Introduction
The current global population is 7.3 billion and is estimated to reach 10 billion by 2050 (UN, 2019). Consequently, such an increase might result in a protein demand twice as much as the current protein production (Godfray et al., 2019). Since conventional meat production systems such as animal agriculture are no longer sustainable, scientists have been searching for alternative protein sources (Goodwin and Shoulders, 2013). Early attempts for meat alternatives were focused on plant-based meat analogues with the use of soy-, wheat-, or fungi-based protein sources (Hoek et al., 2004; Sadler, 2004). Only recently researchers have tried to use cultured muscle cells as alternatives to real meat. Cultured meat, also known as in vitro meat, is a meat analog produced using in vitro cell culture technology where the animal cells are primarily skeletal muscle-derived cells isolated through muscle biopsy and from slaughtered livestock (Choi et al., 2021; Datar and Betti, 2010).
Cultured meat technologies have received a lot of attention because many people think that this technology could supplement or partially replace conventional animal production systems (Post et al., 2020). In fact, conventional animal production system has been the most important part of agriculture. Nonetheless, during last few decades, people and researchers have raised concerns about the conventional animal production system because it may cause several problems, including environmental and social concerns, and animal welfare issues (Post, 2012).
The first cultured meat was produced in 2013 by Mark Post from the Maastricht University, Netherlands, from primary bovine skeletal muscle cells. Since then, several university laboratories and companies have entered this research field (Stephens et al., 2018). Later, another US-based start-up company, Memphis Meats, produced several forms of cultured meat products such as meatballs, beef fajita, chicken, and duck (Stephens et al., 2018). In addition, JUST, a vegan cookie dough and mayonnaise company, announced that they would debut cultured chicken nuggets. Further, a start-up company, Modern Meadow, developed a steak chip made of cultured meat combined with a hydrogel (Marga, 2016; Stephens et al., 2018). Since the introduction of the first cultured meat patty in 2013, several private companies have been founded and focusing on cultured meat production (Choudhury et al., 2020).
Although there are many technological difficulties associated with cultured meat area, at least some of the global problems could be potentially solved through the successful development of this technology (Table 1). Therefore, this review summarized the current issues and technological development about cultured meat production, particularly focusing on three areas: 1) social and economical aspects of cultured meat, 2) biological basis underlying the meat culture of various livestock, and 3) technological approaches for cultured meat production.
| Attributes | Traditional meat | Cultured meat | References |
|---|---|---|---|
| Production system | |||
| Production method | Animal farming | Cell cultivation | (Bhat et al., 2019) |
| Land requirement | High | Low | (Alexander et al., 2017) |
| Location of production | Mostly rural | Rural and urban | (Bhat et al., 2019) |
| Production cost | High | (So far) Very high | (Van der Weele and Tramper, 2014) |
| Production time | Long | Short | (Bhat and Fayaz, 2011) |
| Production yield | Low | High | (Alexander et al., 2017) |
| Greenhouse gas emission | Very high | Low | (Bhat and Fayaz, 2011) |
| Energy requirement | High | High | (Tuomisto and Teixeira de Mattos, 2011) |
| Water and soil pollution | High | Low | (Welin and Van der Weele, 2012) |
| Sustainability | Low | High | (Siegrist and Hartmann, 2020) |
| Characteristics | |||
| Manipulating composition | Impossible | Possible | (Bhat and Fayaz, 2011) |
| Human health | Low | High | (Joshi et al., 2020) |
| Food safety | Low | High | (Joshi et al., 2020) |
| Animal welfare | Low | High | (Mouat and Prince, 2018) |
| Ethical advantage | Low | High | (Mancini and Antonioli, 2020) |
| Consumer acceptance | High | Low | (Siegrist et al., 2018) |
Cultured meat system requires less use of water, land, feed grain, and energy compared with traditional livestock system (Tuomisto and Teixeira de Mattos, 2011). In addition, cultured meat system may exhibit a higher conversion rate transformed into edible meat than traditional livestock system that exhibits 5%–25% conversion rate (Alexander, 2011; Bhat and Hina, 2011). Thus, cultured meat could be an ideal alternative due to its potential sustainability and limited environmental effects. For example, a 20 m3 bioreactor, the largest size for cultured meat production today, could produce 25,600 kg of cultured meat per year (Van der Weele and Tramper, 2014). Assuming no loss during the cultured meat production process, this represents an estimated supply of cultured meat for 2,560 people per year (Van der Weele and Tramper, 2014). The calculation on feeding 2,560 people is based on Van der Weele and Tramper (2014) who assumed that everybody in the world will eat 25–30 grams of cultured meat per person per day (10 kg/year). Considering that such production requires only a few hours of labor per day to maintain the bioreactor, cultured meat production is a potentially low-cost alternative to the current livestock system for meat production (Bhat et al., 2014). In addition, it was reported that the price of cultured meat burger decreased from $325,000 to $11.36 per burger or $80 per kilogram of meat within 2 years (Crew, 2015). Another economic benefits could be found in the distribution of cultured meat. By locating cultured meat production facilities close to the cities, the transport cost can be largely decreased (Bhat et al., 2015). Additionally, in terms of food waste, traditional meat industry has big problem in waste management because whole carcass cannot be used for consumption. However, culture meat system can provide prime cut alone for consumption and further processing and that will be an substantial economical benefit (Stephens et al., 2018).
