Introduction
Depressive disorder, a major cause of disability and early mortality from suicide worldwide, is reported to be more prevalent in women than men (Bangasser and Valentino, 2014; Levinstein and Samuels, 2014; Thompson et al., 2015). Until recently, depression was regarded purely as a mental disorder that was treatable only by psychologists and psychiatrists (Plante, 2005). However, recent studies have revealed that it might be associated with many diseases (Daskalopoulou et al., 2016; Lotfaliany et al., 2018; Milaneschi et al., 2019). Additionally, recent evidence suggests gut microbiota to be involved in inflammation, brain development, and behavior (Guo et al., 2019; Li et al., 2019). Therefore, in order to develop an effective therapeutic strategy, we focused on developing the food sources including functional and nutritional supplement that could alter stress induced behaviors and gut microbiota.
Cheese is a fermented food with a long history (Prajapati and Nair, 2003). In particular, cheeses are rich in energy and nutrition and contain proteins, bioactive peptides, amino acids, fats, fatty acids, vitamins, and minerals (Walther et al., 2008). Additionally, cheese has been reported to be effective in anti-obesity, anti-hypertension, and bone health therapies owing to its ingredients (Gomez-Ruiz et al., 2006; Higurashi et al., 2007; Kato et al., 2002). Milk from Jersey cows contains more milk solids than does Holstein milk, which is advantageous in cheese production, and the cheese yield from Jersey milk is higher than that from Holstein (Bland et al., 2015b; Jensen et al., 2012). Furthermore, the cheese and fermented milk from Jersey cows' milk has been reported to be richer in calcium, phosphorus, milk-fat, and -protein than those prepared from Holsteins milk (Yoo et al., 2019).
In this study, we aimed to evaluate whether the cheese obtained from Jersey or Holstein milk has the stress-reducing effect in terms of behavior and gut microbiota in chronic, unpredictable, mildly stressed (CUMS) mice.
Materials and Methods
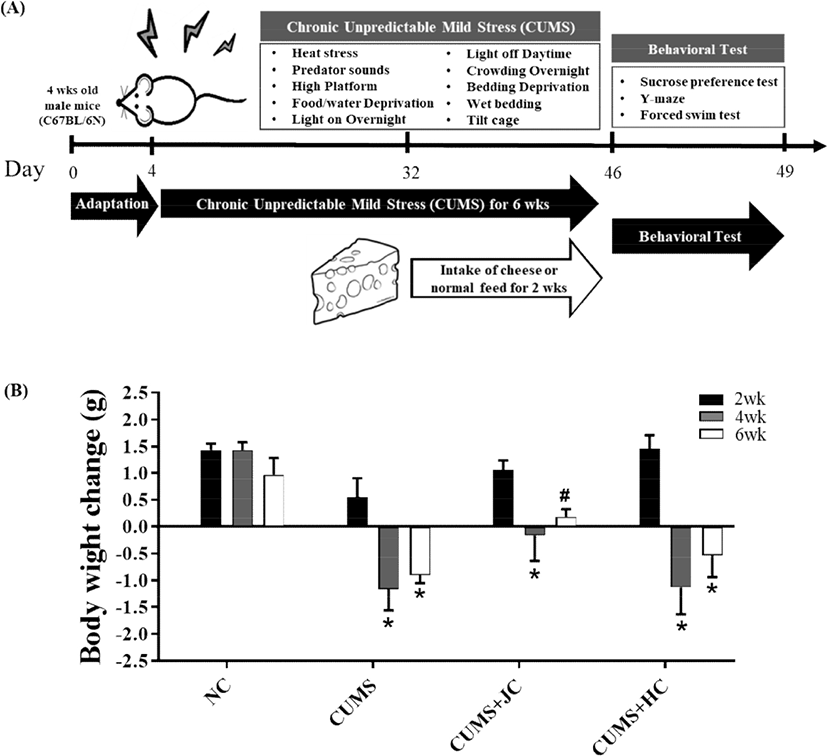
All animal care procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Chonbuk National University, Korea; prior to experiment initiation (2015-05). The mice (C57BL/6N), aged 4 weeks; were housed in a standard animal laboratory with a 12-h light-dark cycle, and fed on a standard diet during adaptation for 4 weeks. In the first set of experiments, 16 mice (8 weeks old) were randomly divided into 4 groups: a control group (not subjected to any stress; NC; n=4), a depression-model group (subjected to CUMS procedure; CUMS; n=4), a group fed on Jersey cheese following CUMS procedure subjection (CUMS-JC; n=4), and a group fed on Holstein cheese following subjection to CUMS procedure (CUMS-HC; n=4). Mice were given a synthetic pellet LabDiet containing 14% fat, 21% protein and 65% carbohydrate (5L79; OrientBio, Inc., Seongnam, Korea) and provided water ad libitum throughout the study. Gouda cheese was prepared by following a previously described method (Yoo et al., 2019). For mice feeding, Jersey Gouda cheese (JC; 44.19% fat and 26.71% protein) or Holstein Gouda cheese (HC; 39.10% fat and 30.17% protein) were ground by adding 5% to standard feed pellets, respectively, mixed and fed to mice for 2 weeks. The feed was freshly prepared and replaced daily, and the unconsumed amount was recorded. The mice body weight was measured weekly. Following 6 weeks of CUMS, all the groups were subjected to a series of behavioral tests. Only one behavior test was conducted daily in the following order: sucrose preference test (SPT), Y-maze test (YMT), and forced swim test (FST). At the end of an experiment, mice fecal samples from all the groups were collected and immediately stored at −80°C.
