Introduction
Since the first prototype cultured meat was reported in 2013 (Post, 2014), several types of cultured meat products have been produced. The prototype was based on a large number of strips containing myotubes and myofibers engineered in a hydrogel. However, colorants, flavor, and texturizers were added to make its muscle-like tissue have a similar appearance to that of traditional meat (TM). This implies that the tissues cultured by extracting satellite cells from livestock muscle tissue are significantly different from those composing TM. Therefore, to make a cultured meat product that is similar to TM, it is necessary to investigate the meat quality and taste characteristics of cultured muscle tissue (CMT).
Cultured meat may be biologically equivalent to TM, but there are still many technical difficulties to solve in order to produce fresh meat-like artificial meat (FAM) using CMT and bio-artificial meat (BAM) using hydrogel/scaffolds with sensory characteristics similar to those of TM (Gholobova et al., 2015; Post and van der Weele, 2014). Consumer acceptance of FAM or BAM is highly dependent upon sensory characteristics such as color, mouthfeel, and taste, and therefore, they are of the utmost importance. Recent studies have compared the appearance and texture of CMT and BAM to those of TM (Furuhashi et al., 2021; Kang et al., 2021; MacQueen et al., 2019), but studies on the taste characteristics are insufficient. The taste panel of the 2013 cultured meat prototype reported that the CMT hamburger patty felt slightly dry due to the lack of fat, but no profound quality or sensory evaluations were performed (Kupferschmidt, 2013). The sensory tests of cultured cells reported in the scientific literature were conducted during the early period of cultured meat experiments and included odor and observation, but no other tests were performed (Benjaminson et al., 2002). In addition, previous literature focused on the sensory properties of cultured meat, which were based on indirect assumptions (Bhat et al., 2019; Chriki and Hocquette, 2020; Fraeye et al., 2020; Ismail et al., 2020b). Currently, it is a challenging task to evaluate the scientific and technical taste characteristics of cultured meat due to the lack of sufficient experimental samples. However, recently, studies investigating the taste characteristics in a small amount of meat tissue using an electronic tongue system have been successfully conducted (Hwang et al., 2018; Hwang et al., 2020; Ismail et al., 2020a). Therefore, in this study, satellite cells extracted from chicken and cattle muscles were cultured, and the taste characteristics of the CMT were compared to those of TM using an electronic tongue system.
Material and Methods
The reagents purchased for isolation and culture of satellite cells included Hanks' Balanced Salt Solution (HBSS, Welgene, Korea), Dulbecco’s phosphate-buffered saline (DPBS; Thermo Fisher Scientific, Waltham, MA, USA), glucose-free Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific), fetal bovine serum (FBS; Thermo Fisher Scientific), GlutamaxTM supplement (Thermo Fisher Scientific), gelatin powder (G9391; Sigma-Aldrich, St. Louis, MO, USA), 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific), horse serum (Thermo Fisher Scientific), antibiotic-antimycotic (×100) (Thermo Fisher Scientific), human recombinant basic fibroblast growth factor (bFGF, 78003; Stemcell Technology, British Columbia, Canada), dimethyl sulfoxide (DMSO; Sigma-Aldrich), and Red Blood Cell Lysis Buffer (Invitrogen, New Zealand). The proliferation medium (PM) was a mixture of 30% FBS, 1% Glutamax, and 5 ng/mL bFGF in glucose-free DMEM and used as a growth medium for the satellite cells. Differentiation media (DM) was composed of 2% horse serum in DMEM for inducing myotubes.
Skeletal muscle samples were derived from several 4 to 6-week-old broiler chickens and several 24 to 27-mon-old Hanwoo (Korean native cattle) steers at a commercial slaughterhouse located at vicinity of Jinju/Korea. All the animals were euthanized following approved humane methods. Several small pieces of the pectoralis major muscle from chickens and the biceps femoris muscle from cattle were removed from the carcasses immediately after slaughter. The collected muscle pieces were sterilized with 70% ethanol, placed in HBSS containing 3% antibiotic-antimycotic (ant-anti), and transported to the cell culture laboratory.
