Introduction
Beijing roasted duck is one of the most well-known Chinese ethnic dishes and is characterized by crispy skin and tender meat (Chen et al., 2009). Generally, the tender meat is formed by the denaturation, aggregation and gelation of proteins under the heating. Myosin is the most abundant and important protein for the tenderization of roasted duck myofibrils (Guo et al., 2017). The properties of myofibrillar proteins, especially myosin, play a crucial part in all technological processes involving heat treatments (Zhou et al., 2014; Zhou et al., 2018). Previous studies have focused on the thermal gelation in pork, chicken and rabbit (An et al., 2018; Brewer et al., 2005; Chin et al., 2009; Xue et al., 2018). The properties of myosin gels have been widely studied with regard to different parts of the animal, animal breeds and ionic strengths (Hollung et al., 2014; Xue et al., 2018), but the age has been minimally researched.
A previous paper (Cross et al., 1972) has reported that the tenderness of roasted sheep fore legs has a significantly negative correlation with chronological age. With the feeding days, there will be changes in the types and contents of flavor substances in poultry meat (Liu et al., 2013), which will influence meat quality such as colour, flavor and texture due to different ages of muscles. As the raw material of Beijing roasted ducks, the Beijing ducks are usually slaughtered at 22 to 45 days. The myosin protein isoforms changed during growth, which affected their thermal stability and gel quality. The thermal stability and gel properties of myosin affect the texture and water retention of duck meat. However, few studies have focused on the myosin gelation properties of duck meat in relation to age. The effects of age on the meat tenderness in other breeds of livestock and poultry meat have been studied (D’Alessandro et al., 2019; Jaborek et al., 2018; Polidori et al., 2017), but how the thermal stability of myosin in Beijing duck affects the texture of the gel has not been studied.
The main objective of the present study was to investigate the properties of myosin gel extracted from Beijing duck meat samples at several common ages, by measuring the gel properties, secondary structure changes and chemical reactions that occur during heating, in order to furtherly understand the role and underlying mechanism of myosin gel, supplying the best choice of raw Beijing duck meat.
Materials and Methods
Beijing ducks were raised at Dong Feng (Hebei, China) under the same conditions, and feed and water were available at all times. During their rearing, six male ducks were chosen randomly at 22 (1.14±0.13 kg), 30 (1.79±0.17 kg), 38 (2.54±0.25 kg) and 46 days (2.99±0.25 kg). Ducks of the same age were slaughtered at Dong Feng (Hebei, China). After slaughtering, both breasts were rapidly removed, trimmed of visible fat and connective tissues, and snap-frozen with liquid nitrogen immediately. All the samples were transported to the laboratory in an ice box and stored at −80°C till use.
The extraction of myosin was according to the literature (Han et al., 2015; Pan et al., 2017) with some modifications. All steps of the extraction were taken at 4°C. The minced meat samples (300 g) were mixed with 1,500 mL cold buffer (0.1 M Tris-HCl, 20 mM EDTA, pH 7.0) and homogenized twice. And then, the mixture was centrifuged at 3,000×g (10 min, 4°C), and the precipitates were resuspended three times with homogenizer (Ultra-Turrax T25, IKA, Staufen, Germany) in three volumes of buffer A (0.1 M KCl, 0.02 M KH2PO4/K2HPO4, 1 mM EGTA and 2 mM MgCl2, pH 7.0). Then, the material was centrifuged at 6,000×g for 10 min and the supernatants were diluted with nine volumes of cold distilled water and precipitated at 4°C overnight. After the supernatant was removed via syphon, the precipitates were centrifugated at 12,000×g for 12 min and resuspended with 3 volumes of 0.1 M KCl (pH 7.0), which was followed by centrifugation (1,500×g for 10 min). Afterwards, the precipitates were resuspended in 0.6 M KCl-phosphate buffer (0.15 M KH2PO4/K2HPO4, pH 6.5) and stirred for 30 min slightly.
The protein concentration was measured by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The final protein concentration was adjusted to 15 mg/mL using the 0.6 M KCl buffer (pH 6.5), and the solution was stored at 4°C until further testing.