CUMS was performed by following a previously described method (Marin et al., 2017). Briefly, the stress paradigm comprised of the following 10 types of irritations: 1) heat stress (45°C, 10 min); 2) predator sounds (10 min); 3) high platform (30 min); 4) food and water deprivation (12 h); 5) light-on overnight; 6) light-off daytime; 7) crowding overnight; 8) bedding deprivation (6 h); 9) wet bedding (6 h); and 10) 45° cage tilt (4 h), for a total of 6 weeks. All the materials used in this experiment were sterilized to prevent contamination. Same stress was not applied for 2 consecutive days, and 3 out of 10 stresses were randomly performed. Mice of the control group were undisturbed except at feeding and cage cleaning times.
The Y-maze test was performed according to a previously study (Kim et al., 2007). Y-maze is a 3–arm horizontal maze (40×3×12 cm, length×width×height), wherein, the 3 arms are symmetrically separated at 120°. Mice were initially placed within one arm, and the arm entry sequence and number of arm entries were recorded manually for each mouse over an 8 min period. Valid alternation is defined as the entries into all 3 arms consecutively (i.e., ABC, CAB, or BCA, but not BAB). The alternation value, expressed as percentage, is calculated by the following equation:
The mice were placed in a tap-water (at 23°C−25°C) filled glass cylinder (23×12 cm; height×diameter). Their swimming behavior was recorded with a video camera. The test lasted for 6 min and the immobility time during the last 4 min, was later analyzed. The time without movement except for slight motions to keep their heads out of water, was recorded.
STP was performed according to a previously described method (Casarotto and Andreatini, 2007). Following 6 weeks of CUMS, the mice were made to adapt to 1% sucrose solution for 24 h, and were simultaneously provided 2 bottles containing 1% sucrose solution and tap water, respectively. The water and sucrose solution were weighed in order to measure their intake after 24 h.
Mice fecal samples (following cheese feeding) were freshly collected and were stored at −80°C for DNA extraction. The total genomic DNA from each fecal sample (200 mg) was extracted using the QIAamp DNA stool minikit (Qiagen, Germany) according to manufacturer's instructions. Microbial community analysis was performed using specific primers (designed in previous studies) (Yang et al., 2015). The polymerase chain reaction (PCR) conditions followed were: 95°C for 10 min, followed by 40 cycles of 95°C (15 s) and 60°C (1 min). Melting-curve analysis was performed thereafter, and the CT (cycle threshold) values and baseline settings were determined by automatic analysis settings. All the fecal samples were analyzed with quantitative PCR (qPCR) assay, and the CT values were used to calculate the proportion of higher bacterial taxa in the feces. The calculation(s) was performed in accordance with a previously described method (Yang et al., 2015).
