Introduction
Bullfrogs (Rana catesbeiana) have been commercially reared in China since the 1950s. In 2019, the production of bullfrog meat in China was 500,000 tons. Bullfrog meat is very popular, for its unique flavour and texture. Cooked bullfrog meat is soft in texture and lightly sweet in flavour (Ramos et al., 2005). Some studies have been carried out on bullfrog meat, including characterization of the volatile constituents, controlling microbial spoilage (Silva et al., 2015), and the colour changes after different slaughter methods (Ramos et al., 2005).
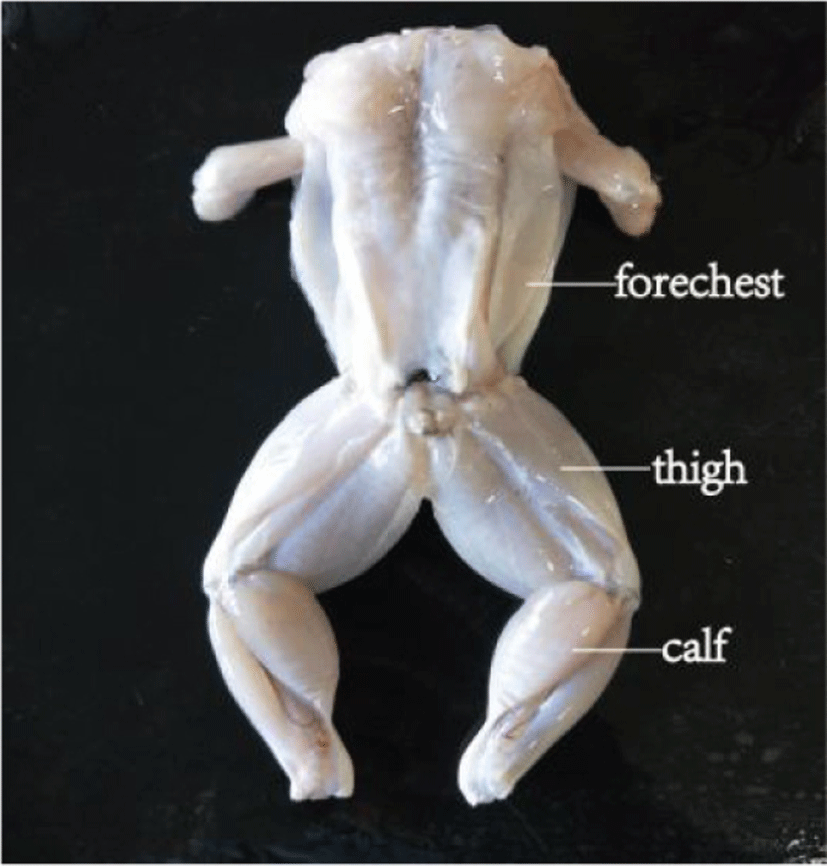
Although production and consumption of bullfrog meat have been growing rapidly, information about its physico-chemical properties is limited. Composition and water loss during cooking are the most important physico-chemical properties of meat, because these factors determine the intrinsic quality attributes and functional properties of the meat (Ssepuuya et al., 2019). It is widely accepted that the composition and cooking loss of meats varies between different body parts (Černíková et al., 2015; Skonberg and Perkins, 2002). The thigh, calf and back meat in bullfrog are the major edible parts (Nóbrega et al., 2007). The aim of this study was to compare the composition (moisture, protein, fat, minerals, etc.) in three types of bullfrog meat (thigh, calf, and fore-chest). These results will provide bullfrog meat producers and consumers with new and useful information about its nutritional value and processing characteristics.
Materials and Methods
All the chemicals and solvents used were of analytical reagent grade or better. Copper sulphate, potassium sulfate, sodium hydroxide, absolute ethanol, absolute methanol, acetic acid, hydrochloric acid and potassium chloride were from Beijing Chemical Works (Beijing, China). Ethylenediaminetetraacetic acid (EDTA) was from Amresco (Solon, OH, USA). Tris, sodium dodecyl sulphate (SDS) and glycine were from Solarbio (Solarbio Technology, Beijing, China). β-mercaptoethanol (β-ME) was from Sigma-Aldrich (St. Louis, MO, USA). BCA Protein Assay Kits were from Thermo Fisher Scientific (Waltham, MA, USA).
