Introduction
Yoghurt made by the starter culture that mainly composed of Stretococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (hereafter L. bulgaricus) is considered as a safe and healthy food (Ashraf and Shah, 2011). It is popularly consumed throughout the world, because of except for the health benefit of the live microorganisms, its favorable flavors and abundant nutrients are important factors for consumer acceptability (Zare et al., 2011). In general, maintaining a high lactic acid bacteria (LAB) survival counts in the industrial processes and products is important, which are beneficial to progression of fermentation and are of industrial interest for yoghurt fermentation. There are plenty of studies proved that microbial growth during fermentation can be greatly influenced by the addition of food ingredients. Zare et al. (2011) investigated the impact of lentil flour on some characteristics of the yoghurt and found that high ration of lentil flour would increase the counts of LAB, improve the physical and rheological properties and enhance the sensory properties. Ye et al. (2012) found that the addition of hawk tea in yoghurt would significantly increase the number of LAB and some characteristic volatile flavor compounds. Cruz et al. (2012) explored the effect of glucose oxidase on postacidification, survival of probiotic microorganisms, productions of aroma compounds in yoghurt.
In China, pork owns the largest share of meat protein consumption (Zhang et al., 2014). As the analyses of the National Health and Nutrition Examination Survey conducted in the United States found that fresh pork is a good source of dietary protein, some essential vitamins and minerals including phosphorous, potassium, zinc, selenium, riboflavin, niacin, thiamine, pyridoxine (vitamin B6), and cobalamin (vitamin B12) (Murphy et al., 2011). Murphy et al. (2012) showed that regular pork consumption improved body composition and had no change in a selection cardiovascular of risk factors. Furthermore, Nolan-Clark et al. (2013) found that pork stimulated the secretion of the gut hormone peptide YY which increases the feeling of fullness, thus pork is beneficial in the maintenance of healthy weight compared with other protein sources.
In this study, proteolytic pork hydrolysate (PPH) was added to milk to investigate the effects of PPH on yoghurt production. The fermentation time, the viable cell counts, the flavor, the free amino acids compounds, and the sensory evaluation of yoghurt were evaluated.
Material and Methods
Fresh lean pork (Sus domesticus, Wal-Mart supermarket, China) was cut into pieces and dealt with colloidal mill, then added deionized water with a rate of 4:1 (w/w) and incubated at 95℃ for 10 min. After cooled, the protease (extracted from stomach of pig, enzyme activity 50,000 U/g, supplemented with 10,000 U per 100 g broth, Shanghai Jinsui bio-technology Co., Ltd, China) was added and incubated at 45℃ for 6 h, and then heated at 95℃ for 10 min again to denature the protease. The partial chemical components of the PPH, including minerals, vitamins and peptides were analyzed by Qingdao Scistd Testing research institute Co., Ltd, China, using atomic absorption spectrophotometry and high performance liquid chromatography (HPLC) (Gentili et al., 2013; Jon, 1980; Tzong-Hsien et al., 2008), respectively
The fermented milk was prepared according to Lucey (2004) with slight modifications. PPH [4.1% (w/v) protein, 1.2% (w/v) Fat] was added into fresh cow milk with 0%, 1%, 5% and 10% (w/w), and skim milk power (34.0% protein, 0.8% fat) and anhydrous milkfat (99.8% fat), respectively, were added into the samples to standardize the levels of protein and fat. The four samples were sheared by a high shear emulsifier (FM300, Fluko, Germany) at 10,000 rpm for 15 min at 40℃, then all the samples (each sample of two replicates) were homogenized under high pressure (20 MPa) at 65℃ and pasteurized by high-temperature short-time (HTST) at 95℃ for 5 min immediately. After cooled to 42℃, all samples were inoculated with 0.05% (w/v) YB 0925-B yoghurt starter culture (Danisco, Denmark). A set of samples were maintained at 37℃ in an MIR-253 incubator (Sanyo, Japan). Another set of samples were monitored automatically (recorded every 5 min) for pH using a Cinac system (Alliance Instruments, France), and the corresponding sample were removed to terminate fermentation until a pH of 4.50±0.03 (the pH end point) was reached. After stirred at 500 rpm for 5 min, they were stored at 4℃.