Results and Discussion
Cheeses are rich in essential nutrients and minerals (Lopez-Exposito et al., 2012). Additionally, despite of the presence of high amounts of saturated- and trans-fatty acid, no clear evidence exists that links cheese intake to any disease (Walther et al., 2008). Due to the presence of high calcium concentration, cheese contributes to bone health and durable tooth formation and maintenance (Edgar et al., 1982; Kato et al., 2002), it positively affects blood pressure and helps in weight loss (Gomez-Ruiz et al., 2006; Higurashi et al., 2007). Recently, it has been reported as a food contributing to nutrition and health, in connection with diseases such as diabetes and cancer (Apostolidis et al., 2007; Yasuda et al., 2012). The association between brain function or mental health and gut microbial community, also referred as the “brain-gut axis”; has been intensively studied and it suggests that diets change gut microbiota, eventually affecting mental health (Bermudez-Humaran et al., 2019; Martin et al., 2018; Perez-Pardo et al., 2018).
Jersey’s milk possesses higher fat, protein, and SNF ratios; than Holstein milk. However, the milk yield is reported to be higher in Holstein than in Jersey cows (White et al., 2001). With respect to this, Jersey is regarded as a dairy breed, characterized by lower milk production than the Holstein, but with optimal milk composition and coagulation ability (Bobbo et al., 2019). Therefore, its milk production serves as a major disadvantage in the dairy industry. Recently, to address this issue, studies have reported an increase in milk yield and milk composition by crossbreeding Holstein and Jersey species (Ferris et al., 2018; Saborio-Montero et al., 2018). Additionally, with respect to dairy production technology, studies have reported to increase the economic efficiency by mixing Jersey- and Holstein-milk (Bland et al., 2015a; Bland et al., 2015b). However, with respect to cheese production, associated with milk composition and coagulation ability; Jersey milk’s use has been observed to be more profitable than Holstein milk (Bland et al., 2015a). However, published reports discussing the differences in functional aspects between Jersey and Holstein cheese are unavailable. Therefore, we attempted to understand how Gouda cheese derived from Jersey or Holstein milk could aid in relieving stress, and whether a difference existed between them, and if it could lead to gut microbial changes.
To investigate whether Gouda cheese from Jersey milk could act as a stress-relief food, a CUMS model was introduced in this study, as displayed by a schematic diagram (Fig. 1A). The body weights of the CUMS, CUMS+JC and CUMS+HC groups were significantly less than the NC group (p<0.05) (data not shown). Changes of body weight were noted to decrease in CUMS, CUMS+JC, and CUMS+HC at 4 weeks, whereas, it increased significantly only in CUMS+JC, at 6 weeks (2 weeks after feeding cheese) (Fig. 1B). Furthermore, food intake increased in both cheese-fed groups, whereas, body weight increase was observed only in the Jersey cheese-fed group (data not shown). For behavioral evaluation, the YMT, FST, and SPF tests were performed to observe the effect of cheese intake on cognitive function and depression-related behaviors in depression model (Fig. 2). The percentage of spontaneous alternation (spatial memory) in mice during YMT is presented in Fig. 2A. A significant decrease in spontaneous alternation was induced by CUMS, as compared to the NC group (p<0.05). Surprisingly Jersey and Holstein cheese-fed mice showed significantly recovered the spatial memories. However, no significant difference among other groups as compared to CUMS mice was observed in immobility time and sucrose preference on FST and SPT, respectively (Fig. 2B-D).