The current livestock system negatively influences the environment, causing environmental sustainability concerns. Although the water used by livestock farming mostly returns to the environment, a significant part of it becomes polluted or evaporates (Melvin, 1995). This pollution is caused by livestock and feed production, as well as product processing, in turn increasing the demand for water (Steinfeld et al., 2006). In order to produce 1 kg of beef, 15,495 L of water would be required, and 99% of such water consumption is used for the growth of grain and roughages (e.g., pasture, dry hay and silage) (Hoekstra and Chapagain, 2006). Only 1% of water (about 155 L) is used for drinking and servicing to livestock. The demand is mostly attributed to the drinking water requirement for the animals, as well as crop and plant growth (Chriki and Hocquette, 2020). Both water pollution and consumption might lead to the destruction of biodiversity through destruction of wildlife habitats (Steinfeld et al., 2006). However, cultured meat technology uses approximately 82%–96% less water than traditional livestock farming (Tuomisto and Teixeira de Mattos, 2011).
In general, livestock production requires 30% of the total land surface—33% of cultivated land for livestock feed and 26% for pasture (Steinfeld et al., 2006). However, cultured meat production systems use only 1% of the land required for traditional livestock production systems (Alexander et al., 2017; Tuomisto and Teixeira de Mattos, 2011). Nevertheless, this assumption is restricted to the production of an algae-based culture medium biomass, and the expense and efficiency of producing different culture media are therefore uncertain. Although cultured meat production systems require lesser land than traditional livestock systems, the cultured meat production system requires at least four times more energy than traditional livestock (Alexander et al., 2017). In detail, cultured meat requires 18–25 GJ/t of direct energy (Tuomisto and Teixeira de Mattos, 2011), while 4.5 GJ/t of direct energy is required to produce traditional meat (MacLeod et al., 2013).
Livestock production consumes direct energy, such as lighting, heating, and cooling, while cultured meat production systems require energy for muscle cell culture, as well as for the sterilization and hydrolysis of biomass material required in the cell culture media (Tuomisto and Teixeira de Mattos, 2011).
Livestock provides a quarter of all the protein content (and 15% of energy) consumed in food, and also contributes to 18% of the global greenhouse gas and 37% of methane emissions into the atmosphere, the values of which are higher than those of global transportation (FAO, 2012; Steinfeld et al., 2006). Cultured meat production would assumably affect less the environment compared to conventional farming. In particular, reducing greenhouse gas emissions would be a significant advantage of cultured meat production. Another potential environment-related advantage of cultured meat production could be the lower land use compared to conventional livestock farming, especially in the case of ruminants (Chriki and Hocquette, 2020).
Recently, approximately 56 billion animals are slaughtered for their meat every year (Dorovskikh, 2015). Hence, the traditional livestock production-related animal welfare is a major worldwide ethic agenda. Cultured meat production systems have been raised as good alternatives to the current meat production systems (Post, 2012). Cultured meat could be an attractive option for vegetarians, vegans, and opponents who reject meat consumption for ethical reasons (Hopkins and Dacey, 2008). According to a previous article, we could expect the following effects of widespread cultured meat production: 1) a significant reduction in animal use, 2) a great reduction in animal suffering, and 3) a variety of cultured meat sources, including those of wild animals (Bhat et al., 2014).