A standard plate count was used to enumerate viable microbiological cells according to Settachaimongkon et al. (2014) with slight modifications. L. bulgaricus and S. thermophilus were grown in de Man, Rogosa, and Sharpe (MRS) medium (Merck, Germany) and M17 medium (Oxoid, UK) supplemented with 0.5% (w/v) lactose (LM 17) at 42℃, respectively. Yoghurt samples were serially diluted using sterilized normal saline to achieve 30 to 300 colonies on proper agar plates, and then cultured under anaerobic conditions (Bugbox Anaerobic System, Ruskinn, UK) with a mixture of 95% (v/v) N2 and 5% CO2 at 37℃ for 48 h.
The yoghurt samples were pre-treated to remove proteins, and free amino acids were extracted as described by Simova et al. (2006). The amino acid content of the samples was analysed using HPLC fitted with a sodium cation exchange amino acid analysis column (4×150 mm, Pickering, USA) and an o-phthaladehyde post-column derivation system (Pickering, USA). The equipment was coupled with a Waters 510 pump, a 7725i manual injector and a 363-fluorescence detector (Varian Inc, USA). The operating conditions used a flow rate of 1.7 mL/min. Elution was performed by applying a linear gradient of 100% solution A over 1 min, followed by 0-100% solution B over the subsequent 48 min (solution A, 0.2 mol/L sodium citrate, pH = 3.0; solution B, 0.2 mol/L sodium borate, pH = 9.8). The excitation and emission wavelengths were 338 nm and 425 nm, respectively.
Headspace solid-phase micro-extraction (SPME) was used to extract the volatile compounds of the yoghurt samples according to Ye et al. (2012). The fiber used for manual extraction was DVB/CAR/PDMS (Supelco, USA).
The samples were prepared for extraction by weighting 10 g of yoghurt into a 20 mL crimp-seal sample vial. The vials were sealed with an aluminum crimp-seal containing a poly-tetrafluoroethylene silicone septum, and then were placed in a 60℃ stirring water bath for 30 min. Then the prepared fiber was inserted through the septum and fully exposed to the headspace for 15 min. The exposed fiber was removed and inserted into the gas chromatography-mass spectrometer (GC-MS) where the volatiles were thermally desorbed.
Analysis of the volatile compounds was performed using an Agilent 7890 (II) gas chromatograph (Agilent Technologies, USA) coupled to an Agilent 5975 series mass selective detector. The SPME fiber was inserted into the injection port that was held at 250℃ and the compounds were thermally desorbed for 4 min under the split conditions (1:10), A DB-Wax column 30 m × 0.25 mm (0.25 μm) (Agilent Technologies, USA) was used to separate the volatile compounds. The temperature of the column was held at 45℃ for 5 min, ramped at 10℃/min to 80℃ and then further heated to 240℃ at the rate of 5℃/min. The carrier gas was helium (1 mL/min). The mass spectrometer was run in the electron impact mode at 70 eV. The mass scan range was 25 to 400 m/z.
Sensory profiling of the yogurt samples was conducted by a trained panel, using conventional profiling. Ten judges from the staff members of technology center of Bright Dairy & Food Co., Ltd. were selected who had successfully passed standardized tests for olfactory and taste sensitivities. The panellists were given a hedonic questionnaire to test taste, texture, color, flavor and overall acceptability of random coded yoghurt samples. Water was used for rinsing between samples. A small period of several minutes was required between samples. They were scored on a scale of 1-5 (1 = poor, 2 = fair, 3 = good, 4 = very good, and 5 = excellent). Each attribute was evaluated in triplicate and the values were then averaged.
All physicochemical experiments were carried out in triplicate and all data were reported as the means or means±standard deviation (SD). The data was analyzed with ANOVA using SPSS Statistics 19 software (IBM, USA). The comparison between means was carried out using the Tukey’s significant difference test (p<0.05).
