Introduction
Honey is a well-known natural sweet food and has been considered an important source of traditional medicine (Eteraf-Oskouei and Najafi, 2013). Honey can be classified by the floral source because honeybees use nectar to produce honey. If a honeybee uses the nectar of many types of flowers to produce honey, it is classified as polyfloral honey, and it is also referred to as wildflower honey; however, if a honeybee uses the nectar of one type of flower to produce honey, it is classified as monofloral honey (Louveaux et al., 1978). Because pollen is a traceable floral source, a melissopalynological analysis is used to identify the types of plant sources used by honeybees for the production of honey. When the pollen of monofloral honey is analyzed in practice, many other types of pollen are often detected because honeybees have access to other types of honey plants even if the beehives are in a field where honeybees have access to only one type of honey plant. Therefore, generally, honey is recognized as monofloral honey when the content of the majority of the pollen is more than 45% of the total pollen (Olga et al., 2012; Soria et al., 2004).
There are several types of monofloral honey worldwide. Because each type of monofloral honey has distinct characteristics, such as flavor, taste, and physiochemical properties, which are derived from their botanical origins, there has been increased consumer demand for a better flavor and specific pharmacological attributes of monofloral honey, and thus the commercial value of monofloral honey has gradually increased (Pires et al., 2009).
In South Korea, more than 70% of annual honey production is comprised of Acacia (Robinia pseudoacacia) honey; however, recently, the total amount of honey production in South Korea has dramatically decreased because climate change has decreased the period of blooming as well as the growth of the acacia flower, resulting in the reduction of total honey production in South Korea (Kohsaka et al., 2017). Therefore, developing a new candidate for a honey plant to compensate for the decrease in Acacia honey production is strongly required in South Korea. The Hovenia (Hovenia dulcis) tree is found in East Asian countries, such as China, Japan, and Korea, and is also reported to be found in the Himalayas up to altitudes of 2,000 m (Hyun et al., 2010). The Hovenia tree prefers to grow in a sunny position, and the blooming period is about 20 days from June to July. The nectar production of the Hovenia flower is higher than that of the Acacia flower (Han et al., 2018; Song et al., 2014). Thus, Hovenia trees have been considered a candidate for honey plants in South Korea. There is a regional report that discusses the antioxidant activity of Hovenia honey produced in South Korea; however, the honey used for the study was harvested in open fields without a pollen analysis, indicating a low reliability regarding the purity of the Hovenia honey used (Paik et al., 2015). Therefore, for a more accurate and reliable evaluation of the value of Hovenia trees as honey plants, an investigation of the physiochemical, antioxidant, and antibacterial properties of Hovenia monofloral honey must be performed before increasing the number of Hovenia trees for the production of honey.
In this study, the physiochemical properties and antioxidant activity of Hovenia monofloral honey, which was prepared using a net house system, was investigated along with the antibacterial activity of Hovenia monofloral honey against foodborne bacteria.
Materials and Methods
Twenty-six Hovenia trees and honeybees (Apis mellifera) were cultured in a net house (23 m×13 m×9 m : W×D×H) constructed by the Korea Forest Research Institute (Suwon, Korea), and Hovenia monofloral honey-1 and Hovenia monofloral honey-2 were harvested on June 21, 2019, and July 2, 2019, respectively. Two types of acacia honey were obtained from the Korea Beekeeping Agricultural Cooperative and the National Institute of Forest Science, respectively, and were used as reference honey. Honey samples were stored at 4°C under a dark condition until analysis.
To determine the moisture content, the honey sample was dried in a dry oven (Wiseven WOF-105, Daihan Scientific, Seoul, Korea) at 105°C until a constant mass was obtained. Ash content was determined by calcinations in an Electric Muffle Furnace (JSMF-270T, JSR, Gongju, Korea) at 600°C until the honey sample reached a constant weight. An electrical conductivity (EC) of 20% (w/v) of a honey solution was measured using an EC meter. The hydroxymethylfurfural (HMF) and the carbon isotope ratio were measured using the standardized method listed in the Korean Food Code (Ministry of Food and Drug Safety of Korea, 2019).
Glucose, fructose, and sucrose content was determined using a high-performance liquid chromatograph (HPLC, Agilent Technologies, Palo Alto, CA, USA) equipped with an Ri-101 detector (Showa Denko K.K., Kawasaki, Japan). Briefly, the honey sample (5 g) was mixed with 25 mL of petroleum ether. Then, 25 mL of distilled water was added, and it was incubated in a water bath at 85°C for 25 min. The sugar solution extracted from the honey was filtered using a 0.45 μm membrane filter. Finally, it was separated using a PhenoSphere NH2 80A column (250 mm×4.6 mm, 5 μm, Phenomenex, Torrance, CA, USA). The mobile phase was composed of 80% acetonitrile. The injection volume of the samples was 20 μL with a flow rate of 1.0 mL/min.