Despite the potential animal welfare- and environment-related merits of cultured meat, the mercantile success of cultured meat greatly depends on consumer perception and various societal concerns, including naturalness, food safety and security issues, framing effect, legislation, religion, and ethics (Chriki and Hocquette, 2020; Mancini and Antonioli, 2020). Hence, the consumer acceptance of cultured meat is highly important but could be controversial. One of the most common cultured meat-related hurdles is its artificial nature. Consumers usually do not easily accept new technologies, such as genetically modified organisms, when they have limited information about the given technology (Bánáti, 2011). In addition, framing effects on cultured meat significantly contribute to consumer attitude, belief, and behavioral intention to cultured meat (Bryant and Dillard, 2019). However, changes in consumer perception by providing positive information could make consumers try, buy, and pay for cultured meat. Continuous evaluation of the changes in consumer perception over time would thus be necessary.
The regulatory structures are important for building consumers’ trust towards cultured meat production and cultured meat itself, including safety and nutritional composition (Laestadius and Caldwell, 2015). Several reports focus on the regulation of cultured meat in the United States and the European Union (Petetin, 2014; Schneider, 2012). However, it is difficult to establish cultured meat-related regulations due to the currently available insufficient information and incomplete technology for cultured meat (Stephens et al., 2018).
There is controversy concerning cultured meat in several religious communities, including Jews, Muslims, and Hindus, due to its nebulous status (Chriki and Hocquette, 2020). In a cultured meat-related consumer acceptance survey targeting 3,030 participants, including Jews, Muslims, and Hindus, most participants responded that they would be willing to eat cultured meat (Bryant et al., 2019). However, religious duties, such as dietary laws (Kosher, Halal, beef-eating restrictions in Hinduism), still need to be discussed (Bryant, 2020).
In the case of food choices, ethical issues become increasingly important. Although cultured meat technology gets closer to actual commercial availability, it is obvious that ethical concerns of cultured meat is not completely solved yet (Dilworth et al., 2015). There are some arguments amongst consumers regarding the ethical issues of cultured meat. Advocates believe that cultured meat systems demand significantly fewer animals for meat production than traditional livestock and could also contribute to stop animal suffering, such as confining in tight space or slaughtering under cruel conditions (Chriki and Hocquette, 2020). In addition, cultured meat might be preferred by people who are interested in reducing their meat consumption for ethical reasons, including vegetarians and vegans (Hopkins and Dacey, 2008). According to a previous report, cultured meat could have a positive impact on a carbon footprint, and this makes a potentially effective strategy to improve awareness of cultured meat (Tomiyama et al., 2020). However, despite of potential advantages of introducing cultured meat, many people concern about food safety regarding unnaturalness perception of cultured meat (Laestadius, 2015; Verbeke et al., 2015). Moreover, some have concerned that cultured meat may aggravate consumer inequality between the rich and the poor (Bonny et al., 2015; Cole and Morgan, 2013; Stephens et al., 2018).
Currently, 32 cultured meat companies exist worldwide, focusing on cultured beef (25%), poultry (22%), pork (19%), seafood (19%), and other exotic meats (15%), such as mouse, kangaroo, and horse (Choudhury et al., 2020). Most of these companies are based in North America (40%), followed by Asia (31%) and Europe (25%). Substantial amount of capital has been invested in cultured meat-related research and development in the past 5 years. Approximately $320 million have presumably been invested in beef and pork (75%) as well as in seafood production (25%) (Choudhury et al., 2020).
