Introduction
Chili pepper is the fruit of a plant belonging to the genus Capsicum that originated in Mexico and spread across the world (Barceloux, 2009). Chili pepper is extensively used in cultural cuisine for a hot and spicy taste, particularly, in Korea and tropical countries. Capsaicinoids, a group of compounds comprising of capsaicin, dihydrocapsaicin, nondihydrocapsaicin, homohydrocapsaicin, etc., are responsible for the pungent taste of chili pepper (Reyes-Escogido Mde et al., 2011). Capsaicinoids have been extensively studied for their biological effects, including anticancer (Park et al., 2014), antidiabetic (Chaiyasit et al., 2009; Okumura et al., 2012), antihypertensive (Harada et al., 2009; Patanè et al., 2010), antiinflammatory (Kim and Lee, 2014), antimicrobial (Sia et al., 2013; Soumya et al., 2012), analgesic (O'Neill et al., 2012; Üçeyler et al., 2014), and antioxidant properties (Materska and Perucka, 2005; Viktorija et al., 2014). Moreover, the consumption of capsaicinoids has been reported to induce antiobesity effects by promoting lipolysis, fullness of satiety or sensation, and energy expenditure, eventually inducing weight loss (Narang et al., 2017).
Health issues are a major concern to the world population, and therefore, food companies make efforts to improve the health claims of their products. However, there are some challenges in the development of functional foods containing health-promoting bioactive compounds as these materials could be unstable and react with other ingredients in food matrix, or present strong odor and/or flavor. Microencapsulation has been used to overcome these problems in the food industry.
Microencapsulation is a process of trapping the material of interest in a wall to produce a small capsule (1-1,000 μm) (Yeo and Kiran, 2005). The material inside the microcapsule, i.e., the core, could be solids, liquids, or gases, whereas the wall, referred to as a shell or a coating, is made up of hard or soft soluble film (Yeo and Kiran, 2005). Microencapsulation is mainly used to increase the stability and shelf-life of the product being encapsulated, facilitate the manipulation of the product, and control the delivery or release of target materials for several industrial applications (Sarao et al., 2017; Wakil et al., 2010). In the food industry, microencapsulation has contributed to the development of functional foods containing natural bioactive ingredients, the bioactivities of which are controlled, protected, and preserved in capsules. For example, omega-3 oils have been microencapsulated to minimize oxidative deterioration (Kaushika et al., 2015), Bifidobacterium longum was microencapsulated to ensure its stability in cheddar cheese during long-time storage (Amine et al., 2014), and lipase was microencapsulated to accelerate the ripening of kashar cheese (Akin et al., 2012).
Although dairy products are generally considered healthy, this status has been challenged multiple times due to high fat content, particularly, saturated fat and cholesterol. Dairy industry has made efforts to improve health concerns about dairy products by reducing fat content (Lobato-Calleros et al., 2001), substituting saturated fat with healthier fat (Lobato-Calleros et al., 2002), and supplementing bioactive ingredients (Choi et al., 2015).
Chili pepper has been rarely used as an ingredient in dairy products, mainly because of the pungent flavor. Moreover, in dairy products, such as cheese and yogurts, chili pepper could inhibit fermentation process owing to antibacterial activities of capsaicinoids. Therefore, to solve this problem, the microcapsules of chili pepper extract were prepared by complex coacervation and mixed in Gouda cheese before ripening. We investigated the physiochemical and sensory characteristics of Gouda cheese containing the microcapsules of chili pepper extract.
Materials and Methods
Chili pepper powder was purchased from Yeong Yang Red Pepper Trade Corporation (Korea). The components of chili pepper powder (100 g) were moisture (13 g), total sugars (38 g), dietary fiber (17.2 g), and capsaicin (60 mg). Arabic gum and gelatin were purchased from Duksan Science Corporation (Korea). All chemicals used in this study were of reagent grade unless otherwise specified.
