Introduction
The demand for commercially sterilized milk typically processed at ultra-high-temperature (UHT) with aseptic packaging has continuously increased, because of its long shelf-life, acceptable sensory quality, and the fact that it has no need for refrigeration (Chavan et al., 2011). Global and Asia-Pacific UHT milk markets are expected to grow annually from 2019 to 2025 by 7.8% and 9.1%, respectively. The sales of UHT milk in Korea also increased by 46.3% for 2018 compared to 2017 (Grand View Research, 2019).
Commercially sterilized UHT milk provides microbial stability, but some physicochemical changes that could occur during storage may negatively influence the quality of UHT milk. Age gelation refers to an undesirable viscosity increase due to formation of a protein network. Gelation is a major quality defect since it critically impairs the fluidity of the milk (Anema, 2019). Although age gelation more readily occurs in concentrated milk, it also limits the shelf-life of commercially sterilized UHT milk (Datta and Deeth, 2001). The detailed mechanisms involved in age gelation have not yet been clarified. However, enzymatic hydrolysis of β-lactoglobulin (β-LG) and κ-casein (κ-CN) complex formed on micelle surfaces during UHT heating can promote the formation of a gel network. Possible major contributors to age-gelation of UHT milk during storage include plasmin, a heat-stable endogenous protease, or a heat-stable exogenous protease (Malmgren et al., 2017; Zhang et al., 2018).
Creaming refers to the formation of a creamy surface on the top of milk. Generally, fat separation is not a problem in fresh homogenized milk or skim milk but fat separation becomes prominent during long-term storage. Agitation of dairy products containing high fat such as whole milk causes aggregation of milk fat globules. Such mechanical stress-induced fat aggregation is important during distribution of dairy products. The aggregated fats resemble butter grains and their presence decreases consumer acceptability and is one of the major reasons for claims against manufacturers by consumers. Since UHT milk has a long shelf life, there are significant chances for an increase in fat aggregation during transportation and distribution. Consumer claims regarding fat aggregation amounted to about 12% (24 cases) of the total number of the milk-related consumer claims (214 cases) of one major company in 2019. Thus, protein proteolysis and subsequent gelation, and cream layer formation during storage are closely related to the quality of commercially sterilized UHT milk (D’Incecco et al., 2018).
Homogenization is a process intended to delay the rise of fat during shelf-life of milk. The sizes of large particles such as fat globules present in milk are dramatically decreased by forcing pressurized milk (8–20 MPa) between pressure valves (Michalski and Januel, 2006). High-pressure homogenization (HPH), which operates at 50–200 MPa, yielded desirable effects on microbial stability and inactivation of endogenous enzymes in milk (Hayes and Kelly, 2003; Pereda et al., 2007). However, ultra high pressure homogenization of milk at 200 and 300 MPa accelerated lipid oxidation, which in turn generates off-flavor during storage (Pereda et al., 2008). The reduced sensory acceptability and high cost of ultra high pressure homogenization limit its use in commercial scale UHT milk production.
The present study was conducted was to examine the effect of commercially-relevant homogenization pressures on the quality characteristics of UHT milk stored under ambient conditions. The three homogenization pressures (20, 25, and 30 MPa) were selected based on the feasibility of their application in commercial UHT milk production. The changes in fat particle size, mechanical stress-induced fat aggregation, plasmin activity, and lipid oxidation were monitored during storage of UHT milk for 16 wk.
Materials and Methods
Commercially sterilized UHT milk was produced at different homogenization pressures (20, 25, and 30 MPa; 2,500 kg each), as shown in Fig. 1. The average proximate composition of UHT milk analyzed using a MilkoScan (Delta instruments, Combiscope 300HP, Drachten, the Netherlands) was as follows: moisture 87.33%, protein 3.25%, fat 3.99%, lactose 4.71%, and minerals 0.72%. The samples were stored at room temperature for 16 wk and were taken at indicated storage period for chemical analysis.