On a clean bench, the muscle pieces were washed once with 70% ethanol and placed in a 100 mL Petri dish (SPL Life Sciences, Pocheon, Korea). Each muscle tissue was rinsed 3 to 5 times with a 4-fold volume of cold phosphate-buffered saline (PBS), followed by the removal of visible adipose and connective tissue. The muscle tissue was placed in a new Petri dish and cut into very small pieces using scissors after spraying with 0.25% trypsin-EDTA. The muscle tissue was minced to look like meat porridge, and approximately 4 g of the minced muscle was transferred into a 50 mL conical tube, and then 5 times the volume of 0.25% trypsin-EDTA was added. The muscle tissue was transferred to a conical tube and incubated in a water bath at 37°C for 30 min while gently inverting every 10 min. The digested muscle tissue was collected by low-speed centrifugation for 5 min at 300×g. After removing the supernatant, 10 mL of PM was added to the precipitated pellet and serially filtered through 100, 70, and 40 μm strainers (Maangchi, kitchenware Korea). Then, the filtered cell suspension was centrifuged for 5 min at 300×g to collect the cell pellet.
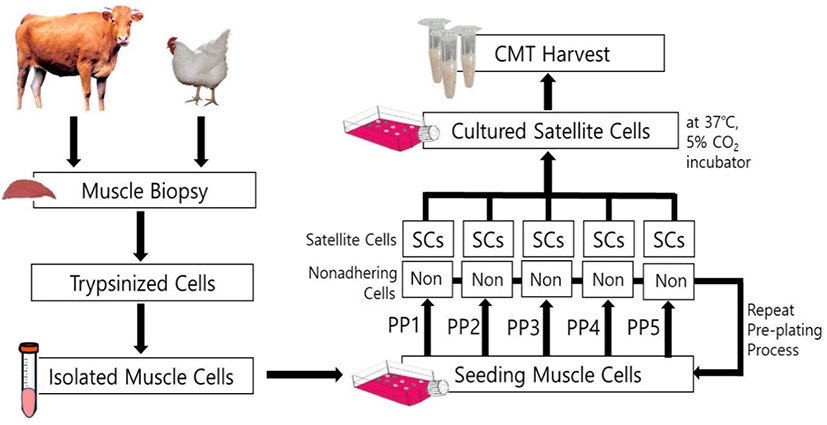
The flow diagram of the cultured muscle satellite cell is shown in Fig. 1. The muscle satellite cells were separated by the pre-plating method utilizing the difference between the cell adhesion rate and the growth rate. The cell pellet obtained in the previous step was re-suspended in PM, and plated onto a cell culture dish that was coated with 0.2% gelatin (#G1393, Sigma-Aldrich). The cell culture dish was incubated at 37°C in the presence of 5% CO2 for 1 h (pre-plating 1, PP1). The fibroblasts quickly adhered to the bottom of the cell culture flask, while the muscle satellite cells remained in the supernatant. The supernatant containing muscle satellite cells was collected in a 15 mL centrifuge tube and centrifuged for 10 min at 500×g. The muscle satellite cell pellet was re-suspended with PM and plated onto a cell culture dish and incubated at 37°C with 5% CO2 for 2 h (pre-plating 2, PP2). The supernatant and non-attached cell suspensions were recovered, centrifuged again, and only the cell pellets were cultured for 24 h (PP3). This pre-plating process was repeated up to PP5 to isolate as pure muscle satellite cells as possible, and all the cells from steps PP1 to PP5 were cultured in PM.
The isolated muscle satellite cells were directly plated onto T75 flasks at a density of 104 cells/mL (approximately 106 cells/flask), and cells of 3 passages were cultured in a 37°C, 5% CO2 incubator for 2 wks. The chicken culture cells were allowed to proliferate for 3 days and the cattle culture cells for 4 days before trypsinization with 0.05% trypsin-EDTA. The cells were grown using PM and the cultures were replaced with fresh PM every 2 days. When the cells reached 80% confluence, PM was replaced with DM (DMEM containing 2% horse serum) for differentiation and the DM was changed daily. After the cells were cultured for 2 weeks, the CMT containing myotubes and/or myofibers was harvested from the flask bottom using a cell scraper. The CMT was washed with a 10% PBS solution and centrifuged for 10 min at 500×g. The CMT obtained by repeating this PBS washing process three times was analyzed for nucleotide-related compounds, amino acid composition, and taste characteristics using an electronic tongue system.