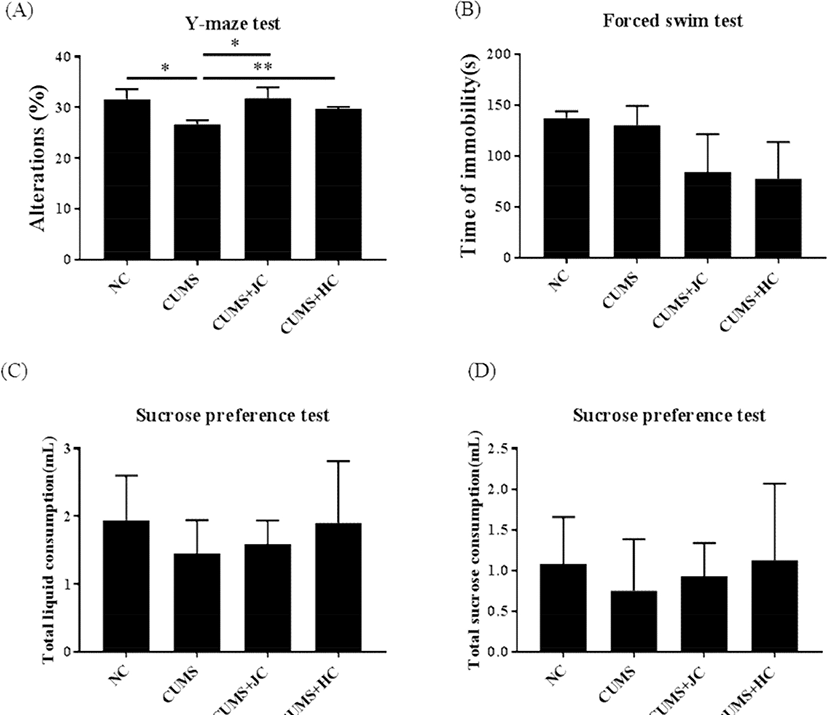
The effects of milk components on brain health have been described widely (Conway et al., 2014; Hernell et al., 2016; Verardo et al., 2017). Nagai (2012) has reported that the risk of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, could be diminished by milk constituents. Another study has reported that milk phospholipids intake in chronically stressed men, ameliorated the stress-induced memory impairment (Schubert et al., 2011). This corroborates the YMT results obtained in this study, which were used as a measure of immediate spatial working memory. Our result showed that cheese intake improved the spatial working memory of CUMS-induced mice regardless of cow species. We found in the previous study that phosphorus and fat contents were significantly higher in Jersey Gouda cheese than in Holstein Gouda cheese (Yoo et al., 2019). So the loss of body weight due to stress was recovered more by Jersey cheese intake rather than Holstein cheese.
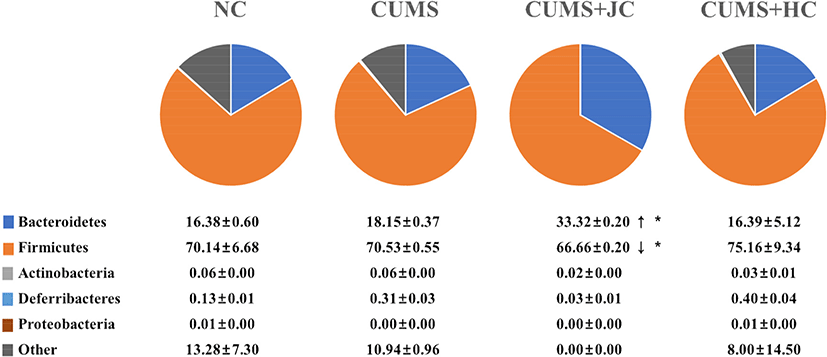
Recent studies have reported acute and chronic stress to be associated with gut microbiota (Guo et al., 2019; Li et al., 2019; Marin et al., 2017). Li et al. (2019) have reported that mice receiving the fecal microbiota of depression-induced mice, exhibited similar behavior against depression. For testing an assumption that the effect of cheese on spatial cognition and weight loss are associated with gut microbiota rebalancing, fecal microbiota community diversity was analyzed. Fecal samples from CUMS model mice and other mice groups were collected, and the relative abundance of bacteria was calculated from the CT values. The composition ratios of the target bacteria are reported as: mean±SD. Further, to make it more clear and to directly reflect the change(s) in predominate bacteria, communities characterization is presented in the form of a pie chart (Fig. 3). The community pie chart analysis displays community composition and species abundance in the 4 mice groups. Bacteroidetes abundance in NC group was noted as 16.38%, however, it was observed to increase to 18.15%; due to stress induction. Whereas, the previous increase in Bacteroidetes of CUMS group was reduced to 16.39%, by Holstein cheese. This finding corroborates previous reports wherein, Bacteroidetes concentration was marked to decrease due to depression and was increased by Bifidobacteriumadolescentis (Guo et al., 2019). However, no significant difference in the microbial community composition of the 3 groups including NC, CUMS, and CUMS+HC; was noted at phylum level. Saccharibacteria, Verrucomicrobia, and Tenericutes were not detected in any of the groups. Interestingly, Bacteroidetes of the CUMS+JC model was noticed to increase significantly as compared to that of CUMS mice (<0.002). Whereas, Firmicutes concentration in CUMS+JC group was observed to decrease when compared to other groups. As a result, Jersey cheese intake completely changed the microbial community composition of the CUMS+JC group, as compared to other groups. In conclusion, cheese intake altered the microbial community and improved the recognition (impaired by CUMS) in mice model. In particular, present study suggested that Jersey cheese played a beneficial role such as a stress reducing food, and provided the functional ingredients. However, further studies on fermentation and the useful components of Jersey cheese will be required for developing an improved functional cheese.