The particle size distributions of samples for various homogenization pressures and storage periods were measured using a particle size analyzer (Mastersizer 2000; Malvern Instruments, Worcs, UK). Milk samples were diluted to appropriate concentrations with distilled water, and average diameter, D3,2 (surface weighted mean diameter) and D4,3 (volume weighted mean diameter) were measured in triplicate.
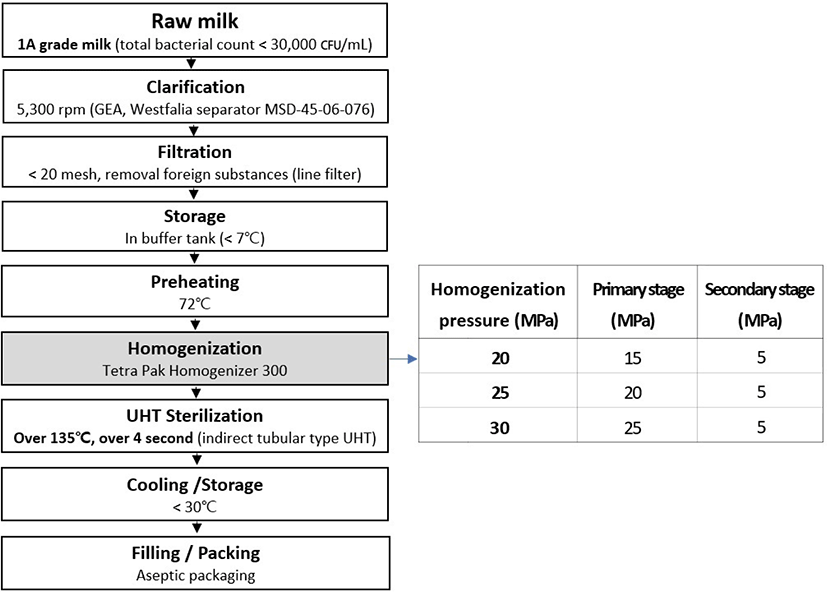
A method to simulate fat aggregation during storage and transport of UHT milk was utilized as follows: UHT milk (200 mL in Tetra Pak; 54.6×38.8×106.5 mm) at the designated storage time was placed on a shaker (Taitec Recipro SR-2w; Fig. 2A) and vertically agitated at 300 rpm for 10 h. To avoid quantitative errors during sample transfer, fluid UHT milk was carefully removed from Tetra Pak and the Tetra Pak was fully opened and dried overnight in a 105°C dry oven (ED53, Binder, Germany) for 24 h. The weight of dried fat aggregates was calculated from the weight difference for the material in the package before and after drying.
Plasmin activity was determined by the method of Politis et al. (1993) with slight modification. Briefly, the casein micelle fraction and milk serum were separated from UHT milk (25 mL) by ultracentrifugation (Optima LE-80K, Beckman Coulter, Fullerton, CA, USA) at 118,000×g for 60 min at 4°C. The separated casein micelle fraction was suspended in Tris buffer (50 mM, pH 8.0) containing NaCl (110 mM) and E-aminocaproic acid (EACA, 50 mM), and plasmin and plasminogen were dissociated by incubation at 20°C for 60 min followed by centrifugation at 30,100×g for 60 min at 4°C. For plasmin activity determination, the recovered supernatant (20 μL) was mixed with Trizma buffer (50 mM, pH 7.4, 190 μL) containing NaCl (110 mM), H-D-valyl-L-leucyl-L-lysine-p-nitroanilide dihydrochloride (0.6 mM), and EACA (2.5 mM). The changes in absorbance of the assay solution were monitored for 30 min at 405 nm using a plate reader (Biotek Instrument, Winooski, VT, USA). The temperature of the reaction plate was maintained at 37°C. For total plasmin activity determination, urokinase (30 U) was added to the assay solution and incubated at 37°C for 60 min to convert the inactive plasminogen to active plasmin. One unit of plasmin activity was defined as the amount of enzyme that causes a rate of change of 0.001 in absorbance per 1 min under the indicated assay conditions (García-Risco et al., 2003).