The myosin solutions were placed in 10 mL beakers (Shubo Company, Chengdu, China) and heated in a water bath (Ronghua Company, Jintan, China) from 25°C to 80°C at the rate of 1°C/min and then incubated at 80°C for 20 min. Afterwards, the beakers were immediately cooled in the ice and stored at 4°C overnight before the tests were made.
Centrifugation was used to analyze the WHC (%) based on the method developed by Pan et al. (2017) with a slight modification. Each gel was centrifuged at 10,000×g for 10 min at room temperature. The WHC was expressed as a percentage of gel weight after centrifugation to the initial gel sample weight. The experiments were conducted in triplicate.
The strength of the gels was analyzed using a TA-XT plus Texture Analyzer (Stable Micro Systems, Godalming, UK) according to the method described by Kotwaliwale et al. (2007) with a slight modification. The samples (10 mm in diameter, 10 mm in height) were compressed using a probe (P50) with a distance of 50% the initial height and 0.05 N trigger force. The pre-test speed was set to 2.0 mm/s, and the test speed and post-test speed were 1.0 mm/s. The experiments were conducted in six replicates.
The thermal stability of myosin was determined by differential scanning calorimetry (DSC) using Q200 controlled by a Texture Analysis 5000 system (TA Instruments, New Castle, DE, USA). Each sample (15 mg) was hermetically sealed in an aluminum pan and heated from 20°C to 90°C with 5°C /min scan rate. An empty pan was used as the reference. The transition temperatures (Tmax) were recorded.
The surface hydrophobicity was measured as described by Yongsawatdigul and Sinsuwan (2007) with some modifications, using 8-anilino-1-naphthalene sulfonate (ANS) as the fluorescent probe. The myosin solutions were diluted to 0.125, 0.25, 0.5, and 1.0 mg/mL. Subsequently, 10 μL of 8.0 mM ANS solution (dissolved in 0.01 M Tris-HCl, pH 7.0) was added to 2 mL of the myosin samples, and the resulting samples were kept in the dark at room temperature for 10 min. The fluorescence was determined with a luminescence spectrophotometer using an excitation wavelength of 374 nm and emission wavelength of 485 nm. The surface hydrophobicity was expressed as the initial slope of the fluorescence intensity against the protein concentration (Excel 2003; Microsoft, Redmond, WA, USA).
The LF-NMR spin–spin relaxation time (T2) measurements were examined according to the method of Han et al. (2009) using an NMR-NMI20 (Niumag, Shanghai, China) system operated at 22 MHz. Two grams samples were placed in glass tubes. Transverse relaxation (T2) was measured with 64 scan repetitions, 10,000 echoes, 6,000 ms between scans, and 300 ms between pulses of 90° and 180°. All treatments were run in triplicate and the tata were collected.
The secondary structure of the samples was determined as described previously (Liu and Zhong, 2013) using an MOS 500 spectrophotometer (Bio-Logic, Claix, France). The heated samples were adjusted to 0.1 mg/mL and placed in a quartz absorption cell with a 0.01 cm optical path length. The spectra of the myosin samples were recorded at 25°C and from 190 to 250 nm at a scanning speed of 100 nm/min, and the scanning interval time was 2 s. Circular dichroism (CD) is represented by the average residue ellipticity [θ] (deg·cm2/dmol). Dicroprot software package (IBCP, Lyon cedex, France) was used to calculate the percentage content of the myosin secondary structure automatically.
A scanning electron microscope (SEM) was used to observe the microstructures of the myosin gel. The gel samples were pre-treated according to the method described by Ma et al. (2012). The gels were were dehydrated in a series of ethanol ratios (30%, 50%, 70%, 90%, 95%, and 100%) and then freeze-dried and coated with gold. Finally, a SEM were used with an accelerating voltage of 15 kV.
The data were analyzed using program of SPSS statistics 23.0 (SPSS, Chicago, IL, USA). The ages (22, 30, 38, and 46 days) were considered as fixed variables, and the duck breast muscles for each age were the random effect. The data were subjected to one-way analysis of variance (ANOVA) followed by Duncan's multiple range test to determine the statistical difference. Pearson correlations were performed to investigate the relationship among the chemical and histological values, WHC and gel strength. The results were presented as the means±SD. Significant differences among the mean values were determined at a level of p<0.05.