The honey sample (1 g) was mixed with nitric acid (5 mL) and incubated for 1 h at room temperature. Then, the mixture was heated at 200°C for 2 h and made 20 mL by adding distilled water. Ca, Cu, Fe, K, Mg, Mn, Na, P, and Zn content was measured using inductively coupled plasma optical emission spectroscopy (720 ICP-OES, Agilent Technologies) equipped with a VistaChip II CCD detector (Agilent Technologies). The detection limit of the mineral content was less than 0.1 mg/L.
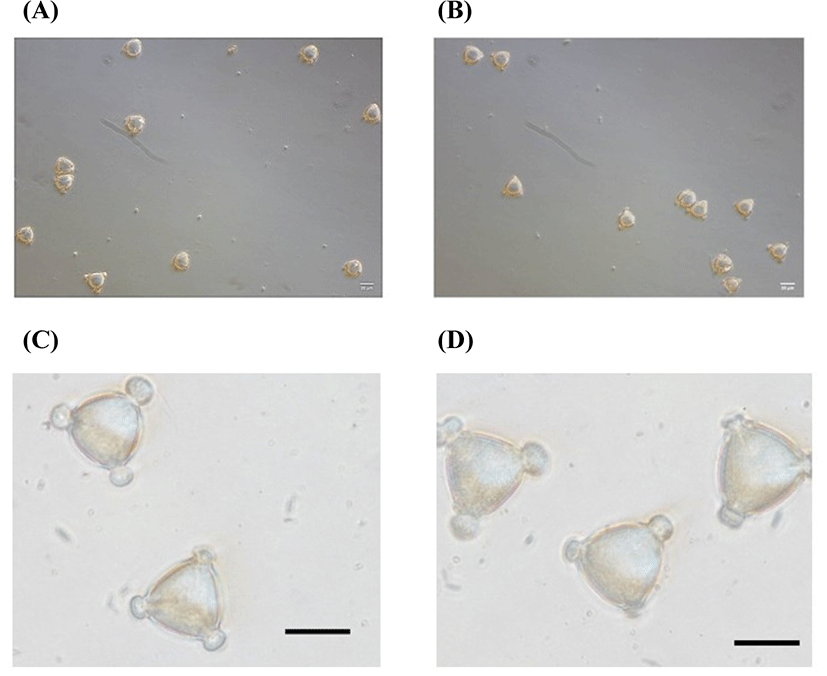
A melissopalynological analysis was performed according to the method of Louveaux (1978) with some modifications. The honey sample (10 g) was diluted in 10 mL of distilled water and incubated at 37°C for 10 min. After the honey solution was centrifuged at 1,500×g for 10 min, the sediment of the honey solution was washed with 5 mL of distilled water and centrifuged again at 1,000×g for 5 min. Then, the sediment was resuspended in 50 μL of 50% (w/v) glycerin. The sediment of the solution was spread on a 22×22 mm area on a slide. More than 300 pollen grains were photographed using a microscope (Nikon Eclipse Ti-S, Tokyo, Japan) and counted.
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of Hovenia honey was determined according to the method of Tuberoso (2013). Five hundred μL of an aqueous honey solution (10%, w/w) was mixed with 2.5 mL of a 200 μM DPPH solution and incubated in the dark for 1 h at room temperature. The absorbance of the solution was measured at 517 nm using a UV/Vis spectrophotometer (Optizen POP, Mecasys, Daejeon, Korea). DPPH scavenging activity was calculated by the following equation:
Where A is the absorption of all reagents, B is the absorption of the honey solution, and C is the absorption of all reagents without the honey solution. Trolox was used to determine the standard curve (25–300 μM, r2=0.995). The antioxidant capacity was expressed as the μmol of Trolox equivalent (TE)/100 g of honey.