Results and Discussion
Partial chemical components of the PPH were determined and the results were shown in Table 1. PPH is rich in some minerals, vitamins especially vitamin B group and small peptides, the rate of peptides which molecular weight below 1450 Da is 66.95% and 31.72% below 330 in total soluble protein.
The acidification-graphs of samples during fermentation were shown in Fig. 1. With low levels (1%) of PPH addition, there was no significant increase in acidification rate. However, when the content of PPH reached 5%, the increased acidifying rate occurred, which the fermentation time was one hour less than that of the control, a time saving of up to 20% compared with the control. This result showed that addition of PPH into fresh cow milk exerted a stimulatory effect on fermentation.
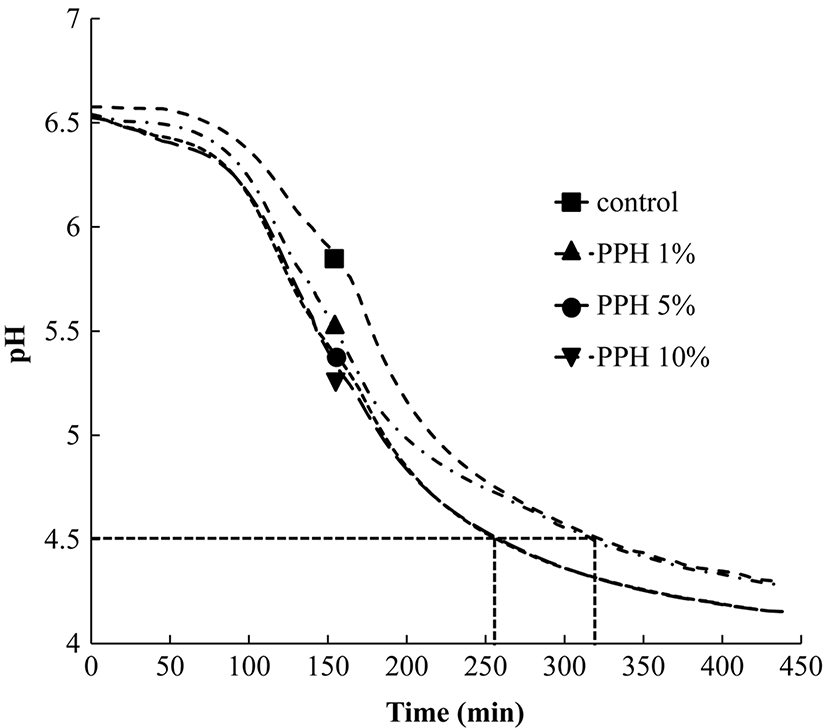
A possible explanation for the stimulatory effect was that the yoghurt starter cultures possessed the ability to utilize compounds supplied by PPH during growth, such as small peptide, vitamins, and minerals. Pork is rich in protein, amino acids, vitamins, minerals, and other constituents (Reig et al., 2013). Smith et al. (1975) investigated the growth of starter cultures in milk was stimulated by amino acids, vitamins and minerals. Liepke et al. (2003) reported that the small peptides of proteolytic human milk proteins are effective growth factors for bifidobacteria. In this study, pork protein was hydrolyzed into small peptides and amino acids by the protease, which both are reasonably considered important factors together with min erals to result in beneficial effect for the yoghurt starter cultures growth.
The viable counts of S. thermophilus and L. bulgaricus in samples during storage were shown in Table 2. These samples with 5% and 10% PPH supplemented contained significantly higher levels of S. thermophilus and L. bulgaricus than the control (p<0.05). However, there was no difference in viable counts of both bacteria between the sample with 1% PPH supplemented and the control (p>0.05), which might due to that the low concentration of PPH was not a sufficient impact on stimulating the growth of both bacteria. There was a similar decrease in viable cell counts of both bacteria during cold storage between the PPH addition samples and the control.