Results
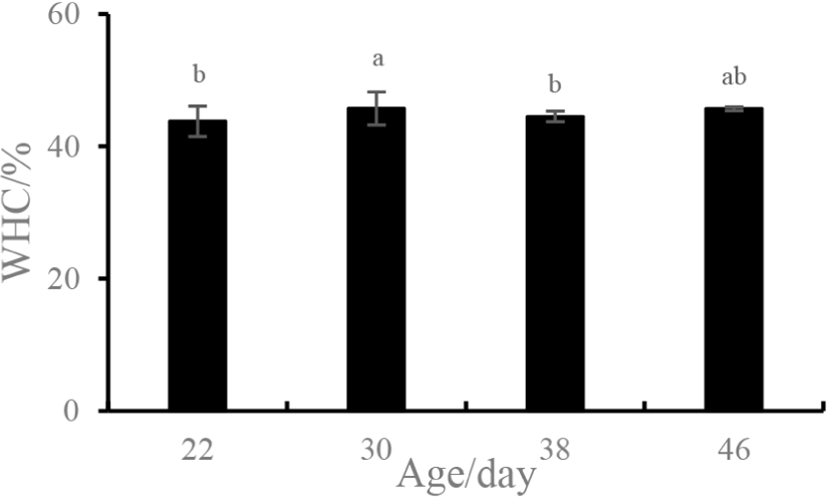
The WHC is one of the most important functional properties of a gel system and is used to indicate a gel’s ability to bind water (Yao et al., 2018). As compared to 22-day-old sample, the WHC of the myosin gel extracted from the 30-day-old sample increased significantly (p<0.05) from 43.77% to 45.71% and then decreased at 38 days (p<0.05) (Fig. 1). However, no difference in the WHC was detected for the samples of 22, 38, and 46 days (p>0.05).
As an important indicator, the gel strength of proteins were generally used to assess food texture (Foegeding et al., 1986). The gel strength of the myosin gel obtained from various ages of ducks is presented in Fig. 2. Initially, the gel strength significantly increased for duck breasts obtained at ages ranging from 22 to 30 days (p<0.05), which was followed by an extreme decrease for the 38-day-old duck breast (54.00 g). However, the gel strength of 46-day-old samples increased again and was similar to that of the 22-day-old groups (p>0.05). Thus, an age of 30 days resulted in the best gel strength (72.56 g).
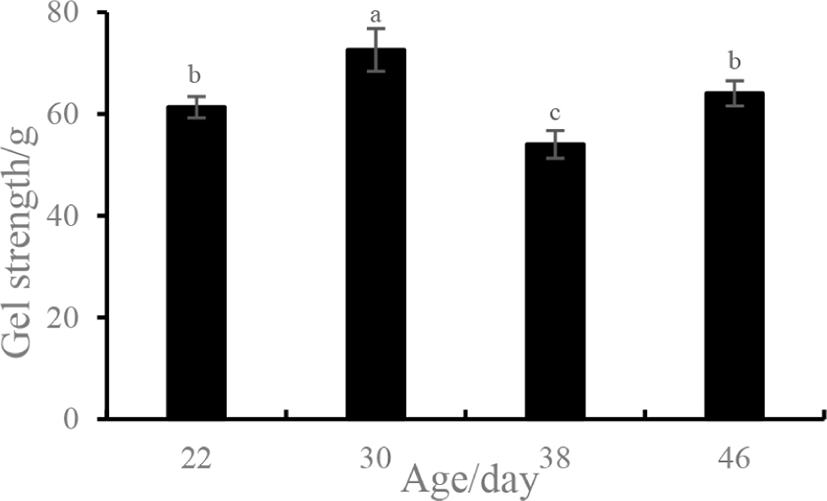
Table 1 shows the T2 relaxation time (ms) and proportion (%) of each relaxation component of the myosin gels extracted from different aged duck breasts. For T22, the relaxation peak decreased from 510.50 ms (22 days) to 377.41 ms (30 days), then increased to 500.52 ms (38 days), and finally decreased to 390.56 (46 days), which further reflected a more compact network structure that limited the water migration in the gel matrix. The proportion of T22 for the 22-day-old sample was significantly lower than that of the 30-day-old sample (p<0.05). There was no significant difference in the proportions of T22 among the samples obtained from the duck breasts aged for 30, 38, and 46 days (p>0.05), but the T22 proportion of the 30-day-old sample is larger than that of the other groups.
