Introduction
Restructured meats are enhancing more popular and considerable improvements for the meat industry. Restructuring implies binding of tiny meat pieces unitedly to form a meat product with the steak and roast meat properties. The objective of producing restructured meats is to capably market low value carcasses of aged animals with poor structure and carcass components. These products sold either precooked or frozen because they do not adhere firmly in the raw state and short shelf life stored in refrigeration (Gadekar et al., 2015).
Recently, consumers have been demanding high quality, fresh and expansive shelf-life refrigerated meats that desire minimal preparation time for instance sous-vide under vacuum process (Pathare and Roskilly, 2016). Sous-vide process is the heating procedure. The raw meat is packed in a vacuum pouch and cooked using water bath at 55°C to 85°C. This procedure desired extended heating times compared to traditional cooking procedures. The lower sous-vide temperature decreases the harm to heat delicate proteins and complements (Baldwin, 2012). It also abates weight losses, maintains the juiciness and increases tenderness of meat. The reached cooking time initiates collagen solubility. The connective tissue tenderization occurs through the intramuscular collagen solubilization inside the moist in sous-vide pack (Pathare and Roskilly, 2016). Sous-vide has nutritional benefit and enhanced texture and flavor that traditional roasting could not bear (Baldwin, 2012).
Although, sous-vide procedure improves organoleptic properties of meat products, the microbiological safety of product stored at cold temperature is concerned. Food safety risk collaborated with sous-vide cooking arises. Low temperature long time of sous-vide procedure could not surrender Listeria monocytogenes, Escherichia coli and Salmonella spp. inhabitants non-viable. These pathogens are able to grow in meat products during default cold temperature storage which is probably to appear at retail, distribution and household environments. (Karyotis et al., 2017). However, one of the most heat resistant bacteria is L. monocytogenes. The D-value at same temperature of L. monocytogenes had higher than those of Salmonella and E. coli (McMinn et al., 2018). Thermal treatment times guidance and requirements for raw meat have been depended on the criterion and globally accepted parameters of heat treatment. For thermal pasteurization, this gives a 6D outcome at a core temperature of 60°C–70°C for 2.4–91.2 min for L. monocytogenes, obtained from observations with all meat (Horn et al., 2015).
Thus, objectives of this study were to assess the survival and thermal-death time curves of L. monocytogenes and analyze the effect of different sous-vide processes (≤ 6D-values of L. monocytogenes at core temperatures of 60°C, 65°C, and 70°C) on their microbiological and physicochemical qualities of restructured goat steak.
Materials and Methods
Goat meat was purchased from a butcher shop located at Bangkok, Thailand. The neck, chump and hind legs meat of male goat carcasses (around 2 year olds) were purchased within 2 to 3 h of slaughter, packed in plastic bags, kept in ice box and brought to the laboratory within 2 h. The meats were chilled at 4°C for 24 h. The meats were trimmed for separating fat and connective tissue. Then trimmed meats were kept in vacuum-sealed bags and stored at –18°C till further use.
L. monocytogenes DMST11256 was attained from the culture collection of the Department of Medical Sciences, Ministry of Public Health, Thailand. This strain was stored at −20°C in mueller hinton broth (MHB, Merck, Darmstadt, Germany) containing 20% (v/v) glycerol. The frozen cultures were cross-linked on mueller hinton agar (MHA, Merck) at 37°C for 24 h. Five colonies were removed to 10 mL MHB and incubated at 37°C for 24 h. Then, 10 mL of culture was removed to 100 mL MHB and incubated at 37°C for 18 h (Tangwatcharin et al., 2018). Cells were harvested by centrifugation at 10,000×g at 4°C for 10 min in Universal 16R refrigerated centrifuge (Hettich Zentrifugen, Tuttlingen, Germany), washed twice in 0.1% (w/v) peptone water (Merck). Finally, pellet was suspended in 10 mL of peptone water for using cocktail of goat marinade (9 Log CFU/mL).