Nucleotide-related compounds including adenosine-5'-monophosphate (AMP), inosine-5'-monophosphate (IMP), inosine, and hypoxanthine were determined using high-performance liquid chromatography (HPLC) by the method of Ismail et al. (2020a). Briefly, muscle tissues (1 g) were minced and homogenized with 20 mL of cold 0.5 M perchloric acid, and the homogenate was centrifuged at 3,000×g for 10 min at 4°C. The supernatant was filtered through Whatman filter paper No. 1, and the residual was mixed with 10 mL of 0.5 M perchloric acid. The filtrate was neutralized to pH 6.0 with 5 N potassium hydroxide (KOH) and centrifuged at 3,000×g for 10 min at 4°C. The pH-adjusted supernatant was made up to a volume of 50 mL by adding 0.5 M perchloric acid (pH 6.0 adjusted with 5 N KOH). The solution was filtered through a 0.45 μm polytetrafluoroethylene syringe filter and stored at –25°C prior to analysis.
Analysis of the nucleotides was done using an HPLC system (Hewlett-Packard HPLC system series 1100, Waldbronn, Germany) with an Eclipse Plus C18 column (4.6×100 mm, 3.5 μm). A 10-μL sample was injected onto the C18 column (4.6×250 mm, 5 μM particle size, AcclaimTM 120; Thermo Fisher Scientific) maintained at 25°C from which the 4 compounds were separated by gradient elution. Mobile phase A consisted of 0.06 M dipotassium hydrogen phosphate and 0.04 M potassium dihydrogen phosphate at pH 7.0. Mobile phase B was a mixture of HPLC-grade water and methanol (20:80 v/v). The flow rate (0.8 mL/min) was consistent throughout the analysis and the elution program was as follows: A mixture of water and methanol (1:1 v/v) was injected and each injection was followed by an injection for a needle-wash program. Nucleotide-related compounds in the samples were identified by comparing their retention time to that of AMP, IMP, inosine, and hypoxanthine standards (Sigma-Aldrich). The concentrations were quantified based on the calibration curves and the data are presented as mmol/kg of the samples.
The amino acid content of the muscle tissues was determined using a Biochrom30 plus (Biochrom, Cambridge, UK) amino acid analyzer. Briefly, approximately 1 g of muscle tissue was minced and homogenized using a homogenizer at 12,000 rpm for 15 s. Then, the homogenate was transferred to a glass bottle, and 10 mL of 6 M HCl was added. After filling with nitrogen, the mixture was hydrolyzed at 110°C for 24 h. The hydrolysate was transferred to a 50-mL volumetric flask and diluted with sodium phosphate buffer in a calibrated tube. The solution was filtered into an auto-sampler vial using a 0.22 μm membrane filter.
An electronic tongue system (INSENT SA402B electric taste sensing system, INSENT, Tokyo, Japan) was used to determine the taste characteristics of the muscle tissue (Hwang et al., 2018). The system was composed of 5 taste sensors and each sensor was attached to a typical artificial lipid membrane. The sensors were named CA0 (to detect sour substances), C00 (to detect bitter substances), AE1 (to detect astringent substances), AAE (to detect umami substances), and CT0 (to detect saltiness substances). All the sensors were pre-conditioned in the reference solution for one day before the measurements.
Each muscle tissue sample was measured after the electric potentials of all the membranes had been stabilized in a standard meat taste (SMT) solution. A synthetic solution containing 0.005% lactic acid (sourness), 0.4% monosodium glutamate (umami), 0.001% quinine hydrochloride (bitterness), 0.05% sodium chloride (saltiness), and 0.8% sucrose (sweetness) was used as the SMT solution. Sample solutions were prepared by extracting 1 g of minced muscle tissue, mixing it in 100 mL of hot distilled water at 95°C, and stirring for 10 min before centrifuging at 3,000×g. After centrifugation, the supernatant was filtered through No. 1 filter paper (Whatman, Little Chalfont, UK) and was ready for analysis by the electronic tongue. All the measurements were made at a room temperature of 28°C.