The extent of proteolysis in UHT milk samples during storage was determined by measuring the peptides soluble in trichloroacetic acid (TCA, 12%). The TCA-soluble extracts were prepared by the method of Kelly and Foley (1997), and the amount of soluble peptides in the supernatant was determined using the BCA assay (Walker, 2009).
A thiobarbituric acid (TBA test) was carried by the method of Hegenauer et al. (1979) to monitor possible changes in lipid oxidation during the storage of UHT milk. Reactions of the TBA test were initiated by mixing 5 mL of milk with freshly made TBA reagent (2.5 mL, 25 mM). The reaction mixture was immediately heated in a boiling water bath for 10 min and cooled in ice water. The reaction mixture was centrifuged at 6,000×g for 5 min after the addition of cyclohexanone (10 mL) and ammonium sulfate (4 M, 1 mL). The absorbance of supernatant was measured at 532 nm using a spectrophotometer (Ultrospec 2100 pro, Amersham Bioscience, Cambridge, UK).
All quantitative determinations were made in triplicate and expressed as mean±SD. Results were analyzed using SPSS Statistics V. 21 (SPSS, Chicago, IL, USA). When data showed significant differences (p<0.05) in two-way analysis of variance (ANOVA), Tukey’s multiple range test was carried out to find significant difference among the means.
Results and Discussion
The particle size distributions for UHT milk produced at different homogenization pressures are shown in Fig. 3. The median particle diameter (D50) of raw milk was about 3.41 μm and it was reduced by 0.358 to 0.648 μm by two-stage homogenization. All particle size parmeters (D50, D3,2, and D4,3) of milk fat globules were significantly decreased as homogenization pressure increased from 20 to 30 MPa (p<0.05). Small fat gobules (<0.4 μm) are not observed in raw milk, which may be due to overlapping by larger fat globules with high volume fraction (Pereda et al., 2007). This implies that casein micelles, which have a typical size of 0.2–0.6 μm (most propbable diameter of 0.22 μm; Gebhardt et al., 2006), play only a marginal role in the particle size distribution, while fat globules are the major contributors in the particle size distribution in whole milk.
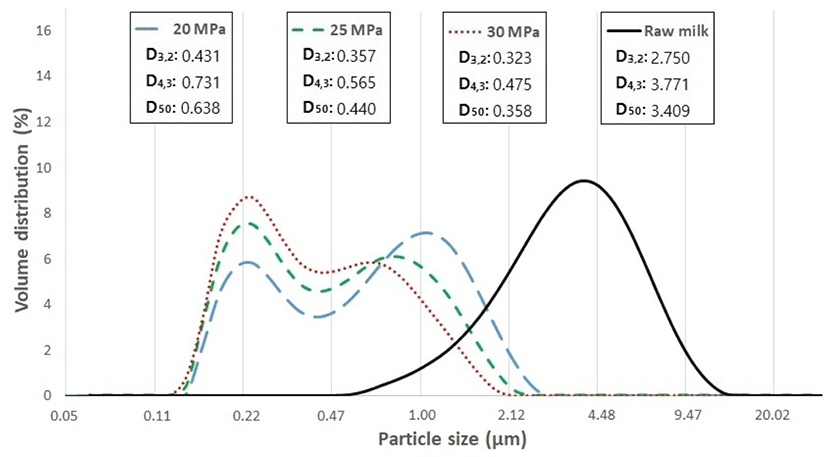
Changes in particle size distribution were monitored during storage at room temperature. The proportion of large fat globules (>1 μm), which was closely related to the fat rising in UHT milk, produced 20, 25, and 30 MPa homogenization pressure was about 22%, 13%, and 6%, respectively (Table 1). The proportion of large fat globules tended to decrease at 8 and 16 wk of storage, regardless of homogenization pressure. This result was probably due to the creaming of milk fat globules at the milk surface. Considering that the majority of the total volume comes from large particles such as fat globules >1 μm, the proportion of small particle (0.5 μm) was increased with the disappearance of larger fat globules. Two-way ANOVA indicated that homogenization pressure led to a significant difference in the proportion of large-sized fat globules all throughout the storage period. An interaction between homogenization pressure and storage time was found, reflecting the proportion of large sized fat globules becoming more prominent as storage increased.