The goat marinade, common recipe, was prepared with goat meats and the ingredients and additives (g/kg): salt, 8.0; pepper powder, 2.0; water, 100.0; vinegar, 20; soy sauce, 10; oyster sauce, 10. Briefly, goat meats were thawed and minced with meat grinder (Model NB-MM12SS, Sun Food Co., Ltd, Thailand) using 12 mm plates. The meat samples were mixed with the other ingredients and additives. The marinade was inoculated with 10 mL of L. monocytogenes cocktail (9 Log CFU/mL). Finally, initial concentration was 7 Log CFU/g in marinade. One kilogram of marinade was added 10 g/kg of sodium caseinate and 1 g/kg of transglutaminase (MTGase) and mixed well. Then mixture was bulk-packed in stainless steel-cylindrical shaped mould (8 cm diameter and 21 cm length). The tubes were chilled at 4°C for 4 h then frozen at –18°C for 12 h. Ten 2 cm thick steaks were cut from each tube (Sorapukdee and Tangwatchrarin, 2018). The steaks were individually vacuum packed in polyamide pouch and stored at 4°C till further use.
Inoculated restructured goat steak sous-vide cooked at different temperature-time combinations were evaluated. Raw samples were divided into 3 groups and comprised 24 pouches/group. The samples were completely immersed in a water bath (Memmert, Buchenbach, Germany) for thermal processing. The water bath was set final temperature at 60°C, 65°C, and 70°C. The water temperature was observed using Type-K thermocouple from a digital thermometer model 52 Series II (Fluke Corp., Washington, DC, USA). Temperature of sample was observed by thermocouples inserted, previous sealing, in a center non-inoculated pouch. The time required for the control sample to arrive to each target cook temperature (come-up-time) was recorded. The final core temperature of control sample was reached (time 0) for each target cook temperature. At predestined times, the samples were removed and submerge into container bearing ice/water slurry and determined within 30 min. At 60°C, 65°C, and 70°C, samples were removed every 7, 2 min and 30 seconds, respectively, for 7 time points/temperature. The microbiological quality was analyzed.
The number of L. monocytogenes survival in sous-vided samples was evaluated. Briefly, each sample was aseptically opened, taken a random into stomach bag containing peptone water and mixed using a stomacher (BagMixer 400 model VW, Interscience, France). After 10 fold serial dilutions, suspensions were plated on Listeria selective agar (Oxford formulation) containing 1% (v/v) modified Listeria selective supplement (Oxoid, Ltd., UK) and incubated at 37°C for 48 h (BAM, 2017). Then gram stain, motility, catalase and hemolysis tests and carbohydrate fermentation series were analyzed. The data were recorded as CFU/g after cooking.
To estimate D-values, three samples were determined for every time point and experiments were independently executed triple. Microsoft Excel 2013 software (Microsoft Corporation, 2013) was used for making survival curves. In each temperature, D-values (1 log reduction of microbial) were created as the reciprocal slope of a linear equation fitted to the survival curves. For z-value calculation (1 log reduction of the D-value), Microsoft Excel 2013 software was used for assessing linear regression of mean log D-values vs. their corresponding heating temperatures. The z-value was determined by creating the complete value of the opposite slope.
The preparations of marinated and restructured goat steak were used method reported before except 110 g/kg of water for ingredients and without inoculation. The non-inoculated restructured goat steaks were cooked at 60°C, 65°C, and 70°C in sous-vide cooking equipment (Sous-vide Immersion Circulator SV100, Cuisine Craft Co., Ltd., Thailand). Temperature of sample was observed by thermocouples inserted, previous sealing, in the center pouch. The final core temperature of control sample was reached (time 0). The samples were immersed in sous-vide cooking equipment until samples reached for 46, 12, and 3 min, respectively. Then samples were removed, submerge into the container holding the ice-water slurry and determined within 30 min for microbiological analysis. For other analyses, the sous-vided samples were cooled at 25°C for 30 min and weight. Then physicochemical and sensory attributes were determined.
After sous-vide, the samples were grilled at 120°C–150°C using an electrical griddle (HOM-1122371, Homemate Co. Ltd., Thailand) All grilled sous-vided samples were turned over every 3 min at each surface until core temperature at 70°C for 2 min, as observed by probes of Type-K thermocouple from a digital thermometer model 52 Series II (Fluke Corp., Everett, WA, USA). The grilled sous-vided samples were cooled at 25°C for 30 min and weighed. Then physicochemical and sensory attributes were estimated.