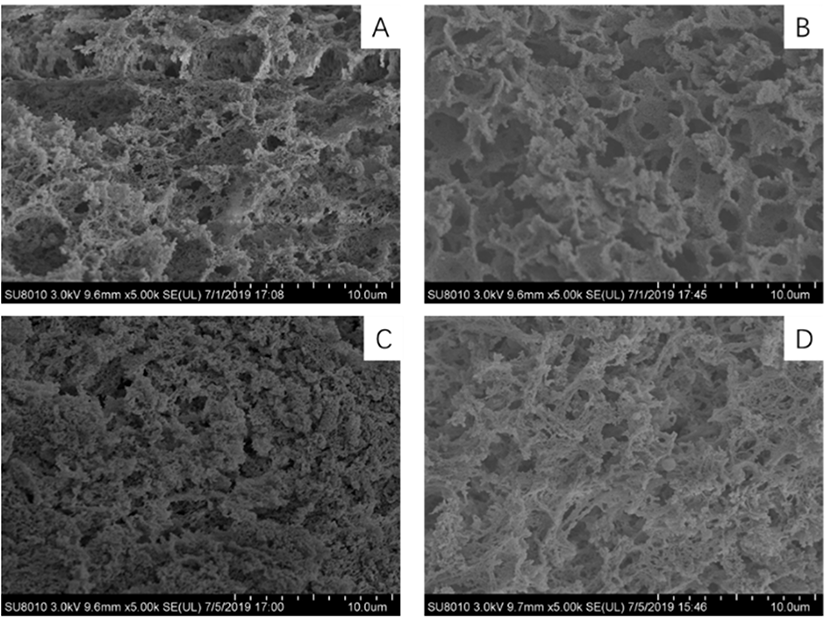
The microstructures of the myosin gel extracted from the duck breasts of different ages are illustrated in Fig. 3. As shown in Fig. 3B, the three-dimensional network structure of the myosin gel from the 30-day-old duck breast was more compact and fine compared to that of the other groups, which formed smooth, continuous and high-density protein gel matrices. The microstructure of the myosin gel obtained from the 38- and 46-day-old duck breasts had rougher appearances and more disordered gel matrices (Fig. 3C and D). The microstructure of the 22-day-old myosin gel had a disordered structure with large cavities or voids. The result suggested that age influences the connectivity of the protein strands in the gel network, in which the alignment or aggregation of the proteins was augmented.
The temperatures of denaturation of the myosin were presented in Table 2. There were two absorption peaks, with one occurring at approximately 45°C and the other at 56°C, and these peaks corresponded to the denaturation of the head and tail, respectively. Table 2 showed that there was no significant effect on the Tmax1 of myosin (p>0.05), while the Tmax2 of both the myosin extracted from 30- and 38-day-old duck breast is significantly higher than that of 22-day-old sample (p<0.05).
| Ages/d | Number of peaks | Denaturation temperature | |
|---|---|---|---|
| Tmax1/°C | Tmax2/°C | ||
| 22 | 2 | 45.38±0.14a | 55.57±0.25b |
| 30 | 2 | 45.46±0.20a | 56.21±0.28a |
| 38 | 2 | 45.61±0.22a | 56.24±0.20a |
| 46 | 2 | 45.60±0.47a | 55.51±0.98ab |
The protein surface hydrophobicity was used to evaluate the denaturation degree of proteins, which reflected the relative content of hydrophobic amino acids on the surface of protein molecules (Chelh et al., 2006). As shown in Fig. 4, as the heating temperature was increased, the relative fluorescence intensity of myosin first slightly increased from 25°C to 50°C and then rapidly decreased from 50°C to 70°C (p<0.05); after 70°C was reached, the surface hydrophobicity was stable (p>0.05).