The aquaculture area (Beijing Jinlai Yongfa Aquatic Products) for bullfrogs was approximately 10 acres, divided into shallow ponds 32 m2 in area. The ponds were surrounded by bamboo poles and nylon nets. Tadpoles were introduced into the ponds in March, at a stocking-density of 180 /m2 and took 80 days to grow into froglets; the average water temperature was 22°C during this time. When tadpoles had grown into froglets, the stocking density was changed to 140 /m2. The bullfrogs were ready to eat after 260 days and those selected for this study (n=21), had an average weight of 250 g. After slaughter and dissection (slaughtered in Hengwang, Jining, China), the by-products (head, viscera, skin, front, and back toes) were discarded and the edible parts divided into fore-chest, thigh, calf, and trunk (Fig. 1). Fore-chest meat from seven bullfrogs was combined, washed twice with chilled deionized water, then drained and minced for further experimentation (Bones have been removed). Thigh and calf meat were treated in the same way.
The combined batches of fore-chest, thigh and calf meat and combined trunk meat were weighed and compared. The average proportion of each type of meat was calculated as a percentage.
The meat samples were subjected to proximate analysis, including moisture, protein, fat and ash contents, following the relevant international standards. Moisture content was determined by oven (DHG-9076A, Jinghong Experimental Equipment, Shanghai, China) drying of 2.5 g of sample at 105°C to constant weight, according to AOAC method 950.46 (AOAC, 2007). The protein content was estimated from the nitrogen content, using the standard Kjeldahl procedure in AOAC method 981.10 (AOAC, 2007). Total crude fat was analyzed by fat extraction using a Soxhlet extraction instrument, with anhydrous diethyl ether as the non-polar solvent, according to AOAC method 960.39 (AOAC, 2007). Ash content was determined by combustion to constant weight at 550°C, according to AOAC method 938.08 (AOAC, 2007).
Amino acid composition was determined by hydrolyzing the samples in 6 M hydrochloric acid at 110°C for 22 h, in an evacuated sealed ampoule. Excess acid was removed by flash evaporation under reduced pressure (Usydus et al., 2009). The amino acid composition was determined by the external standard method, using an amino acid analyzer (L-8500A, Hitachi, Ibaraki, Japan).
Contents of K, P, Na, Mg, Ca, Zn, Fe, Cu, and Mn were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES), using an iCAP 6300 instrument (Thermo Fisher Scientific), according to AOAC method 969.23 (AOAC, 2007). The samples (0.5 g) were weighed into nitric acid-perchloric acid (10:1) mixed solution and heated using an electric hot plate until smoke was produced and the solutions became colourless, transparent, or slightly yellow. The digested samples were transferred to a volumetric flask, the volume was made up to 25 mL with deionized water and the solutions analyzed by ICP-OES. The concentration of mineral was calculated and expressed as mg/kg sample.
The different protein components were extracted and separated as described previously (Saito et al., 1983), with some modifications. All protein extractions were performed on samples cooled on ice and all solutions used were maintained at approximately 4°C. Briefly, the minced meat (20 g) was added to 100 mL of low salt buffer (0.05 M KCl, 20 mM Tris-HCl, pH 7.5) and homogenized at 5,054×g for 2 min using an Ultra-Turrax T25 homogenizer (IKA, Staufen, Germany). After standing at 4°C for 90 min, the samples were centrifuged at 11,962×g for 20 min, using a refrigerated centrifuge (LYNX4000, Thermo Fisher Scientific). The supernatant (water-soluble protein) was stored at 4°C until needed. The precipitates were washed twice with low salt buffer. Subsequently, the pellets were extracted for 18 h at 4°C after homogenization at 5,054×g for 2 min with 100 mL of high salt buffer (0.6 M KCl, 20 mM Tris-HCl, pH 7.5), followed by centrifugation at 11,962×g and 4°C for 20 min. The supernatant (salt-soluble protein) was stored at 4°C. The precipitates were washed twice with high salt buffer. The final precipitate (insoluble protein) was collected and stored at 4°C.