The 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity of Hovenia honey was determined according to the methods of Tuberoso (2013). The ABTS radical solution was prepared by reacting 10 mL of 2 mM ABTS in PBS with 0.1 mL of 70 mM potassium persulfate. After 16–24 h of incubation in the dark at room temperature, the ABTS radical solution was diluted with PBS to obtain the absorbency of 0.7±0.1 at 734 nm. The 70 μL aqueous honey solution (5%, w/w) and the 1.8 mL ABTS radical solution was mixed and incubated for 6 min in the dark at room temperature. Finally, the absorbance of the solution was measured at 734 nm using a UV/Vis spectrophotometer (Optizen POP, Mecasys). The ABTS radical scavenging activity was calculated by the following equation:
Where A is the absorption of all reagents, B is the absorption of the honey solution, and C is the absorption of all reagents without the honey solution. Trolox was used to determine the standard curve (100–500 μM, r2=0.999). The antioxidant capacity was expressed as the μmol of Trolox equivalent (TE)/100 g of honey.
The total phenolic content of Hovenia honey was determined using the Folin-Cioalteu method (Meda et al., 2005). The 0.5 mL aqueous honey solution (10%, w/w) was mixed with 2 mL of the 0.2 N Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA) and incubated at room temperature for 6 min. Then, a 1.5 mL of 7% (w/v) sodium carbonate solution was added and incubated for 2 h at room temperature. Finally, the absorbance of the solution was measured at 750 nm using a UV/Vis spectrophotometer (Optizen POP, Mecasys). Gallic acid (Sigma-Aldrich) was used to determine the standard curve (12.5–200 μg/mL, r2=0.999). The total phenolic content was expressed as the mg of gallic acid equivalent (GAE)/100 g of honey.
The total flavonoid content of Hovenia honey was determined according to the methods of Kim et al. (2005). A 0.5mL aqueous honey solution (20%, w/w), 0.1 mL of 10% (w/v) aluminum nitrate solution, 0.1 mL of 1M potassium acetate solution, 0.5 mL of 80% (v/v) ethanol, and 2.8 mL of distilled water were mixed and incubated for 40 min at room temperature. The absorbance of the solution was measured at 415 nm using a UV/Vis spectrophotometer (Optizen POP, Mecasys). Quercetin (Sigma-Aldrich) was used to determine the standard curve (10-80 μg/mL, r2=0.999). The total flavonoid content was expressed as the mg quercetin equivalent (QE)/100 g of honey.
Four foodborne pathogens were used in the test. The gram negative bacteria, Escherichia coli O157:H7 (ATCC 35150) and Salmonella Typhimurium (KCTC 1925), were grown in Luria Bertani Broth (Difco, Michigan, MI, USA) at 37°C in an incubator. The gram positive bacteria, Staphylococcus aureus (ATCC 29213) and Listria monocytogenes (ACTC 3569), were grown in Brain Heart Infusion Broth (MB cell, Seoul, Korea) at 37°C in an incubator. The minimum inhibitory concentration (MIC) of Hovenia honey was determined by the broth micro-dilution method in 96-well microplates (Bucekova et al., 2018). The 50% (w/v) honey stock solution in a broth medium was diluted at different concentrations ranging from 1.56 to 50% (w/v). 90 μL of the honey solution was dispensed into each well. The bacterial cultures were diluted to the 105 CFU/mL using a broth medium. Then, 10 μL of the diluted bacterial culture was mixed to test the honey solution and was incubated at 37°C for 18 h. The absorbance of the culture medium was measured at 490 nm using a micro absorbance spectrophotometer (iMarkTM Microplate Reader, Hercules, CA, USA).
Results and Discussion
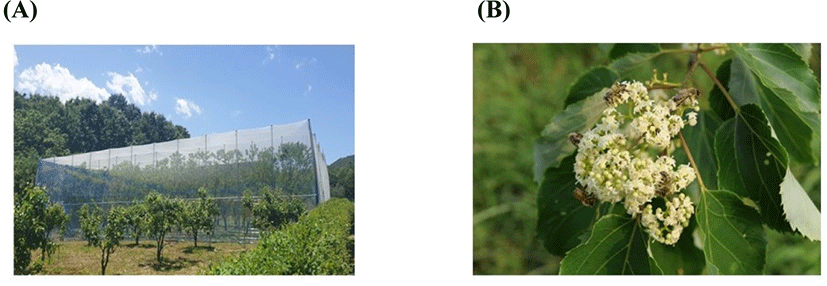
To produce Hovenia monofloral honey, honeybees and Hovenia trees were bred in a net house (Fig. 1), and honey was harvested on two different day. The purity was determined by a pollen analysis, and both honeys showed more than 95% of Hovenia pollen content, which indicated that the honey produced in the net house was Hovenia monofloral honey (Fig. 2). Because there is no dense, open area with Hovenia trees to produce Hovenia monofloral honey in Korea, even though the honey was harvested near Hovenia trees during the Hovenia blossom season, it may contain several other types of pollen, and the content of major pollen may be less than 45%. In general, when the major content of pollen is less than 45%, the honey is classified as multifloral honey. Thus, the net house, which can produce high-purity monofloral honey, could be an optimal small-scale system used to estimate the potential of the honey plant. Therefore, Hovenia monofloral honey harvested in a net house system is a good source to evaluate the potential of the Hovenia tree as a honey plant.