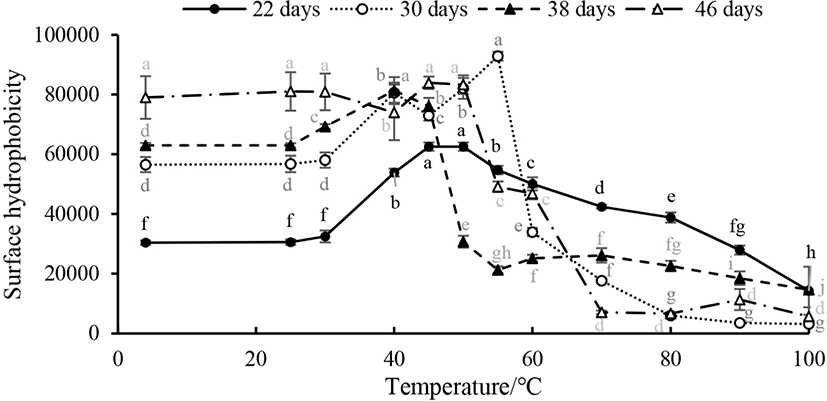
Table 3 shows the content of the chemical forces of the myosin extracted from different ages of duck breast and heated at 80°C. The content of hydrogen bonds in the myosin obtained from a 30-day-old duck breast was 0.36±0.04, which is significantly lower than that of the duck breasts of other ages. Ionic bonds are the main force that maintains the natural structure of myosin, but heating can break the ionic bonds between protein molecules, leading to protein aggregation and gelation. Moreover, the content of disulfide bonds in myosin extracted from 30-day-old duck meat was significantly higher than that of the others (Table 3).
CD is a common method used to study the secondary structure of proteins. As seen in Fig. 5, the protein in the myosin gel is dominated by an α-helix structure. As the heating temperature gradually increases, the content of α-helices in the myosin gels obtained from duck breasts of all ages decreases gradually, and the rate of decline is the largest in the range of 40°C–70°C, indicating that this is the temperature range in which the myosin secondary structure is most sensitive to changes. The content of α-helices decreased from 94.81% (25°C) before heating to 16.73% at 100°C, indicating that the myosin tail gradually extended with the increase of the temperature, and the protein molecules unfolded. Neighboring myosin molecules entangle with each other, resulting in the aggregation of proteins and the formation of large myosin aggregates. The frequency of β-folding and irregular curling increased significantly. When heated to the gel formation temperature (80°C), the α-helix structure content of the samples obtained from the 22-day-old duck was significantly higher than that of other days of age.
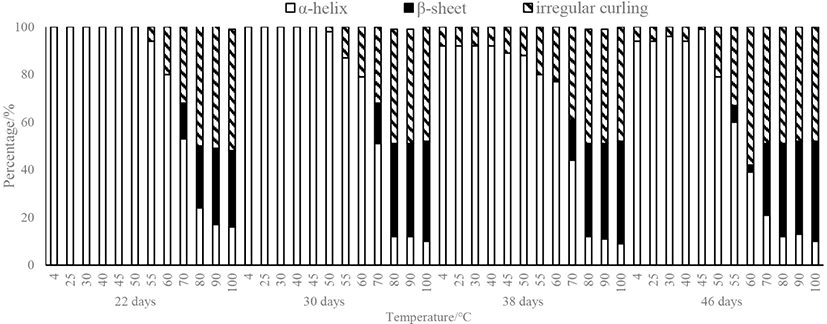
Table 4 shows the correlation coefficients for the chemical and histological values, WHC and gel strength. The WHC of the myosin gel was negatively related to the T2 relaxation time (R=−0.97) and surface hydrophobicity (R=−0.99) while positively associated (R=0.98) with the ionic bonds. The ionic bonds were negatively related to the T2 relaxation time (R=−0.96) and surface hydrophobicity (R=−0.97). However, there was no correlation found between the gel strength and the other parameters. Differences in the populations involved or the portions of the population sampled probably explain these differences in magnitude and/or direction of the assumed relationships.