Different lowercase letters in the same row indicates significant difference (p<0.05) between means; Control, fermented milk without proteolytic pork hydrolysate (PPH); PPH1%, fermented milk containing 1% PPH; PPH5%, fermented milk containing 5% PPH; PPH10%, fermented milk containing 10% PPH.
Several studies had confirmed the decrease in viability of probiotics attributable to acid accumulation, interaction with starter cultures, levels of dissolved oxygen and hydrogen peroxide, and storage conditions during storage (Donkor et al., 2006; Talwalkar and Kailasapathy, 2003). In general, LAB possess a efficient scavenging mechanism, such as superoxide dismutase (SOD) and hydroperoxidase, which scavenge superoxide radicals and hydrogen peroxide, respectively, and thus prevent the formation of the hydroxyl radical to avoid damage (Talwalkar and Kailasapathy, 2003). Other way is adding antioxidant compounds, such as flavonoid constituents, antioxidant phenolic constituents, which could increase the antioxidant capacity of medium to improve the LAB viability during cold storage. Shah et al. (2010) found that antioxidant such as green tea, grape extract and vitamin C improved the stability of L. rhamnosus, Bifidobacterium lactis and L. paracasei in model fruit juices. Najgebauer-Lejko (2014) reported that green tea which is rich in antioxidant phenolic constituents that are considered important contributing factors to protect L. acidophilus during milk fermentation. Hervert-Hernández et al. (2009) found polyphenols possessing antioxidant functions had a stimulatory effect on stability of L. acidophilus. Our works demonstrated that although the PPH contained some small peptide, vitamins, and minerals and other constituents had positive effects on the growth (Fig. 1) but lack of some effective constituents which was working a function on maintenance of the both bacteria viability during storage.
The volatile compounds detected in raw milk (RM), the raw milk with 5% PPH (RMP) and their fermented samples were given in Table 3. In RM, 11 volatile components were identified, including alcohols, aldehydes, ketones and esters. In RMP, besides the above 11 volatile components were identified, five esters were also detected (Table 3), which should attributed to the PPH addition. The main flavor compounds in yoghurt can be categorized into alcohols, aldehydes, ketones and acids. Acids are considered as the major components causing the sour taste in yoghurt (Fayed, 2015). The amounts of propanoic acid, butanoic acid, hexanoic acid, octanoic acid, and decanoic acid in fermented milk supplemented with 5% PPH (FMP) were significantly higher than that in the control yoghurt (fermented milk supplemented without PPH; FMNP) (p<0.05). The content of disulfide dimethyl which is rich in pork, was detected in FMP with the metabolism of thiamine (Masuda et al., 2012). Dimethyl sulfide that detected in FMP was also contributed to the aroma of the end product, however, which was not detected in FMNP that was different from the results of Molimard and Spinnler (1996). That would be due to that the starter culture which was the most important factor of flavor was different or the concentration of disulfide dimethyl in FMNP was too low to identified (Al-Attabi et al., 2014).
Different lowercase letters in the same row indicates significant difference (p<0.05) between means; ND, not detected; RM, raw milk; RMP, raw milk containing 5% proteolytic pork hydrolysate (PPH); FMNP, fermented milk without PPH; FMP, fermented milk containing 5% PPH.
The amounts of hexanal, octanal, decanal, benzaldehyde, 2-heptanone, nonanone, 2-undecanone, 2-hexadecanone were not significantly difference between FMP and FMNP (p>0.05). Alcohol, Aldehyde and diacetyl are also important to the flavor of yoghurt (Cheng, 2010). There were more contents of alcohol and diacetyl in FMP compared with FMNP, but the aldehyde content was the opposite: more was found in FMNP. Aldehyde is labile and has a high level of activity, which part of the aldehyde might have been reduced to alcohol or other compounds. In short, adding PPH into milk increased the volatile compounds for FMP, thus gave an enhanced flavor in the end product.