Desalting was performed as described previously (Tian and Shi, 2019). In brief, dialysis tubing (MWCO 3500, MD77; Solarbio, China) was boiled in water with 2% NaHCO3 and 1 mM EDTA. 2Na for 10 min, washed with distilled water, then boiled in water with 1 mM EDTA. 2Na for 10 min and rinsed thoroughly with pure water. The extracted and separated protein solutions were added to the dialysis bags, so that they were no more than half-full. Then the dialysis bags were put into chilled pure water for two days. During the dialysis period, 1% AgNO3 was used to detect chloride ions and the dialysate was replaced until no white precipitate was produced. The desalted proteins were freeze-dried for further experimentation.
The protein composition of the different bullfrog meat types was determined by SDS-PAGE, using a 4% stacking gel and a 12% running gel (Nakajima et al., 2020). The water-soluble, salt-soluble and insoluble proteins were diluted to 1.5 mg/mL with low salt buffer, high salt buffer and 6 M urea, respectively. The protein concentration was determined using a BCA Protein Assay Kit. To denature the protein, 5× loading buffer (400 μL) was added to each protein sample (100 μL), vortex mixed and heated in boiling water for 5–10 min. After centrifugation at 4,000×g for 5 min, the supernatants were collected for further analysis. Marker (5 μL) and sample protein (20 μL) were loaded onto the gel and subjected to electrophoresis at a constant voltage of 80 V. When the bromophenol blue band ran out of the end of the stacking gel, the voltage was adjusted to 120 V. After separation, the gels were stained with 0.25% (w/v) Coomassie Brilliant Blue R-250 in 45.4% (v/v) methanol and 9.2% (v/v) acetic acid for 15–20 min and destained with 5% (v/v) methanol and 7.5% (v/v) acetic acid; the destaining solution was replaced every 30 minutes for the first 2 hours, until the protein bands were clearly visible. Subsequently the gels were scanned with an Automatic chemiluminescence gel imager and analyzed by Quantity One software (Bio-Rad, Hercules, CA, USA).
Results
The weight ratio between bullfrog fore-chest, thigh, calf, and trunk was determined (Table 1). The relative proportions of fore-chest, thigh, calf, and trunk meat were 12.85%, 40.82%, 19.17%, and 27.17%, respectively, i.e., thigh was the main component, followed by trunk, calf and fore-chest. Trunk was the second most abundant part, but contained relatively little meat, therefore thigh, calf and fore-chest were the main edible meats available from bullfrog and subsequent experiments were performed to determine their physico-chemical properties.
| Position | Fore-chest | Thigh | Calf | Trunk |
|---|---|---|---|---|
| Ratio | 12.85±1.15d | 40.82±1.63a | 19.17±1.10c | 27.17±1.88b |
The proximate compositions (moisture, total proteins, crude fats, and ash) of the different bullfrog meats were determined (Table 2). The ranges found were: moisture, 76.88%–83.43%; total proteins, 15.32%–21.17%; crude fats, 0.62%–1.44%; ash 0.64%–0.85%. Compared with poultry, mutton and beef, bullfrog meat has lower fat content (Chen et al., 2016). The lowest moisture content (76.88%) and the highest fat content (1.44%) were determined in thigh meat (p<0.05). The highest moisture content (83.43%) and the lowest fat content (0.62%) were found in fore-chest meat (p<0.05). The highest protein content (21.17%) in comparison with the other parts of the bullfrog body (p<0.05) was found in thigh meat. There were significant differences in the proximate composition of different parts of bullfrog meat. The findings concerning proximate composition of thigh and calf meat are in agreement with previous reports (Nóbrega et al., 2007; Tian and Shi, 2019; Zhang et al., 2007) who also found low fat content and high protein content. However, only a general comparison is possible, because the previous studies combined meat from the whole leg, rather than separating meat from thigh and calf, as in this study. In addition, the fat content in thigh found in our study was slightly higher than other studies, which may be related to sampling seasons, regional, individual size, etc.
| Parameters | Fore-chest | Thigh | Calf |
|---|---|---|---|
| Moisture | 83.43±1.05a | 76.88±1.64c | 78.43±1.11b |
| Protein | 15.32±1.08c | 21.17±1.72a | 17.66±0.98b |
| Fat | 0.62±0.08c | 1.44±0.13a | 0.98±0.11b |
| Ash | 0.64±0.07b | 0.76±0.10a | 0.85±0.13a |
The high water content of bullfrog meat made it more tender and softer than other meat. Inaddition, the fat content in bullfrog meat was low, especially in fore-chest meat which was a good choice for people who require low fat intake but high protein intake.