Discussion
The result of DSC showed that the myosin extracted from both the 30- and 38-day-old breast is more stable than that extracted from duck breasts of other ages. The stability, morphological structure and functional properties of the protein had a great effect on the hydrophobic properties. In this study, the surface hydrophobicity increased from 25°C to 55°C, and continued heating lead surface hydrophobicity to decrease, which is similar to Promeyrat’s report (Promeyrat et al., 2010). This change was due to the hydrophobic side chains of the protein, which were exposed to the water environment and caused protein aggregation, the formation of disulfide bonds. And then hydrophobic groups embedded within the myosin, so that the surface hydrophobicity decreased. The surface hydrophobicity (ANS-S0) of the myosin extracted from different ages of duck reached its maximum value at different temperature points. The myosin ANS-S0 of the 22- and 38-day-old duck reached a maximum value at 45°C. At 46 days old, ANS-S0 reached its maximum value at 50°C, while at 30 days old, ANS-S0 reached its maximum value at 55°C. The release of myosin light chain may be the reason for the increase of surface hydrophobicity during heating, forming hydrophobic patches at the head-head interactions of myosin heads that occurred at 40°C (Sharp and Offer, 1992).
With regard to the secondary structure displayed in Fig. 4, the content of α-helices presented between 4°C and 40°C stabilized at approximately 100% or 94% for myosin, but decreased immediately upon heating from 40°C to 80°C and remained stable at 12% for temperatures greater than 80°C, which was obtained from 38- and 46-day-old duck breast. This result implied that the myosin rod containing abundant α-helical structures did not participate in the aggregation under 40°C. The content of β-sheets increased as the temperature ascended from 25°C to 100°C. From 40°C to 70°C, it had a significant change from approximately 0% to 15%. When the temperatures reached above 80°C, the content of β-sheets only increased slightly but not significantly. The content of random coils increased during heating, and the increase in the area occurred mainly in the temperature range from 40°C to 80°C. Many studies have found that in the process of heat-induced formation of myosin gel, there is a widespread decrease in the content of α-helix and increase of the content of β-sheet and random coil (Li-Chan and Nakai, 1991). Liu et al. (2008) studied the variations in the myosin secondary structure extracted from porcine and concluded that the α-helix content reduced from 90% to 40% during heating, while the β-sheet content increased nonlinearly (Liu et al., 2008). Xu et al. (2011) explored the process of heat-induced gel of pork myofibrillar proteins, and the results had a similar tendency, which was consistent with our study.
The correlation coefficients exposed that the WHC of the myosin gel was negatively related to the T2 relaxation time (p<0.05) and surface hydrophobicity (p<0.01) and positively associated (p<0.05) with the ionic bonds. As all knows, the WHC of a gel is closely relevant to the morphology of the matrix and cavity in the three-dimensional network, as well as the interactions between protein and water that existed in the gel matrix (Han et al., 2014). Water will be entrapped in the gel network as a result of the protein cross-linking and aggregation during gelling (Tintchev et al., 2013). In addition, the fine gel microstructure was conducive to the stability of the immobilized and bound water.
Compared to the other samples, the results showed that the myosin extracted from the 30- and 46-day-old duck breasts had significantly smaller T22 values (p<0.05) and higher proportions of T22, which were meant that these samples contained more bound water and were stable during transformation. It has been proposed that myosin heavy chains (MHC) have various properties when derived from different types of muscle (Lopez-Diaz et al., 2003). It was generally believed that the ability of MHCs to cross-link depended on the location of the MHC in one or more specific sites of myosin, and any structural or functional differences in these sites would result in different gel properties from different fish myosin (Chan et al., 1993).