The microbiological analyses were executed in two replicates before and after sous-vide processes. Briefly, the sample was randomized and homogenized in peptone water using stomacher. The homogenate was serially diluted with saline solution and 1 mL of each dilution was grown in different growth media under the following conditions. The following media and incubated conditions were enumerated: (1) were cultivated on plate count agar (PCA, Merck) then incubated aerobically and anaerobically at 37°C for 48 h and 7°C for 7 d for aerobic and anaerobic mesophilic and psychrotrophic counts, respectively (Roldan et al., 2013); (2) PDA (Merck) incubated at 25°C for 5 d for yeast and mold counts (BAM, 2001a); (3) crystal-violet neutral-red bile dextrose agar (Merck) incubated at 37°C for 24 h for Enterobacteriaceae count (Tosukhowong et al., 2011); (4) De Man, Rogosa and Sharpe agar (Merck) incubated anaerobically at 30°C for 48 h for lactic acid bacteria (LAB) count (Tangwatcharin et al., 2019); (5) Streptomycin thallous acetate actidione agar (Oxoid, UK) at 20°C for 72 h for Brochothrix thermosphacta count (Roldan et al., 2013); (6) Baird Parker agar (Merck) to which egg-yolk tellurite emulsion 20% (Merck) incubated at 37°C for 24–48 h for S. aureus count (BAM, 2001b); (7) Tryptic soy agar (Merck) supplemented with polymyxin-B-sulfate (Dumex-Alpharma Fine Chem. Div., Copenhagen, Denmark) incubated at 37°C for 24–48 h for Bacillus spp. count (Turner et al., 1996). Then, coagulase test for S. aureus was determined. Typical colonies for each selective media were counted in plates. L. monocytogenes, Salmonella spp., Coliform, E. coli and Clostridium spp., were also researched by enrichment and MPN method using BAM (2017), ISO 6579 (2002), BAM (2002) and Turchi et al. (2016), respectively.
The pH of cooked samples was directly measured at three different locations of around sample using portable pH meter (SevenGo SG2, Mettler Toledo, Switzerland).
The raw, sous-vided and grilled sous-vided samples were weighed. The weights of sample were used to calculate the sous-vide loss (%) and grilling loss (%).
To measured steak surfaces before and after cooking, a drawing computer program, AutoCAD 2000 (Autodesk Inc., San Rafael, CA, USA) was used. Surface shrinkage was calculated as surface change (%) due to cooking in sous-vide cooking equipment and on electrical griddle (Serrano et al., 2006).
The color was investigated on the cut surface of samples after cooking in sous-vide cooking equipment and on electrical griddle. The CIE L* (lightness), CIE a* (redness) and CIE b* (yellowness) values were determined by using a HunterLab MiniScan EZ 4000L (Hunter Associates Laboratory, USA). The reading means were obtained on five locations in each sample. The chroma (C) and hue angle (h°) were calculated according to the equations:
Shear force and TPA were analysed using an Instron universal testing machine model 3344 (Instron Engineering Co., USA). For shear force, ten rectangular cubes (15×15×35 mm) of sous-vided and grilled sous-vided samples were taken. Each sample was cut and nonintersect to the fiber direction making allowance for possible. Each sample was sheared with a 50-kg load cell using a cross-head speed of 60 mm/min with a WBSF device. The maximum force (N) was recorded (Sorapukdee and Tangwatcharin, 2018). For TPA, sous-vided and grilled sous-vided samples were cut into cubes (15×15×15 mm). TPA was estimated by a cylindrical aluminum probe, 55 mm inner diameter. The consecutive testing conditions of TPA parameters were crosshead speed was 1.0 mm/s and compressed two times to 40% of their original height, results were automatically stored data by the Blue-hill 2 software (Instron Engineering Corp., USA). Hardness (N), cohesiveness (ratio), gumminess (N), springiness (ratio) and chewiness (N) were estimated for the force-time curves generated for each sample using the method of Bourne (1978). These determinations were estimated triplicate for each sample.
The data were determined by the general linear model procedure to study the effects of different sous-vide processes. Least squares means were computed and separated (p<0.05) using the PDIFF option of GLM. All statistical determinations were executed using SAS v. 9.0 (SAS Institute Inc., 2002). Pearson’s correlation coefficients were carried out to determine the relationship among variables using the CORR procedure.