Li et al. (2018) reported that there was no difference in the mean diameter of fat globules of 2% fat UHT milk produced at three levels (13.8, 20.7, and 27.6 MPa) of 2-stage homogenization pressure during storage for 8 wk at 4°C. However, they did not report changes in the proportion of large fat globules, and the storage time in their study was shorter than in the present study. Moreover, fat content and storage temperature also affect the results of fat particle size distribution.
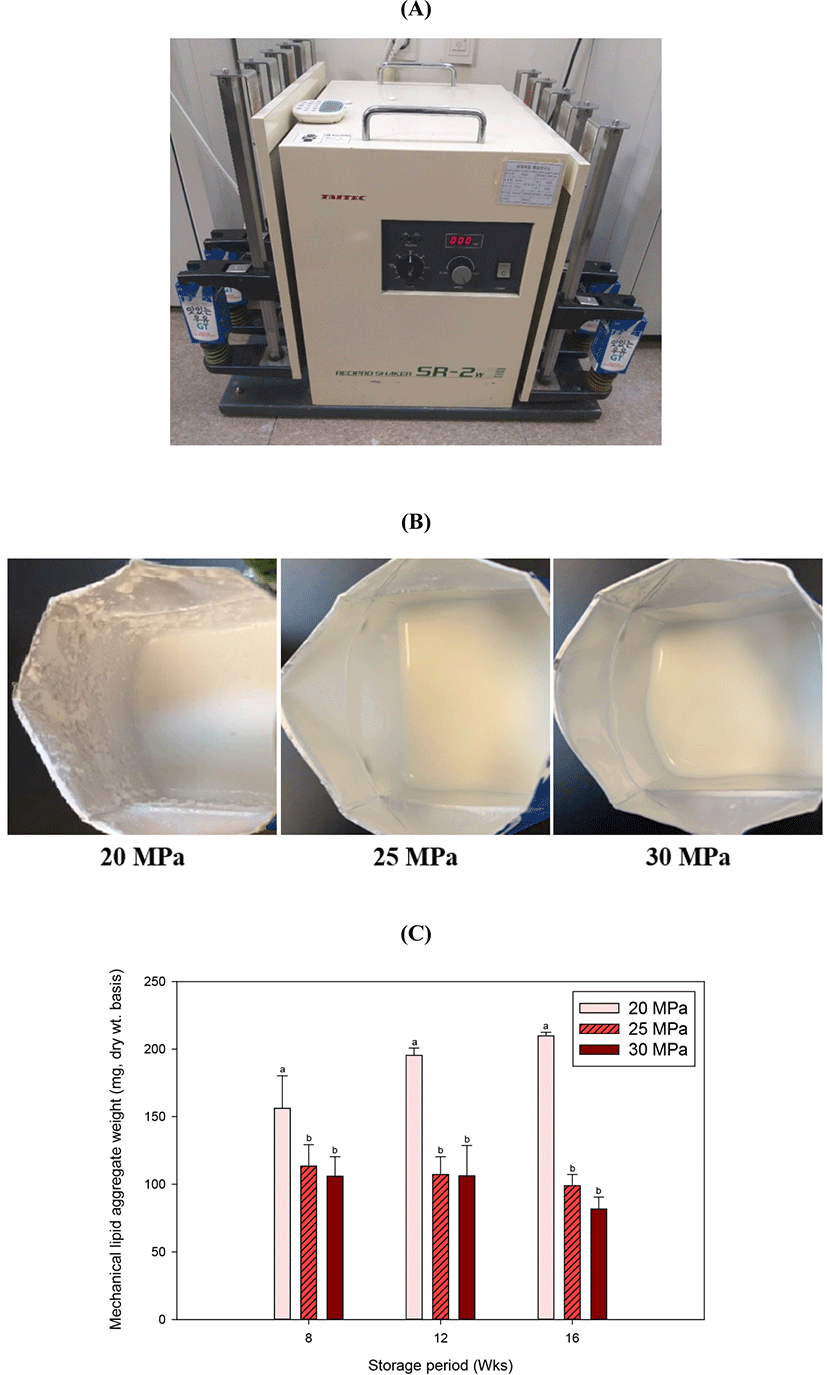
Mechanical stress-induced fat aggregation simulates aggregation of milk fat globules in milk resulting from agitation during distribution. Mechanical stress-induced fat aggregates were negligible up to 6 wk of storage, but a significant difference was observed at 8 wk of storage. The fat aggregates in milk produced at 20 MPa was significantly higher than those of milk produced either 25 or 30 MPa, while no difference was found between the milk produced at 25 MPa and the milk produced at 30 MPa. This difference was maintained all throughout the storage period (Fig. 2C). In milk produced at 20 MPa the amount of fat aggregates increased as the storage period increased, but there were no changes in the other milk samples. This difference was probably due to the difference in particle size distribution of milk fat since the aggregation rate increases with the size of the fat globules. The chances for collision increased for larger fat globules, and also stronger deformation of fat globules can occur in larger fat globules (Fredrick et al., 2010).