All assays were performed in triplicate. The results of the chemical analyses and taste evaluations were recorded as the mean and SEM. Statistical analyses were performed by analysis of variance (ANOVA) using SPSS (SPSS 16.0, Chicago, IL, USA), and the differences between the mean values were obtained by Duncan’s multiple range test. Significance was defined as p<0.05.
Results and Discussion
The composition of amino acids in CMT derived from chicken and cattle and TM is summarized in Table 1. There was a significant difference in the composition of all amino acids (in percentage) except valine and tyrosine between CMT and TM (p<0.05). Both chicken and cattle CMT had higher amounts of serine, proline, alanine, and arginine than TM, whereas TM showed a higher content of aspartic acid, isoleucine, leucine and lysine (p<0.05). There was no significant difference in the amount of threonine, cysteine, phenylalanine, and histidine between CMT and TM from chicken, but the amounts in CMT were lower than in TM from cattle (p<0.05). Notably, the amount of glutamic acid was not significantly different between CMT and TM from cattle, but that of CMT was lower than TM from chicken (p<0.05). In addition, there were differences in the amino acid composition between chicken CMT and cattle CMT. The amount of aspartic acid, threonine, isoleucine, phenylalanine, and lysine was higher in chicken CMT, whereas the amount of alanine, cysteine, methionine, and arginine was higher in cattle CMT (p<0.05).
The amino acid composition of meat is important because it determines not only the nutritional quality but also taste, especially taste sensations. Amino acids are known to elicit complex tastes, and the overall taste of amino acids was found to be a combination of basic tastes, such as sourness, sweetness, saltiness, bitterness, and umami (Kawai et al., 2012). Consequently, during the production of cultured meat with similar taste attributes, the amino acid composition of TM must be simulated. In particular, since the umami attribute of meat is important for good taste, simulating glutamic acid and aspartic acid, which are known to be closely correlated to umami is important (Fuke and Konosu, 1991; Kawai et al., 2002). The current findings in cattle showed that the glutamic acid content in CMT was within the normal range of that of TM with no significant difference. However, since the aspartic acid content of CMT is lower than that of TM, it is considered necessary to find a method to increase the ratio of aspartic acid when culturing cattle satellite cells. In the case of chicken, the amount of aspartic acid and glutamic acid in CMT was significantly lower than that of TM, so when culturing chicken satellite cells, it is necessary to find a way to increase the proportions of glutamic acid and aspartic acid.
Meat sweetness is related to glycine, alanine, threonine, proline, and serine whereas bitterness is related to isoleucine, leucine, cysteine, lysine, tyrosine, phenylalanine, valine, arginine, methionine, and histidine (Kawai et al., 2012). In this study, the significantly higher amounts of serine, proline, glycine, and alanine in CMT than in TM suggest that CMT may be sweeter than TM. In addition, the results showed significantly lower amounts of isoleucine, leucine, phenylalanine, histidine, and lysine in CMT than in TM, indicating that CMT is more bitter than TM. Therefore, to produce cultured meat that is similar to TM, satellite cells should be cultured in a culture medium that can increase bitterness and reduce the sweetness. Unless special amino acids are added to the culture medium and taken up by the satellite cells, the amino acid composition of cultured meat will not be similar to that of TM, and eventually, the cultured meat will exhibit a different taste sensation compared to TM.
The difference in the nucleotide-related compound content of CMT and TM derived from chicken and cattle is shown in Fig. 2. Among the nucleotide-related compounds, only a considerable of IMP was detected, whereas AMP, inosine, and hypoxanthine were measured in trace amounts. In particular, these contents were less than 0.2 mmol/kg in CMT. The IMP content was 0.81 and 1.98 mmol/kg for chicken CMT and cattle CMT, respectively, and there was no significant difference between the two CMTs (p>0.05). However, the IMP contents of the CMTs derived from chicken and cattle were significantly lower than those of TM, and the IMP content of chicken TM was significantly higher than that of cattle TM (p<0.05).