The physiochemical properties of Hovenia monofloral honey produced in the net house were investigated to determine the potential of the type of honey. As showed in Table 1, Hovenia monofloral honey was composed of glucose (29.0±0.42%), moisture (18.9±0.28%), fructose (35.9±0.78%), reducing sugar (64.9±0.35%), sucrose (3.9±1.63%), and ash (0.1±0.00%). The contents of HMF were not detected in Hovenia monofloral honey, and the carbon isotope ratio was −26.6±0.14 ‰. All contents of Hovenia monofloral honey were in the range of the international standards by Codex Alimentarius (2001) as well as the food code legislated by the Ministry of Food and Drug Safety of Korea (MFDS, 2019). In addition, the mineral contents of Hovenia monofloral honey included calcium (20.1±1.06 mg/L), potassium (407.5±3.11 mg/L), magnesium (10.7±1.1 mg/L), sodium (1.8±0.28 mg/L), manganese (2.9±0.14 mg/L), phosphorus (20.6±1.77 mg/L), and zinc (13.9±15.1 mg/L) (Table 2). These data suggest that Hovenia monofloral honey produced in a net house system could be used as a primary honey source, and the net house used in this study could be used for the large-scale production of Hovenia monofloral honey as well as other monofloral blossom honeys.
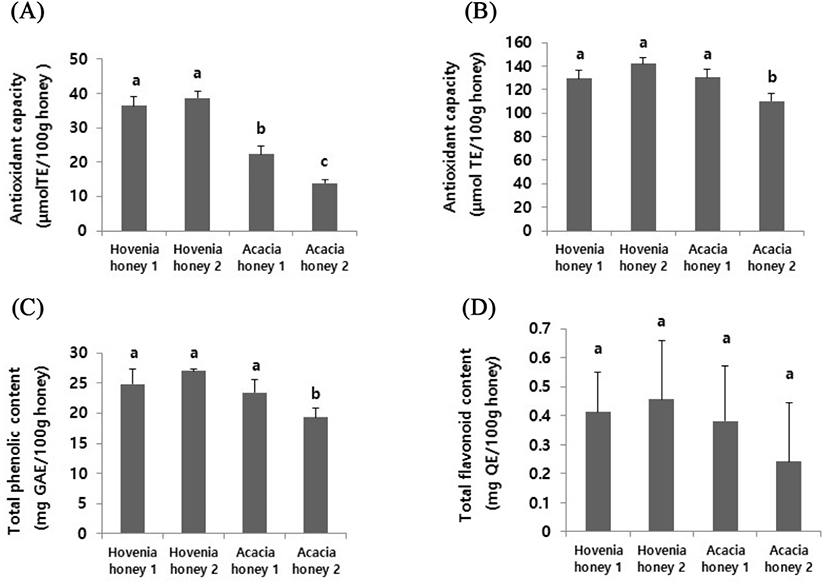
The DPPH and ABTS radical scavenger activity of Hovenia monofloral honey was then evaluated and compared with that of acacia honey. The two types of acacia honey used in this study were identified as monofloral honeys by a pollen analysis (data not shown). As shown in Fig. 3, both Hovenia monofloral honey-1 (36.3±2.71 μmol TE/100 g honey) and -2 (38.7±1.86 μmol TE/100 g honey) showed a significantly (p<0.05) higher DPPH radical scavenger activity than that of Acacia honey-1 (22.4±2.13 μmol TE/100 g honey) and -2 (13.9±0.95 μmol TE/100 g honey); however, although the ABTS radical scavenger activity of the Acacia honey-2 sample (110.4±6.63 μmol TE/100 g honey) was slightly lower than that of Hovenia monofloral honeys, the Acacia honey 1 sample (130.6±6.74 μmol TE/100 g honey) showed a similar ABTS radical scavenger activity as that of Hovenia monofloral honeys (Hovenia monofloral honey 1; 129.5±7.07 μmol TE/100 g honey, Hovenia monofloral honey 2; 141.9±5.49 μmol TE/100 g honey). These results indicate that Hovenia monofloral honey has a higher antioxidant activity than Acacia honey when tested with a DPPH radical but not with an ABTS radical. Interestingly, the amounts of total phenol and total flavonoid were not significantly different between Hovenia monofloral honey and Acacia honey (Fig. 3C and 3D). Therefore, as the DPPH assay was used to identify the role of hydrophobic antioxidants in the samples (Arnao et al., 2000), the data suggested that the type of hydrophobic antioxidants may be different between Hovenia monofloral honey and Acacia honey. A detailed analysis of the single components of Hovenia monofloral honey will be performed in a future study.