In fore-chest meat, the content of insoluble protein was the highest (55.59%), followed by the water-soluble protein (25.31%) and the salt-soluble protein (19.12%) (p<0.05) (Table 3). In thigh meat, the salt-soluble protein fraction was the most abundant, comprising approximately 54.72% of the protein, followed by the water-soluble and insoluble protein fractions in decreasing order. The contents of the protein fractions in calf were similar to that of fore-chest meat, with the highest insoluble protein content, followed by water-soluble protein and salt-soluble protein, accounting for 57.02%, 22.35%, and 20.64% of the total protein, respectively. There was no significant difference between the protein fractions of fore-chest and calf meat, but the proportions of salt-soluble and insoluble proteins in thigh meat were significantly different from those of fore-chest and calf meat. The insoluble protein was mainly collagen and elastin. The salt-soluble protein is a major contributor to the gel strength of meat products (Rabiey and Britten, 2009) and may provide different texture characteristics to different parts of bullfrog meat. The water-soluble, salt-soluble and insoluble protein contents of bullfrog whole leg meat were 10.21%, 25.68%, and 64.11%, respectively (Tian and Shi, 2019), similar to the results of calf meat in this study. In addition, a s for some shellfish and fish proteins, the major component of thigh meat was salt-soluble protein (Liu et al., 2012; Zheng et al., 2012).
The total amino acid content of the different bullfrog meat parts were in the range of 15.30%–19.41% (Table 4) and were consistent with the crude protein content (Table 2). The most abundant amino acids were glutamic acid, aspartic acid and lysine, which are generally the most abundant amino acids found in aquatic organisms (Özden and Erkan, 2011). In addition, aspartic and glutamic acid are abundant in other kinds of meat, such as beef, pork, poultry, eland and/or foal (Bartoň et al., 2014; Lorenzo and Pateiro, 2013; Okrouhlá et al., 2018). Lysine is an essential amino acid which is limiting in many foods (Friedman, 1996) and another essential amino acid, leucine, was abundant in bullfrog meat. The concentrations of most amino acids were similar in thigh and calf and slightly higher than those in fore-chest. Thigh meat was richer in essential amino acids when compared with the fore-chest and calf muscle. Amino acids are the most important taste compounds in food, and bullfrog meat is favoured for its unique flavour. All three parts of bullfrog meat contained high levels of flavour amino acids, all of which were above 42%. The glycine content of bullfrog is lower than beef and chicken, and the alanine content is close to chicken, but higher than beef. Branched-chain amino acids have unique physiological functions that can eliminate or reduce the symptoms of hepatic encephalopathy and improve nutritional status and fatigue disorders (Kawamura-Yasui et al., 1999). Branched-chain amino acid contents were similar in the fore-chest, thigh and calf (18.61%, 19.04%, and 18.98%, respectively).
Protein patterns of salt-soluble protein, water-soluble protein and insoluble protein fractions from bullfrog meat were analyzed by SDS-PAGE (Fig. 2). The molecular weights (MWs) of the major salt-soluble protein bands were in the 17–200 kDa range, consisting of eight polypeptides (200, 100, 75–63, 42, 42–35, 35, 20, 20–17 kDa), with the 35 kDa band being the most abundant. The protein patterns of the fore-chest and thigh meat were similar, whereas those of calf meat were slightly different, including bands at 200, 135, 135–100, 42, 42–35, 35, 20, and 43 kDa. The salt-soluble protein fraction mainly consisted of the myofibrillar proteins, which were composed of myosin heavy chain (MHC), light chain, actin and α-actinin, among other proteins. MHC and actin appeared at 200 and 42 kDa, respectively (Zheng et al., 2012). The protein band with a MW of 100 kDa may be paramyosin, which is found in shellfish muscle (Katayama et al., 2002). The absence of bands at 200 and 20 kDa, in the calf samples, may result from degradation of myofibrillar proteins. As the salt concentration in the extracting solution increased, the salt ions may bind to oppositely charged amino acid residues, which would result in disruption of intra- and/or inter-molecular ionic bonding. The degradation of myofibrillar proteins was likely to occur because they are susceptible to unfolding and denaturation, which may lead to the loss of MHC (Lin and Park, 1996).