Homogenization results in substantial changes in the size and structure of fat globules. Milk fat globules are surrounded by a milk fat globule membrane (MFGM), which mainly consist of membrane specific glycoproteins and phospholipids with complex structures (Dewettinck et al., 2008). Homogenization disrupts MFGM so that milk plasma proteins are incorporated in the fat globule surface, since the original extent of MFGM is not enough to cover the newly increased surface area (Lee and Sherbon, 2002). The composition of the newly organized milk fat globule surface plays an important role, because proteins adsorbed on the interfacial layer exert steric and electrostatic repulsive forces that retards the rate of aggregation of fat droplets (Fredrick et al., 2010).
The role of individual components of MFGM on emulsion stability is still uncertain, and even more complex players (caseins and whey proteins) are involved after homogenization of milk. These issues should be further studied in the future. Cano-Ruiz and Richter (1997) reported that the protein load onto the fat surface area was increased by homogenization (30, 60, and 90 MPa), whereas the protein composition in newly formed membrane was not significantly altered at different homogenization pressures. However, they used higher homogenization pressures than in our study (20, 25, and 30 MPa). Currently, most countries including Korea use two-stage homogenization at 20 MPa for the production of UHT milk. This result suggests that an increase of homogenization pressure up to 25 and 30 MPa can provide a positive effect in minimizing undesirable mechanical stress-induced fat aggregation, especially in long shelf life UHT milk products.
In addition, there is currently no standard method to assess the amount of fat globule disruption and aggregation caused by agitation. The method evaluated in the present study was able to determine agitation-induced fat aggregation quantitatively with good reproducibility. This method can effectively predict the potential for problematic fat aggregation during the distribution of UHT milk.
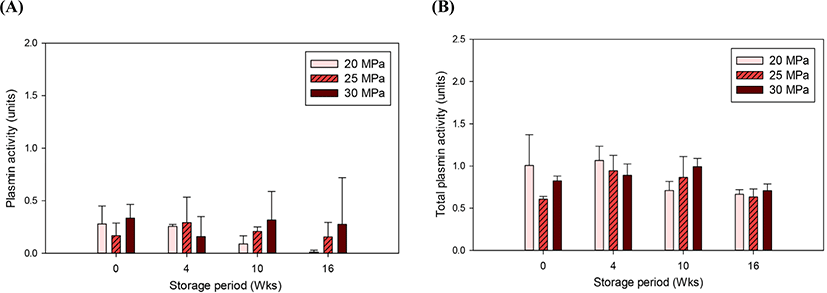
Plasmin is a highly heat-resistant native protease in milk, and significant plasmin activity is still remains after UHT processes (Kennedy and Kelly, 1997). The changes in plasmin activity of UHT milk were monitored during 16 wk of storage. As shown in Fig. 4A, no significant changes were found due to either the homogenization pressure or the storage period. Total plasmin activity of UHT milk, which was measured after plasminogen activation, also showed no difference (Fig. 4B). García-Risco et al. (2003) examined the effect of high pressure on the plasmin activity of skim milk. Treatment with a high pressure (200 or 400 MPa for 15 min) caused disintegration of the casein micelle structure and increased the solubilization of caseins and plasmin in serum. However, they did not find a difference in plasmin-induced casein proteolysis between the unpressurized milk and pressurized milk. Li et al. (2018) reported that in 2% fat milk, homogenization pressures applied similar to those in the present study (13.8–27.6 MPa) did not result in significant structural changes in proteins for up to 8 wk of storage at 4°C. Homogenization prior to indirect UHT sterilization make UHT milk less vulnerable to proteolysis. The pressure-induced unfolded β-LG may have complexed with plasmin and/or had increased interaction with κ-CN at the casein micelle surface (García-Risco et al., 2002; Scollard et al., 2000). In both cases, plasmin-mediated proteolysis can be inhibited. It has been reported that plasmin-induced hydrolysis of the κ-CN and β- LG complex at the casein micelle surface facilitates age gelation in UHT milk (Datta and Deeth, 2001) but no hint of age gelation or viscosity increase was not observed in the present study.