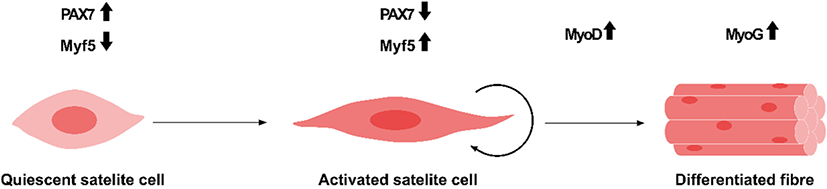
Meat from industrial animals, including cattle, pigs, poultry, and fish, consists mainly of skeletal muscles, fibroblasts, and adipose cells (Dodson et al., 2015). In addition, meat can also provide vitamin B12 and heme iron, which are essential for human nutrition. Skeletal muscle cells are multinucleated and striated cells, which fulfill the basic function of muscle contraction. Moreover, skeletal muscles are able to regenerate and recover minor damage in the muscle tissue (Laumonier and Menetrey, 2016). Their self-renewal ability is due to stem cells, i.e., satellite cells that reside within the skeletal muscle tissue. As the number of satellite cells reportedly remains constant after multiple injuries, these cells are considered stem cells that could most certainly be maintained by self-renewal (Shi and Garry, 2006). Under normal conditions, satellite cells are quiescent but could be activated by intrinsic or extrinsic cues, such as muscle injury. The quiescent state of satellite cells is maintained by the negative cell cycle and growth factor regulation and the expression of tumor suppressors, such as retinoblastoma protein (Rb) (Dumont et al., 2015). Up-regulated Notch signaling is also a quiescent satellite cell marker. Therefore, Notch down-regulation is a prerequisite for myogenic differentiation (Brack et al., 2008). Moreover, myogenic factor 5 (Myf5), myogenic determination (MyoD), and myogenin (Myog) are critical factors that are expressed from activated satellite cell under muscle stimulus, and therefore, they are committed myogenic progenitor markers (Dumont et al., 2015). Active proliferating satellite cells (quiescent cells) – expressing high levels of paired box 7 (Pax7), and concurrently negative for Myf5 and MyoD – are crucial for maintaining stemness (Fig. 1).
Satellite cells were first isolated in vitro by Richard Bischoff in 1974 (Bischoff, 1974). Since the discovery of muscle satellite cell isolation and proliferation methods (Bischoff, 1975), various modified protocols have been developed to isolate satellite cells more efficiently from multiple livestock, such as chicken (Yablonka-Reuveni et al., 1987), horse (Greene and Raub, 1992), cow (Dodson et al., 1987), sheep (Dodson et al., 1986), fish (Greenlee et al., 1995), and pig (Doumit and Merkel, 1992). Using isolated satellite cells, researchers were able to understand further the underlying processes of muscle formation and development (Allen et al., 1979). Recently, scientists have used stem cells and muscle culture technology to develop lab-grown meat, cultured in a laboratory incubator using isolated skeletal muscle and satellite cells (Bischoff, 1975).
Although not yet on the market and much more expensive than farmed meat, cultured meat offers multiple advantages over conventional meat. Cultured meat is a clean meat, free of possible pathogens (Kadim et al., 2015), environmentally friendly due to its lack of need for large space to raise livestock, and significantly less global gas emission compared to conventional livestock farming (Tuomisto and Teixeira de Mattos, 2011). Several startup companies are currently emerging around the world, and research on the production of high-quality, low-cost culture meat production is underway (Table 2).
Chicken muscle satellite cell in vitro isolation and differentiation was described in 1983 by Matsuda et al. (1983). Yablonka-Reuveni et al. (1987) obtained chicken pectoralis cells differentiated from satellite cells, isolated by centrifugation through a Percoll density gradient (Yablonka-Reuveni et al., 1987). Satellite cells play a crucial role in the muscle growth of post-hatch broiler chicken and in muscle maintenance and repair after muscle injury. Since the stem cell properties of muscle satellite cells, the proliferation and differentiation potential of chicken satellite cells have been evaluated in detail. In general, when skeletal muscle is damaged, new muscle fibers derived from pre-existing quiescent satellite cells replace the damaged area and reconstruct the muscle structure (Feldman and Stockdale, 1991). Feldman and Stockdale et al. suggested that chicken satellite cells isolated from the fast muscle (pectoralis major) part would be differentiated only into fast fibers, whereas satellite cells isolated from the slow muscle (anterior latissimus dorsi) part could mostly differentiate into fast muscles but, to a small extent, also into slow muscles (Feldman and Stockdale, 1991).