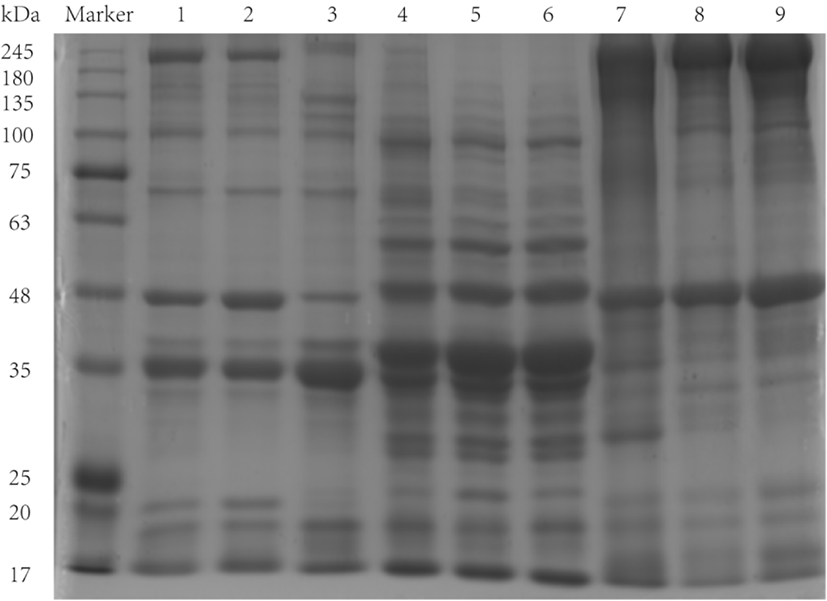
The water-soluble protein bands were mainly distributed between 100 and 17 kDa, with the bands at 48–35 kDa dominant; the bands were continuous, indicating that the water-soluble protein fraction was composed of many proteins with similar MWs. The MW of insoluble protein was mainly distributed between 245 and 17 kDa. Proteins with MWs of 245–180 and 42 kDa predominated in the insoluble protein fraction. There were differences in protein bands between different parts of bullfrog meat. The fore-chest had major dense bands at 35 and 35–25 kDa, whereas thigh and calf did not have any especially dense bands.
The mineral contents in different parts of bullfrog meat were showed in Table 5. The main components were K, P, Na, Mg and Ca, Zn, Fe, Cu and Mn wereminor components. In all samples, potassium was the most abundant mineral, followed by phosphorus, sodium, magnesium and calcium. There were also differences between meat from the different parts of the animal. Fore-chest meat had lower potassium, phosphorus and magnesium contents, compared with thigh meatand calf meat (p<0.05). Thigh meat and calf meat had lower contents of sodium, calcium and zinc than fore-chest meat (p<0.05). Minerals are an important part of normal life processes, for all animals. Potassium contributes to the intracellular ion-balance, sodium constitutes the main extracellular cation, assuming an important role in acid-base balances. Phosphorus is involved in phospholipids andphosphoproteins (Mahmoud et al., 2008; Matsubara et al., 2003). Zinc, iron, copper, and manganese are essential cofactors for various metalloenzymes (Kawamura-Yasui et al., 1999). Divalent metal ions have pro-oxidant activity (Thanonkaew et al., 2006). Similar phosphorus contents were found previously in bullfrog legs (Han et al., 1991).
Conclusion
The results of this study showed that there were differences in nutritional components in different parts of bullfrog meat. The moisture content of fore-chest meat was higher than other parts of the bullfrog body, and the fat conent was the lowest. There was no significant difference in mineral content between thigh meat and calf meat. In addition, the protein and the essential amino acid content of thigh meat were higher than other parts. The insoluble protein content in the fore-chest meat was higher than that in the calf meat and thigh meat, while the salt-soluble protein content in the calf meat was the highest, which may lead to different texture structures in different parts of bullfrog meat, thus affecting the taste, which will need further study. In summary, this paper will provide important information on the nutritional components of the three parts of bullfrog meat (thigh, calf and chest), and can provide new useful information on the nutritional quality of bullfrog meat for meat producers and consumers.