The extent of proteolysis in UHT milk during storage was determined by quantification of the TCA-soluble peptides. As shown in Fig. 5, there was no significant difference in TCA-soluble peptides for UHT milk produced at different homogenization pressures. However, proteolysis was continuously increased with storage time. This result suggests that plasmin activity did not differ significantly in the range of tested homogenization pressures, and it was consistent with the result of plasmin activity in Fig. 4.
The production of small peptides by proteolysis is likely to affect the sensory quality of UHT milk. Caseins are sensitve to proteolysis, and accumulation of peptides, especially from αS1- and αS2-CN by plasmin, caused bitterness to develop in directly heated UHT milk (> 150°C, <0.2 s) during 14 wk of storage at 20°C (Rauh et al., 2014). Topçu et al. (2006) reported that TCA souble nitrogen significantly incresaed in UHT milk during storage, which is consistent with the present study. They also maintained that UHT milk with a high somatic cell count (SCC: 621,000/mL) resulted in a greater extent of proteolysis and higher proteinase and plasmin activity than those of UHT milk with a low SCC (212,000/mL). The high SCC also increased susceptability to gelation for UHT milk (Datta and Deeth, 2001). However, the quality of raw milk used for the present was first grade of milk (SCC: 137,000/mL; total bacterial count: <30,000 CFU/mL), and the contribution of bacterial proteinase should be low.
The changes in TBARS of UHT milk during storage were determined as an indicator of lipid oxidation. As shown in Table 2, the homogenization pressures used in this study did not influence oxidation susceptibility and no significant difference was found during the storage. In general, sensitivity to lipid oxidation can be increased by homogenization because of increased fat surface area. On the other hand, homogenization of milk decreases the sensitivity of milk to autoxidation, which might be due to transfer of phospholipids containing the high unsaturated fatty acids from MFGM to milk serum (Walstra et al., 2005). Pereda et al. (2008) reported that ultrahigh homogenization pressure (300 MPa) accelerated production of TBARS. They suggested that incomplete coverage of the fat surface with caseins due to extensively increased surface area might be responsible for the increased lipid oxidation. Based on this result, homogenization pressure tested in the present study did not affect the production of lipid oxidation-mediated off-flavor in UHT milk.
Conclusion
The increase of homogenization pressure from 20 MPa (the typical homogenization pressure employed in the Korea dairy industry) to 25–30 MPa, mechanical stress-induced fat aggregation significantly decreased without affecting the susceptibility to lipid oxidation. Minimization of mechanical stress-induced fat aggregation by the control of homogenization pressure has significant promise to reduce undesirable consumer claims within the dairy industry. The testing method described for the determination of mechanical stress-induced fat aggregation is unique and can be used effectively to predict possible agitation-induced fat aggregation in long shelf-life dairy products, such as commercially sterilized whole milk. There were no significant differences in plasmin activity or TCA (12%, w/v) soluble peptides, which related to the age gelation of UHT milk. As subsequent research, the impact of changes in homogenization pressure on sensory attributes of UHT milk and dairy products is currently under study.