Cultured meat has not yet been formally commercialized and sold, but many companies have promoted it as various prototype foods such as hamburgers, bacon, and nuggets. Artificial chicken meat was presented by JUST, a vegan food company, in 2018, through a promotional video (JUST, 2018). They showed a footage of clean chicken meat that was created using cell cultures (JUST, 2018). Moreover, JUST successfully manufactured a cell-cultured chicken nugget product in 2019 at the cost of 50 dollars per nugget (Savvides, 2020). A food technology company, Memphis Meats (Berkeley, CA, USA), published a similar promotion video introducing the concept of a cultured meat product in 2016 (Memphis Meats, 2016a). In the following year, Memphis Meats was able to successfully manufacture and introduce a cultured chicken meat product (Memphis Meats, 2017). Future Meat Technologies, a start-up company founded in 2018 and based in Israel, also created cell-cultured chicken meat. This company managed to reduce production costs to 150 dollars per pound of chicken (Lucas, 2019). However, even these small pieces of foods, such as artificial nuggets, require the United States Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) approval (Savvides, 2020). The commercialization of these products has not yet realized.
During embryonic development, proliferating myoblasts differentiate into myotubes, followed by further maturation and differentiation into mature muscle fibers (Braun and Gautel, 2011). Adal and Cheng studied the structure of duck muscle cells as early as 1980 and showed that the duck muscle spindle consists of several muscle fibers and a capsule surrounding them (Adal and Cheng, 1980). In 1986, stromal mesenchymal cells in the iris of a duck reportedly migrated towards the muscle of the iris and became iridial skeletal muscles (Yamashita and Sohal, 1986). Muscle-specific microRNAs, called MyomiRs, are expressed in the muscle cells, although they are also expressed in several other tissues (McCarthy, 2008). Li et al. found detected 279 novel miRNAs in the breast muscle of ducks, indicating the importance of miRNAs in muscle development and maturation (Li et al., 2020). Among these, miRNA-1 and miRNA-133 have been suggested to be crucial factors for duck skeletal muscle proliferation and differentiation. miRNA-1 reportedly promoted myogenesis by targeting the transcriptional repressor histone deacetylase 4 (HDAC4), and miRNA-133 reportedly inhibited serum response factor (SRF) and TGFBR1 expression, increasing myoblast proliferation (Wu et al., 2019).
During duck embryonic development, MyoD expression in both the breast and leg muscles tended to increase gradually, and MyoD expression level in the breast muscle was higher than that in the leg muscle (Li et al., 2010; Li et al., 2014). However, Li et al. suggested that MyoD expression in the breast muscle was consistent but decreased in leg muscle during early embryonic development (Li et al., 2014; Li et al., 2010). They also showed that the MyoD and Myf6 gene expressions correlated with that in the leg muscle. However, insulin-like growth factor-1 (IGF-1) induced the expression of MyoD and Myf5 and increased muscle hypertrophy (Liu et al., 2012). IGF-1 is known to stimulate skeletal muscle (Musarò et al., 2001).
Similarly to cultured chicken meat, Memphis Meats also produced cultured duck meat, which was cooked and presented, followed by product tasting (Memphis Meats, 2017). Moreover, a French start-up company Gourmey, was able to cultivate duck egg cells with slightly adjusted nutrients to mimic the effect of force-feeding in order to create artificial foie gras, which they refer to as ‘ethical foie gras’ (Gourmey, 2020; Southey, 2020). In 2020, the vegan food company JUST managed to produce duck chorizo and pâté completely based on cultured duck cells (Purdy, 2020).
Beef has long been studied in various ways. Several biological aspects of muscles have been studied for the basic understanding of the mechanisms underlying cellular proliferation, and many scientific findings have been reported related to muscle development and proliferation in meat animals (Allen et al., 1979; Dayton and White, 2008; Wojtczak, 1979). In meat animals, the fetal stage of muscle development is crucial since the number of muscle fibers does not change after birth (Zhu et al., 2004). Therefore, the postnatal muscle develops by enlarging the muscle fiber size (Karunaratne et al., 2005; Stickland, 1978). Satellite cells located under the basal lamina of the muscle fibers are crucial for muscle growth after birth. Major satellite cells differentiate into the myogenic lineage, but a small population of satellite cells could also differentiate into fibroblasts or adipocytes, which comprise the skeletal muscle tissue. Understanding the mechanisms underlying satellite cell-related muscle growth and differentiation would enable further improvements in cultured meat production (Rubin, 2019).