The microencapsulation was performed by complex coacervation as described below. To prepare pepper extract, chili pepper powder (particle size: 16-20 mesh) was mixed with olive oil (Carbonell, Spain) in the ratio of 1:5 (w/w), stirred at 500 rpm for 1 h at room temperature, and filtered through Whatman filter paper No. 4. The pepper extract was stored in a vacuum jacket until use. Gum acacia (48.0 g; Duksan, Korea) was completely dispersed in 460 g water, heated at 80°C for 2 h, and then cooled down to 50°C. Then, 72.0 g gelatin (Junsei, Japan) was completely dispersed in 690 g water, heated at 80°C for 2 h, and then cooled down to 50°C. A total of 375 g capsaicin extract was dispersed in 450 g gum acacia solution and emulsified by stirring at 5,000 rpm and 400 rpm (IKA, T25 Basic, Germany) for 5 min to form 50-μm and 800-μm capsules, respectively. Then, this emulsion was gently transferred to 20 g gelatin solution maintaining the speed at 300 rpm and the temperature at 45°C. The liquid was adjusted to pH 4.9 with citric acid (10% aqueous solution) and diluted with 80 mL water (45°C). Then, the system was slowly cooled from 45°C to 30°C in about 75 min and then rapidly cooled from 30°C to 10°C in ice water. While maintaining the system at about 10°C and stirring at 300 rpm, the pH was adjusted to 9 with sodium hydroxide (5% aqueous solution), and 3 g glutaraldehyde solution (25% aqueous solution) was added for cross-linking. The process was continued for about 18 h at 10°C to stabilize the cross-linked capsules. The capsules were harvested and thoroughly rinsed with water. The capsule slurry was preserved in sterilized bottles with 0.05% sodium benzoate.
Raw milk was obtained from the dairy farm of Chungnam National University (Korea). It was pasteurized at 65°C for 30 min, cooled to 32°C, and inoculated with CHN-11 DVS (Direct Vat Set) starter (Danisco, Denmark) at 2.2 g/100 L. After 1-h incubation, 19 g liquid rennet (Christian Hansen, Denmark) was added to obtain curd. The curd was cut into 1.0 cm3 cubes, settled for 10 min, and then stirred at 32°C for 20 min. After 30% of the whey was drained, vat temperature was raised to 39°C and more whey was drained while stirring the curd for 30 min. The pepper microcapsules (0.5% or 1.0%) were mixed to the curd before molding. The curds were transferred to 1 kg round containers and pressed with weight. The curd was soaked in 19% NaCl solution for 3 h and matured in a cooling chamber (12±0.5°C, 85-90% relative humidity (RH)) for 4 wk, and coated with plastic.
The structure and shape of microcapsules were observed under a bright field microscope (Axioplan 2, Carl Zeiss, Germany). In detail, 2 mg microcapsules were dispersed in 1 mL 0.1 M sodium carbonate buffer solution (pH 9) and mixed with 50 μL FITC solution (1 mg/mL, DMSO), which were incubated under dark conditions for 4 h to complete the reaction. The capsules ware washed, dried, and observed under the microscope.
The cheese samples were homogenized (IKA, T25 Basic, Germany) with sterile saline in the ratio of 1:2 at 13,000 rpm for 1 min. The homogenates were used to measure pH value and bacterial number. The pH of the sample was measured using a pH meter (Precisa pH 900-9050, Switzerland). To enumerate lactic acid bacteria (LAB), 1.0 mL homogenized sample was serially diluted with sterilized saline and spread on BCP agar (BD Difco, USA). The agar plates were incubated at 37°C for 48-72 h and the colonies were counted.
The water-soluble fraction of the cheeses was prepared according to the method of Kuchroo and Fox (1982). Total FAAs from the water-soluble extracts were determined by a Biochrom series 30 Amino Acid Analyzer (Biochrom Ltd., Cambridge Science Park, UK) as described by Siragusa et al.(2007) by reversed-phase high-performance liquid chromatography (HPLC, 1200 Series Agilent chromatograph, Agilent Technologies, USA) using a Waters Nova Pack C18 column (3.9 × 300 mm, Waters Corporation, USA) and UV detection (254 nm).
Texture profiles of cheese samples were obtained and analyzed using a Texture Analyzer (Model TAXT2i, SMS Co., UK) with a load force of 50 kg and a crosshead speed of 2 mm/s. Cube-shaped samples (2.0 cm3) were prepared. Texture profiles were measured in quintuples.