The structure and physicochemical properties of gelation depended on the relative rates of protein denaturation and aggregation. With the increase in temperature, the α-helix content in myosin decreased gradually, while the β-sheet content tended to increase. The loss of α-helical structures possibly lead to the exposure of active groups such as hydrophobic and sulfhydryl groups, resulting in temperature induced cross-linking, and shaped a three-dimensional network capable of retain water. When heated to 80°C, more α-helices were removed, leading to the space between the molecules becoming smaller and reducing the space available to retain water and reducing water retention. Moreover, the content of disulfide bonds in myosin obtained from 30-day-old duck meat was significantly higher than that of the others (Table 3), which is the main covalent bond that forms during heat-induced protein gelation. Thus, the higher content of β-sheets and disulfide bonds from 30-day-old duck resulted in higher gel WHC.
The finer network with more protein cross-linking was associated to the increased WHC, as indicated by the higher WHC (Fig. 2). Chantrapornchai and Mcclements (2002) reported that fine-stranded networks with comparatively small pores had high WHC, while the particulate networks containing relatively large pores had low WHC (Chantrapornchai and Mcclements, 2002). Joachim and Koehler (2005) also reported that the microstructure was determined by the spreading of the water relaxation times: the low relaxation time was closely related to the fine gel microstructure. The T22 relaxation time for the gel obtained from 30-day-old duck meat was significantly lower than that of the 20 and 38-day-old duck meat (p<0.05), demonstrating that the water binding degree in the protein network structure was increased at this age, which could be concluded by the size of the pores and the aggregation state of the gel structure. The latter gel was denser and more uniform, with smaller pores which possess stronger interaction with water in the gel.
A higher gel strength was associated with an increased breaking force, and a more rigid gel was the result of a denser network (Buamard and Benjakul, 2015). Myosin gels were supported by a network structure that was composed of S–S and hydrophobic bonds that formed as a consequence of heating, and with the increased in the moisture content, the covalent bonds could not be damaged easily by heating; therefore, the network structure also could not be readily broken. As the increase in the denaturation degree of protein, the hydrophobic groups exposed more, and protein molecules aggregated in a gel matrix by enhancing the interactions of the proteins. Considering the endpoint temperature (80°C) of the gel, the content of disulfide bonds in the myosin obtained from 30-day-old duck meat was notably higher as compared to the myosin obtained from the other age of duck meat (p<0.05), indicating that myosin formed more disulfide bonds at this temperature, making proteins bound more tightly. During the process of protein thermal denaturation, there more hydrophobic groups and sulfhydryl groups exposed. The exposed hydrophobic groups were grouped by their surface hydrophobicity, and the sulfhydryl groups linked to each other to form disulfide bonds. These interactions contributed to the formation of gel networks.
The higher α-helix content which are rich in sulfhydryl group, was higher in myosin tail than that of myosin head region. With the increase in temperature, the myosin tail region might be destroyed, and lentigo maligna melanoma (LMM) might deform, which caused α-helices lessening. Then, the interaction between the hydrophobic groups in the tail of the myosin promotes the formation of the network. In the previous studies it was noted that the damage of α-helices is interrelated to the unfolding of myosin, as determined by a study on different types of fish myosin (Chan et al., 1992). Nevertheless, it has been proposed that the development of the β-sheet structure might be an essential for the accumulation of cross-linked gel network with strong hydrogen bonding among molecules at high temperature, leading to the irreversible aggregation of proteins and the maintenance of the gel network (Han et al., 2015). Even though the temperature did not alter the content of secondary structure of myosin gel whereas, some of the secondary protein structures it can possibly vary (Sanchez-Gonzalez et al., 2008). When heated to the gel formation temperature (80°C), the α-helix content in the sample derived from the 22-day-old duck meat is significantly higher than that of the samples obtained from the other ages of duck meat, which indicated that the myosin aggregation degree is weak, and the formation of a network structure in the sample obtained from the 22-day-old duck meat was inferior to those of the other samples.
Conclusion
Present study demonstrated that the thermal stability was significantly improved for 30-day-old duck breast, while which myosin gel had a compact and ordered three-dimensional network structure with small and regular cavities. Overall, myosin gels obtained from the 30-day-old duck breast had better properties than the gels obtained from the other ages’ duck breasts, which can be considered as the selection criteria for the raw material of Beijing roasted ducks.