Controlling nutrient supplementation and several signaling factors is important for skeletal muscle growth and marbling. For example, skeletal muscle growth is enhanced by the activation of the Wingless and Int (Wnt) signaling, while it inhibits adipogenesis (Du et al., 2010). β-catenin, which is stabilized by Wnt signaling, positively regulates myogenic genes, such as Pax3, MyoD, and Myf5 (Ridgeway and Skerjanc, 2001). For commercial applications, marbling could be controlled by the activation and repression of the Wnt/β-catenin signaling during culture, in order to produce higher-quality meat.
Mosa Meat, a Dutch start-up company, was the first to promote cultured beef in public. This beef was generated by culturing and differentiating stem cells obtained from a cow and was formulated into muscle strips. Mosa Meat cooked the cultured meat at a conference, then organized a tasting party (BBC news, 2013). Mosa Meat now creates cell-cultured meats that are more cost-effective than before, and it has now developed a bovine serum-free medium (Kateman, 2020). Memphis Meats, a start-up company based in California, showed the first meatballs made from cell-cultured beef in 2016. The company is now building a pilot plant for cultured beef and chicken meat (Memphis Meats, 2016b; Shaffer, 2020).
Doumit and Merkel suggested that porcine myogenic satellite cells could be isolated from porcine skeletal muscle and developed an optimized medium for porcine satellite cells (Doumit and Merkel, 1992). This culture condition has been improved with slight modifications (Mau et al., 2008; Metzger et al., 2020). For the in vitro culture of skeletal muscle, satellite cells or muscle fibers could be isolated from muscle tissues to induce growth and differentiation (Mau et al., 2008; Metzger et al., 2020). Pax7 is a critical marker for functional satellite cells in several species, including mice, humans, cattle, and pigs (Ding et al., 2017). IGF-1 also affects pig satellite cells through the mechanistic target of rapamycin (mTOR) pathway (Han et al., 2008). Neural cell adhesion molecule (NCAM), also called CD56, and cluster of differentiation molecule 34 (CD34) have been suggested as myogenic cell markers in swine skeletal muscles (Perruchot et al., 2013). Lactate dehydrogenase A (LDHA) and coatomer protein complex, subunit beta 1 (COPB1) were also suggested to be involved in pig muscle development (Qiu et al., 2010). RNA-seq analysis using the pig longissimus dorsi muscle revealed that long non-coding RNAs are involved in muscle growth and fat deposition (Chen et al., 2019). As shown in mice, the number of satellite cells decreases with age and during long-term culturing due to the loss of their self-renewal and differentiation potentials (Ding et al., 2017).
Meatable, a Dutch start-up company, produced cell-cultured pork meat using stem cell technology, which allowed the company to easily extract specific cell types required to produce meat (Brodwin, 2018a). Another startup company in San Francisco, New Age Meats, successfully produced prototype pork sausage made from fat and muscle cell culture from a live pig sample (Brodwin, 2018b).
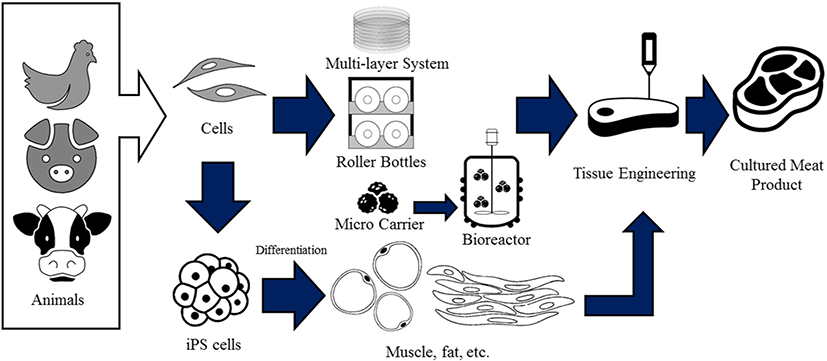
Tissue engineering-based cultured meat production largely depends on large-scale cell culture technologies, which could provide a significant amount of cells, allowing meat production (Verbruggen et al., 2018). Large-scale cell production systems also aim at producing as many cells as possible with the least of the required resources. Minimal handling and a short culture period for a sufficient number of harvested cells are also commonly considered factors for efficient cell mass production (Moritz et al., 2015). Several cell types are potentially viable options for cultured meat production, including myogenic satellite cells, embryonic stem cells, and induced pluripotent stem (iPS) cells (Kadim et al., 2015). Among these various cell types, myogenic satellite cells are widely used as the promising option due to their efficient differentiation into myotubes (Arshad et al., 2017). A variety of methods and bioreactors are used to expand anchorage-dependent cells (Merten, 2015). Each technology has its own merits, but in common, these platforms provide an attachment surface area for the cells while assuring gas and nutrient exchange in parallel (Tavassoli et al., 2018).