The sensory panel was composed of 10 trained judges. For training of the panels a descriptive work and three preliminary tests were performed on the attributes of gouda cheese. The panelists evaluated sensory characteristics of Gouda cheese, such as appearance, flavor, texture, and taste, using 5-point hedonic scale (1 = very bad, 3 = moderate, 5 = very good). Prior to sensory evaluation, Gouda cheeses ripened for 6 mon were held for 3 h at room temperature and then cut in representative rectangular slices (10 g). The samples were served in a random order with a 3-digit code. Water and cracker were provided to clean the mouth between tasting.
Results and Discussion
Microcapsules of pepper extract were prepared and their structures and shapes were observed by microscopy (Fig. 1). Each microcapsule of different diameter, i.e., ~50 μm and ~800 μm, was prepared by complex coacervation technique, in which encapsulated pepper oil forms droplets into a colloidal solution forming complex polysaccharide (gum acacia)-protein (gelatin) coacervates (Lamoudi et al., 2015). Both the types of microcapsules maintained stable morphology during the freezing and thawing processes, presumably due to the cross-linking, which modifies the structure and property of microcapsules to a hard texture and stabilizes the wall materials (Leclercq et al., 2009). The pepper microcapsules with ~50 μm diameters had regular spherical shape with smooth surface, whereas the capsules of ~800 μm diameters had irregular morphology. This result is not surprising as previous studies have reported that the ratio of core/wall material was closely related to the shape and stability of microcapsules (Aziz et al., 2014; Yu et al., 2012).
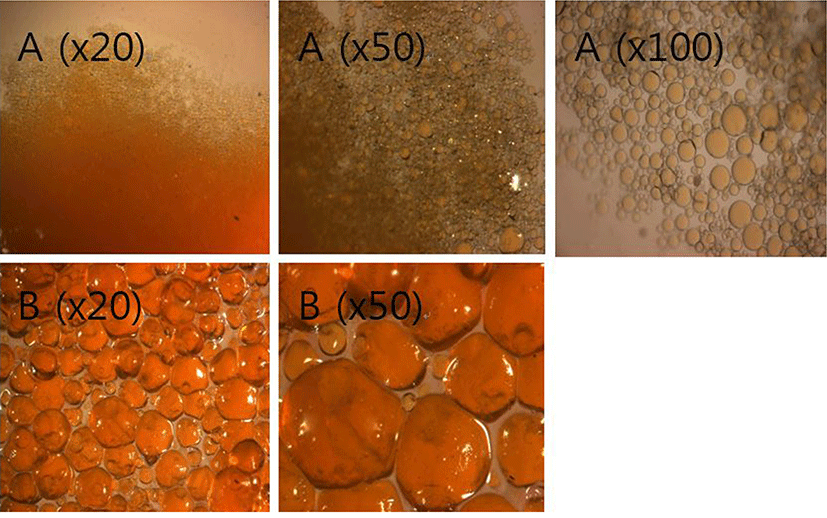
As a preliminary step, we prepared two different types of Gouda cheese supplemented with pepper extract microcapsules of ~50 μm and ~800 μm diameters. Eventually, the capsules of ~800-μm diameter were chosen for further process because they were stable enough and showed better appearance than that showed by ~50 μm diameter capsules when mixed in cheese.
The changes in pH values and LAB content of the Gouda cheeses during ripening are shown in Table 1. The pH values of the Gouda cheese supplemented with pepper extract microcapsules (0.5% or 1%, w/w) were not significantly different than those of the control cheese at each time point. During the ripening period, the pH value of each sample was not changed significantly and was maintained at 5.1-5.3 considered as an optimal pH range for the taste and flavor of Gouda cheese.
Values are Means±standard deviation (SD) of three separate determinations.
A,BValues sharing same capital letter within a row are not significantly different by Duncan’s multiple-range test (p<0.05).
a,bValues sharing same lowercase letter within a column are not significantly different by Duncan’s multiple-range test (p<0.05).
The LAB content did not show significant difference among Gouda cheese samples and was not significantly influenced by the ripening period. Although the antimicrobial activities of capsaicinoids in chili pepper have been extensively studied (Marini et al., 2015; Omolo et al., 2014), their impact on microorganisms still remains controversial and depends on strains and experimental conditions (Marini et al., 2015; Sharma et al., 2013). In this study, however, the addition of pepper extract microcapsules (0.5 or 1%, w/w) did not affect the growth of LAB in Gouda cheese during ripening.