The free amino acids of RM, RMP and their yoghurt samples are listed in Table 4. The concentration of total free amino acids in RMP were higher than that in RM (29.08 mg/kg for RM vs 103.08 mg/kg for RMB, p<0.05), which should attributed to the PPH addition. After fermentation by L. bulgaricus and S. thermophilus, the total free amino acids content increased in both samples (66.01 mg/kg for FMNP and 160.87 mg/kg for FMP). The increment of free amino acids in FMP was much higher than that in FMNP (57.79 mg/kg for FMP vs 36.93 mg/kg for FMNP, p<0.05), which suggested that the addition of PPH stimulated the proteolytic activity of L. bulgaricus and/or S. thermophilus. The increasing tendency of free amino acids that cause by hydrolysis action of LAB was expounded in yoghurt (Serra et al., 2009).
Different lowercase letters in the same row indicates significant difference (p<0.05) between means; ND, not detected; Control, milk or fermented milk without proteolytic pork hydrolysate (PPH); PPH5%, milk or fermented milk containing 5% PPH; RM, raw milk; RMP, raw milk containing 5% proteolytic pork hydrolysate (PPH); FMNP, fermented milk without PPH; FMP, fermented milk containing 5% PPH.
Amino acids and small peptides are frequent nitrogen sources for LAB growth (Toit et al., 2011), for example, methionine is relation to the metabolism of pyruvate and is also an important precursor of superoxide dismutase (SOD) that is able to scavenge O2− (Abreu and Cabelli, 2010; Irmler et al., 2008), thus the free methionine is considered important contributing factor to antioxidant activity for LAB viability. Furthermore, amino acids are major precursors for volatile aroma compounds in fermented dairy products. Through various pathways, amino acids are decarboxylated (to amines and CO2), deaminated (to α-keto acids and ammonia), transaminated (to new amino acids) and desulfated (to form various sulfur compounds), and further metabolized into a range of branched- and short-chain fatty acids, esters, aldehydes and other constituents. For example, LAB has the capacity to generate aldehydes from alanine, and cysteine gives rise to volatile sulfur compounds (Cheng, 2010). With the activity of threonine aldolase, threonine could be translated to acetaldehyde (Chaves et al., 2002). With the increase of the free amino acids, the higher content of flavor compounds was obtained in FMP.
The sensory scores of FMP and FMNP are shown in Table 5. The scores of taste, flavor and overall acceptability of FMP were higher than those of FMNP (p<0.05), which should attributed to the PPH supplemented to improve the fermentation metabolism of L. bulgaricus and S. thermophilus, thus enhanced the contents of free amino acids and volatile flavor compounds. There were no significant difference observed for texture and color tested.
Different lowercase letters in the same row indicates significant difference (p<0.05). FMNP, fermented milk without proteolytic pork hydrolysate (PPH); FMP, fermented milk containing 5% PPH.
In generally, protein hydrolysate prepared by protease had extremely bitter taste. However, the bitter taste of FMP was no significant difference compared with FMNP. This was probably due to that there are some affinity between the bitter peptides and milk protein (Tamura et al., 1990), and the increase of protein hydrolysis degree during yoghurt manufacture process by LAB fermentation (Aubes-Dafau et al., 1995), thus caused the decreasing concentration of bitter peptides.
Conclusions
LAB is fastidious microorganisms that require an exogenous source of amino acids or peptides. In this study, the PPH was added to cow milk to investigate the influence of PPH on yoghurt production by L. bulgaricus and S. thermophilus. These results indicated that the PPH significantly stimulated the growth and acidification of the both bacterial strains. The less fermentation time was achieved. The viable cell counts, the contents of essential and total free amino acids, the scores of taste, flavor and overall acceptability in PPH-supplemented yoghurt were higher than those of the control. Furthermore, the contents of some characteristic compounds including acids, alcohols, aldehydes, ketones and esters which the important flavor substances in yoghurt were richer as a result of PPH supplementation. These results suggested that the PPH harbors a great potential application in yoghurt industrial process.