As cell culture is a major step in the production of cultured meat, the choice of the appropriate culture dish or vessel is pivotal. T-flasks commonly used in cell culture provide a surface area of 20–225 cm2. In the case of large-scale cultures that require a significantly larger surface area than that, multiple T-flasks could be used. A multi-tray system has been developed as an alternative high-surface area provided within a single unit. Although this system has multiple trays that provide multiple cell attachment surfaces, handling multiple T-flasks might be labor-intensive as each T-flask must be managed individually (Rafiq et al., 2013).
Roller bottles were devised by Gey in 1933, aiming at low-cost maintenance of a large number of cell populations while using less culture medium (Gey, 1933; Melero-Martin and Al-Rubeai, 2007). Roller bottles, placed in a gas-tight chamber or a case with no chamber, could be sealed to keep the cells and medium from drying. This system also requires a slow driving mechanism, allowing the bottles with cells to slowly rotate, enabling the medium to cover cells evenly, and allowing greater gas exchange (Melero-Martin and Al-Rubeai, 2007). Roller bottles could offer a surface attachment area of up to 350,000 cm2. Compared to T-flasks or multi-tray cultures, roller bottles provide superior applications for anchorage (Rafiq et al., 2013). However, the real-time monitoring of the roller bottle system is difficult, and handling several roller bottles simultaneously is laborious (Tavassoli et al., 2018). To overcome these shortcomings, relevant efforts have been made to automate the roller bottle-based culture process (Kunitake et al., 1997). Roller bottles have been used to culture chicken muscle cells in large scale (Wesson et al., 1949) which may be applied for chicken meat production. According to USDA, roller bottle incubator systems drastically improved swine muscle cell production output, providing enough cells for three-dimensional (3D) fabrication of cellular sheets for in vitro meat engineering (Marga, 2012).
Culturing cells in suspension provide more output than monolayer culture systems, but adhesion to a specific culture surface is crucial for the anchorage-dependent cells to proliferate without losing their cellular properties (Grinnell, 1978). In order to mass-culture anchorage-dependent cells, microcarriers are used to establish suspension cultures (Rafiq et al., 2013). In 1967, Van Wezel described the concept of “micro-carriers” using dextran particles for developing large-scale cell cultures in a stirred suspension (Van Wezel, 1967). These dextran particles are micro-sized beads that display positively-charged surfaces and attract animal cells that contain negatively charged membranes (Van Wezel, 1967). Various materials could be used as microcarriers, including dextran, cellulose, gelatin, and plastic (Stanbury et al., 2013). These microcarriers might be solid or porous, and the materials could be selected according to culture intention and cell type (Tavassoli et al., 2018). Food compliance should be considered in situations where microcarriers are used in the production of edible meat. Although several researchers have focused on the development of microcarriers suitable for human stem cells, microcarriers for myoblast expansion or cultured meat production are yet to be developed (Bodiou et al., 2020). The separation process of the cultured cells from the microcarriers is the final step, in which cultured cells are used for subsequent applications (Nienow et al., 2014). Microcarriers could be separated from the cultured cells by using enzymes or mechanical forces, known to be a challenging procedure (Verbruggen et al., 2018). Microcarriers made of thermo-responsive materials could temperature-dependently change their surface properties and dislodge the attached cells, which could be subsequently filtered (Bodiou et al., 2020). Biodegradable microcarriers are also being widely used as they do not require fastidious harvesting procedures (Lam et al., 2017). Various edible polymers and hydrogels might be used as bases for edible microcarriers (Ali and Ahmed, 2018). Edible microcarriers might not need a cell dissociation step as the whole structure is safe for ingestion (Bodiou et al., 2020). Myogenic satellite cells could be cultured in suspension with biodegradable or edible microcarriers (Moritz et al., 2015). Recently, satellite cells have been grown and differentiated in suspension culture systems using biodegradable scaffolds for the development of cultured meat. This process requires cells to be anchored to scaffold surfaces, which could be provided by tissue engineering constructs (Post, 2012).