The FAA contents during ripening of Gouda cheese are presented in Table 2. During ripening, no significant differences in the total FAA content were observed among the sample groups at each time point. However, the amount of FAA was increased during ripening process in all of the cheese groups (p<0.05). These results demonstrated that the proteolysis in cheese samples was not inhibited by supplementing the microcapsules of pepper extract. Presumably, microencapsulation maintained the protease activity in Gouda cheese preventing the direct contact of chili pepper extract.
Values are Means±standard deviation (SD) of three separate determinations.
A,BValues sharing same capital letter within a row are not significantly different by Duncan’s multiple-range test (p<0.05).
a,bValues sharing same lowercase letter within a column are not significantly different by Duncan’s multiple-range test (p<0.05).
After 6 mon of ripening, the texture characteristics of Gouda cheese in terms of hardness, cohesiveness, springiness, chewiness, and gumminess are shown in Table 3. The addition of microcapsules of pepper extract (0.5 or 1%, w/w) to Gouda cheese significantly decreased its hardness in a concentration dependent manner (p<0.05). In terms of cohesiveness, springiness, chewiness, and gumminess, the differences were not significant among the groups.
Values are Means±standard deviation (SD) of five separate determinations.
A,BValues sharing same capital letter within a row are not significantly different by Duncan’s multiple-range test (p<0.05).
Overall, during 6 mon of ripening, the addition of microcapsules of pepper extract did not affect the texture properties of Gouda cheese compared to those of control, except for hardness. Previous studies reported that the fat content affects physicochemical and sensory attributes of cheese (Felfoul et al., 2015). The property of fat was also related to the hardness of cheese (Felfoul et al., 2015). Regarding hardness, our result is consistent with that of the study by Felfoul et al. (2015), who prepared Gouda cheese by replacing milk fat with olive oil emulsion.
The results of the sensory evaluations of Gouda cheese are shown in Table 4. The sensory attributes, such as taste, flavor, texture, and appearance, of Gouda cheese ripened for 6 mon at 12°C were evaluated. A significant difference (p<0.05) was observed in taste and texture between the control and the sample group supplemented with 1% pepper extract capsule. The supplementation of 1% pepper extract capsule negatively affected taste and texture of Gouda cheese compared to that of the control group. Some innovative trials have been performed in the dairy industry to obtain better quality cheese (Choi et al., 2015; Felfoul et al., 2015; Kim et al., 2012; Lobato-Calleros et al., 2002). Unfortunately, however, many of those trials changed the sensory attributes of cheese and deteriorated the quality of cheese. For example, the Gouda cheese prepared using olive oil emulsion as fat replacer received significantly lower scores for appearance and overall impression (Felfoul et al., 2015). The supplementation of traditional fruit liquors also negatively affected the sensory characteristics of Gouda cheese (Choi et al., 2015). In this study, however, supplementation with 0.5% pepper extract capsule showed no adverse effects on the sensory attributes of Gouda cheese.
Values are Means±standard deviation (SD) of ten separate determinations.
a,bValues sharing same lowercase letter within a column are not significantly different by Duncan’s multiple-range test (p<0.05).
Conclusions
Our primary intention on this study was to improve the quality of gouda cheese in terms of health concerns by substituting with healthier fat and supplementing bioactive ingredients. Microencapsulation was applied to prepare Gouda cheese supplemented with chili pepper extract. The influence of pepper extract microcapsules on the physicochemical and sensory properties of Gouda cheeses was related to the concentration of microcapsules. Although 1% supplementation of pepper extract microcapsules significantly decreased hardness (p<0.05) and negatively affected sensory attributes in terms of taste and texture (p<0.05), supplementation with 0.5% extract did not affect the characteristics of Gouda cheese during ripening. In conclusion, chili pepper extract could be supplemented as functional ingredient by microencapsulation without affecting the physicochemical and sensory properties of Gouda cheese. Presumably, microencapsulation could expand the application of chili pepper to the development of functional foods in the dairy industry.