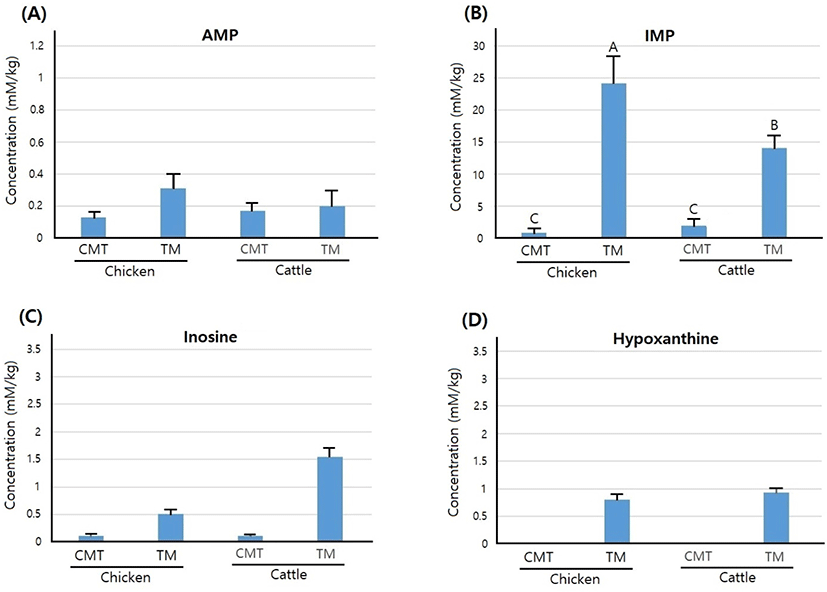
IMP contributes to several meat taste characteristics including delicious, umami, and brothy taste (Fuke and Konosu, 1991; Kawai et al., 2002; Shi et al., 2017). The sensory properties of the meat IMP content, which is directly proportional to umami taste, is considered an important contributor to umami sensation (Fuke and Konosu, 1991). The IMP content, which can positively affect meat preference, is released from the meats in the early stages of moist cooking and the amounts vary depending upon the species or the type of livestock (Okumura et al., 1996; Sasaki et al., 2005; Sasaki et al., 2007). The IMP content may differ even among the muscles of a livestock individual due to differences in muscle fiber composition, which, in turn, affects meat taste (Chikuni et al., 2010; Hwang et al., 2018). The present study showed that the IMP content of CMT was significantly lower than that of TM from both chicken and cattle. Therefore, it is expected that the umami intensity of CMT was weaker than that of TM due to the lower concentration of IMP.
In normal postmortem muscles, ATP is hydrolyzed to ADP, followed by ADP conversion to AMP and AMP to IMP by the action of several enzymes such as phosphoglycerate kinase, adenylate kinase, and AMP deaminase (Pearson and Young, 1989). Therefore, it is assumed that the low IMP concentration in CMT was because the cultured muscle fibers did not yet have enough ATP or enzymes for postmortem metabolism. Due to the limited studies available, more trials on CMT with similar umami concentration as in TM are needed.
The application of an electronic tongue system has been tested previously and successfully employed in testing the quality of meat and meat analogs (Hwang et al., 2020; Ismail et al., 2020a; Sabikun et al., 2021; Zhang et al., 2015). In this study, the CMT and TM umami, bitterness, sourness, astringency, richness, and saltiness were investigated, as shown in Fig. 3. When the relative intensity values of the taste components were computed from the SMT solution, both CMT and TM showed positive values for bitterness and richness, but negative values for sourness and saltiness. In addition, in both chicken and cattle CMTs, the umami values were positive, whereas that of TM was negative, and the astringency values showed the opposite tendency as the umami values. As a result, there were significant differences in umami, bitterness, sourness, and astringency between CMT and TM (p<0.05). The umami, bitterness, and sourness values of CMT were significantly lower than those of TM from both chicken and cattle, whereas the astringency value was higher in CMT compared to TM (p< 0.05). However, there was no significant difference in saltiness between CMT and TM (p> 0.05).