Obtaining tissue structure from muscle cell would be efficient way for creating cultured meat. However, normally growing cells in a dish to get tissue-like structure is very challenging. For cells to form an appropriate structure, scaffolds are utilized. Scaffolds molded into desirable shape may provide physical support for muscle cell anchorage (Ben-Arye and Levenberg, 2019). Cells are highly niche dependent, and scaffolds aim to provide cells propriate niche-resembling environment for growth (Zeltinger et al., 2001). Hydrogel is often used as scaffold base material to mimic cell niche. Hydrogel engineered into porous structure mimics extracellular matrix (ECM) as it provides cells with permeable anchorage fit for water, gas, and nutrient exchange. Such 3D scaffolds can be utilized by simply seeding the cells onto finished structure or mixing cells into bioink and 3D printing cell encapsulated mixture to form cell-laden scaffold (Hwang et al., 2010). Several types of base materials are used for tissue engineering. Collagen, fibrin, and alginate are utilized as hydrogel, but to make gels more biologically like actual tissues, bioinks using decelluarized extracellular matrix (dECM) are introduced (Choi et al., 2016b). Bioinks made with dECM contains more tissue-specific factors including growth factors, adhesive proteins, compared to general hydrogels, and is believed to be more fit for tissue engineering (Kim et al., 2020). Though no case of producing cultured meat by scaffolding cells have been reported, but research show cell-laden 3D printed structures could be used for tissue transplantation (Liu et al., 2019), and myoblasts are also capable of being 3D printed and cultured (Choi et al., 2019). Decellularizing plant tissues for 3D cellulose scaffolds are also available. Plant tissues are abundant, easy to obtain and economically cheap. Culturing muscle cells on decellularized plant scaffold stimulate growth, proliferation, and differentiation, while providing myotube alignment due to natural plant cellulose patterns (Cheng et al., 2020).
The ultimate goal of cultured meat is to produce edible meat products without directly involving animals, not to obtain and proliferate the meat taken from livestock. To do this, pluripotent stem cells might offer the best option as they could differentiate into muscle, fat, and other cell types that could enhance the real meat flavor. Among the two pluripotent stem cell types, embryonic stem and iPS cells (ESCs and iPSCs, respectively), iPSCs seem to be more suitable as they are easy to establish and offer the advantage of a non-embryo-based alternative. To date, iPSCs from various livestock have been established, including cattle (Han et al., 2011), pigs (Wu et al., 2009), and chicken (Choi et al., 2016a). Although human and mouse iPSCs exhibit limitless self-renewal potential, livestock iPS cells lose stemness during long-term culture in the present culture system (Choi et al., 2016). Therefore, the culture medium should be improved for long-term livestock iPSC culture. Since muscle tissue is a complex structure of multiple different cell types, reliable muscle, fat, myoglobin, etc., differentiation protocols should be established, as well as a technique for forming a 3D structure for multiple cell types (Fig. 2). Using the tissue engineering technology or bioprinting system, muscle cells and various supportive cell types could be cultured on the same 3D scaffold to form complex tissues that mimic in vivo skeletal muscle structure (Krieger et al., 2018). Recently, a 3D engineered scaffold was used for bovine satellite cells, which were proliferated on the 3D scaffold by submerging them into a myogenic growth medium. Bovine smooth muscle cells and endothelial cells are differentiated on the scaffold to form cell-based meat products, which are reported to be suitable for consumption as food products (Ben-Arye et al., 2020).