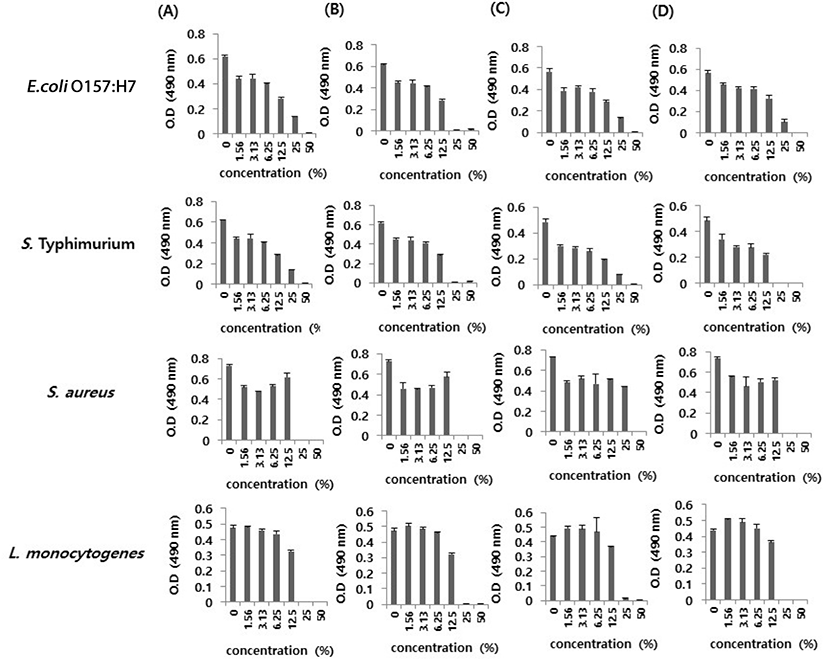
To estimate the antibacterial activity of Hovenia monofloral honey, the minimum inhibitory activity of Hovenia monofloral honey against four foodborne bacteria, including E. coli O157:H7, S. Typhimurium, S. aureus, and L. monocytogenes was evaluated (Fig. 4 and Table 3). Hovenia monofloral honey showed MIC values of 25%–50% (w/v) against two gram negative foodborne bacteria, E. coli O157:H7 and S. Typhimurium, and MIC value of 25% (w/v) against two gram positive foodborne bacterial, S. aureus and L. monocytogenes. The MIC values are similar to that of Acacia honey (Fig. 4 and Table 3), suggesting that Hovenia monofloral honey produced in a net house system has a strong antibacterial activity and can be used for food preservation against food pathogens. Furthermore, the MIC values of artificial honey which constituted with sugars (glucose: 33.5 g, fructose: 40.5 g, sucrose: 1.5 g, maltose: 7.5 g in DW: 17 mL) against foodborne bacteria were more than 50% (w/v) (data not shown) indicated that a part of antibacterial activity of Hovenia monofloral honey was derived from honey constituents other than sugar such as phenols and flavonoids.
| MIC (%, w/v) | ||||
|---|---|---|---|---|
| Gram negative | Gram positive | |||
| E. coli O157:H7 | S. Typhimurium | S. aureus | L. monocytogenes | |
| HMH-1 | 50 | 50 | 25 | 25 |
| HMH-2 | 25 | 25 | 25 | 25 |
| Acacia honey-1 | 50 | 50 | 50 | 25 |
| Acacia honey-2 | 50 | 25 | 25 | 25 |
Conclusion
In this study, high-purity Hovenia monofloral honey was produced using a net house system, and its physiochemical properties, such as the contents of sugar, minerals, total phenoic acid, and total flavonoids, were evaluated. Hovenia monofloral honey showed DPPH and ABTS radical scavenger activities and antibacterial activity against gram positive and gram negative foodborne bacteria. To the best of our knowledge, this is the first evaluation of Hovenia monofloral honey, and it can be used to evaluate the potential of the Hovenia tree as a honey plant. Furthermore, because the amount of nectar per flower bud of the Hovenia tree is higher than that of the Acacia (Robinia pseudoacacia) tree, the Hovenia tree could be a candidate to compensate for the loss of the Acacia tree as a honey plant.













