors for bone homeostasis. Alteration of diversity and composition of gut microbiome influences on gut barrier permeability and metabolites, which can trigger inflammatory reaction of specific immune cells including TH17 cells associated with bone degradation via stimulating the differenciation of osteoclasts in bone marrow (Cooney et al., 2021; Sato et al., 2006). Probiotics including L. plantarum are well known to present positive effects on gut health via modulation of gut microbiome, increase of short-chain fatty acids (SCFAs), improvement of gut barrier integrity, etc., which could be closely related to bone health (Hemarajata and Versalovic, 2013).
In our study, after OVX in rats, we evaluated BMD and other parameters for 20 weeks through micro-CT. The OVX/MGE 3038 group displayed an increased BMD, Tb.Th, Tb.N, and BV/TV, and a reduction in Tb.Sp in comparison with the rats of OVX group, although no significant differences were observed statistically. Furthermore, in the case of Tb.Th values no significant differences were noticed between the control group and OVX/MGE 3038 group (data not shown).
Bone remodels to new bone usually achieve by replacement of damaged and old tissue. Osteoclasts and osteoblasts co-work simultaneously in the remodeling unit. Bone remodeling is most protrusive on surface of the cancellous bone, where osteoblast induces new bone formation with the removal of the old and damaged tissues from the eroded surface through osteoclasts (Abdul-Majeed et al., 2012). During the bone resorption stage, serum CTX, making a good bone resorption marker that generally well-reflects osteoclast activity, is increased by osteoclasts. In this study, the CTX levels in the OVX group showed the maximum level and in comparison to the control group displayed significant difference statistically. However, the CTX levels in the OVX/MGE 3038 group were not significantly different from the control group and have significant difference from the OVX group (p<0.05; Fig. 3). During the bone formation process, OC levels in the serum increases (Ivaska et al., 2004; Romero Barco et al., 2012; Rosen et al., 2000). Osteoblasts and osteocytes secrete serum OC that serve as a marker for the bone formation (Szulc and Delmas, 2008). In the present study, the serum level of OC in the rats of OVX group was highest among the three groups and significant difference was observed in comparison with the rats in the control and OVX/MGE 3038 groups (p<0.001; Fig. 3). Furthermore, RANKL was the highest in the OVX group, with significant difference between OVX and control group. However, there was no difference between control and OVX/MGE 3038 group (p>0.05). So, we thought that the LAB strain can improve the osteoporosis via prevention of the activation of the osteoclasts. Taken together, OVX-induced osteoporotic rats showed strong activation of osteoclasts and coupling of osteoblast activation, in which MGE 3038 administration showed preventive effects. Therefore, MGE 3038 may prove beneficial effect on osteoporosis prevention.
As with any study, there are limitations to take into consideration. We did not evaluate changes in the colon, which should include measures of SCFAs, luminal pH, and mineral absorption (Moslehi-Jenabian et al., 2010; Parvaneh et al., 2014).
Conclusion
L. plantarum MGE 3038 is thought to be helpful in preventing and treating osteoporosis in ovariectomized animal model. Nevertheless the results are preliminary and have limitations to fully understand the mechanisms underlying the effects. In the future, we will identify the active components from the probiotic combined with gut microbiome composition and analyze more sophisticated biomarkers including ratios of osteoblast vs. osteoclast.