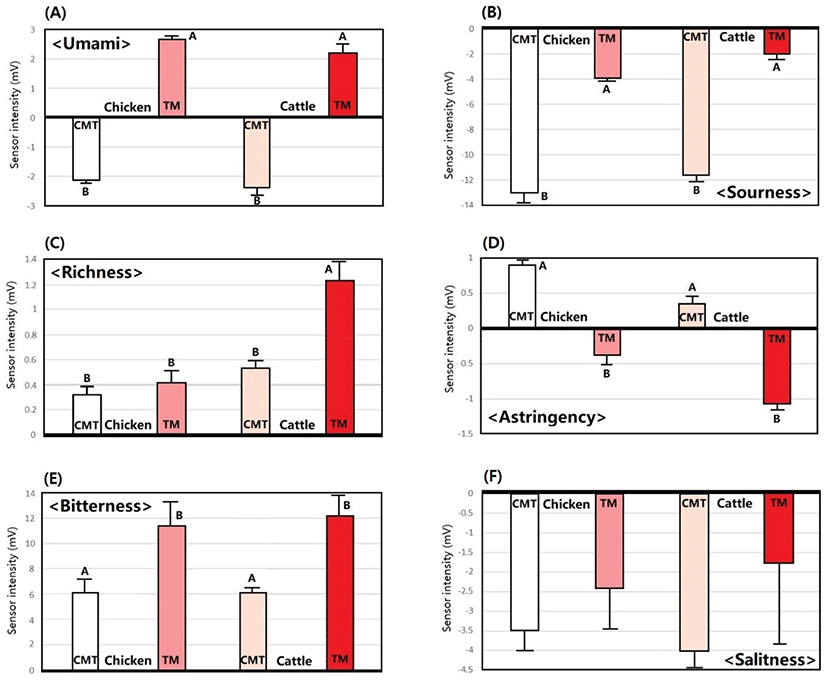
The lower umami value of CMT was expected because CMT showed a lower content of glutamic acid and aspartic acid, and a lower concentration of IMP compared to TM (Table 1 and Fig. 2). Because a strong umami synergistic interaction occurs between glutamic and aspartic acid and IMP (Kawai et al., 2002), the high glutamic acid, aspartic acid, and IMP content of TM would have increased the umami intensity in TM. The intensity of meat flavor was highly correlated with the bitterness value in the electronic tongue system (Ismail et al., 2020a), and TM had a higher bitterness value than that of CMT probably because the muscle fiber differentiation in TM is more mature and contains other tissues, such as blood vessels and connective tissues. In addition, CM was found to have a weaker sour taste than TM, which may be the result of less lactic acid accumulation because CMT had less postmortem energy metabolism than TM. Consequently, cattle TM showed higher richness and umami and lower astringency intensity than CMT. However, the richness of chicken TM was not different from that of CMT, suggesting that the richness values were strongly affected by mature muscle fiber differentiation or the ratio of red muscle fiber in the muscles.
Since cultured meat myoblasts are essentially the same cells as those that produce livestock muscle, it is possible to produce a biochemical composition in cultured meat tissue that is similar to that of TM. If so, the taste should be the same. However, the results of this study showed that in CMT, the differentiation to red muscle fiber was lower, so its taste characteristics were significantly different from those of TM. In particular, the weak umami and bitterness, which are important factors in perceiving deliciousness in CMT, should be improved in cultured meat products in the future. Perhaps the control of the composition of the culture medium to one that can promote the differentiation into red muscle fibers or the development of culture processes such as electrical stimulation would help to improve the taste of cultured meat. Supplementation with additives capable of enhancing umami or bitterness during the process of manufacturing FAM products using CMT would also be a viable method.
Conclusion
The composition of amino acid and nucleotide-related compounds, and taste characteristics of CMT obtained by culturing satellite cells isolated from chicken and cattle were investigated and compared to those of TM. CMT showed lower glutamic acid, aspartic acid, and IMP contents than TM, which were closely related to the umami taste of meat. The intensity of umami and bitterness assessed by the electronic tongue system was also weaker in CMT than in TM. The results of this study suggest that it is necessary to adjust the composition of the growth medium to a composition that could increase the umami intensity and the bitterness of CMT and develop a culture method of satellite cells that could produce cultured meat with a taste similar to that of traditional meat.