Results and Discussion
The restructured goat steaks inoculated L. monocytogenes were cooked in water bath at final core temperature of 60°C, 65°C, or 70°C. The initial number of L. monocytogenes before cooking ranged between 7.34–7.57 Log CFU/g. The final core temperature of control sample was reached (time 0). Come-up times ranged from 30–35 min. Due to higher come-up times with higher core temperatures, initial number of L. monocytogenes affected by heating before time 0. There was a decrease in initial number of L. monocytogenes as core temperature rise. At time 0, the counts of L. monocytogenes were ranged between 7.22–7.32, 7.00–7.14 and 6.55–6.75 Log CFU/g at 60°C, 65°C, or 70°C, respectively. At these temperatures, the samples were demonstrated linear decline in the log numbers of survival cells with time. The D-values of L. monocytogenes in different heated samples at 60°C to 70°C accessed by linear regression are shown in Fig. 1A-C. At three temperatures, regression curves for L. monocytogenes inactivation were fitted with R2 values of more than 0.99. D-values of L. monocytogenes in samples were 7.72, 1.99, and 0.46 min at 60°C, 65°C, and 70°C, respectively.
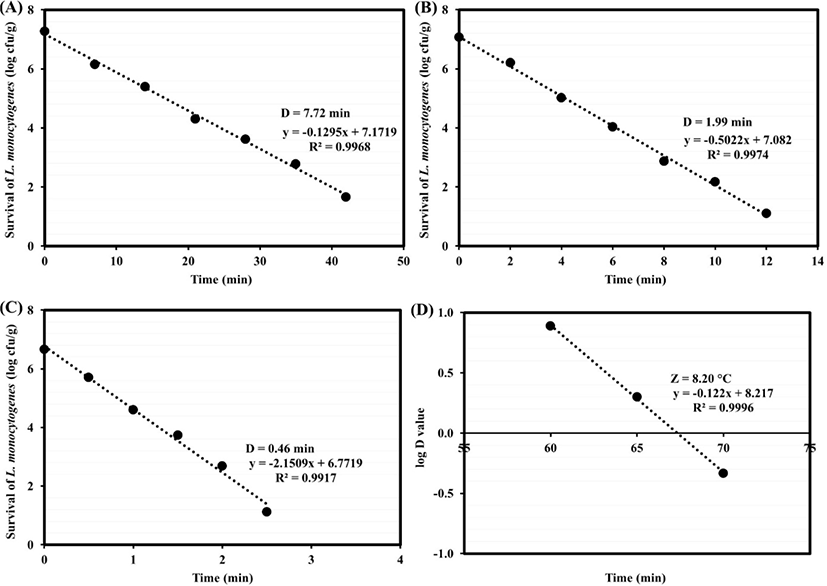
In previous study, the D-values at 60°C of L. monocytogenes was 5.61 min (Murphy et al., 2004). The D-values at 65.6°C and 71.1°C of L. monocytogenes in uncured roast beef were 7.25 and 0.34 min, respectively, using water bath (McMinn et al., 2018). In another study, the resistance of five-strain L. monocytogenes cocktails was estimated in chicken breast piece and cooked in water bath. The D-values were achieved using linear regression analysis which there were 45.05 and 7.28 min at 55°C and 60°C, respectively (Karyotis et al., 2017). These differences of D-values may be due to influence heat resistance, including variations between strains, diversities of sample sizes, meat types and additives, and the heat protection differences from environment (Bolton et al., 2000).
In this study, long time/ low temperature for cooking objected to simulate the conditions confronted in retail food service that appear in the food industry preparing sous-vide processed foods. Horn et al. (2015) gave the D-values at 60°C, 65°C, and 70°C of L. monocytogenes were 15.2, 2.4, and 0.4 min, respectively, in all meats. Then their 6D-values as thermal pasteurization, were 91.2, 14.4, and 2.4 min, respectively. In this study, the 6D-values of L. monocytogenes were 46.32, 11.94, and 2.76 min, respectively, in 2 cm thick restructured goat steak. Thus, sous-vide times (≥ 6D of L. monocytogenes) were approximately 47, 12, and 3 min, respectively and required in the next experiment.
To calculate z-values, the D-values achieved in restructured goat steak were used for plotting thermal-death time curves. The z-value of L. monocytogenes was 8.20°C (Fig. 1D). L. monocytogenes demonstrate uniform heat lethality kinetic with the thermal-death time curves. Heat resistance of L. monocytogenes increased two-fold when the rates of temperature rise were lower than 0.5°C/min (Quintavalla and Campanini, 1991). In consonance with suggestion of Kregel (2002), due to system of heat shock response and heat-shock proteins formation, the lower is the temperature rise rate, the greater is the heat resistance. Horn et al. (2015) reported that z-value of L. monocytogenes in all meat was 6.25°C. The increased heat resistance in this study may be due to the differences of quantifying heat resistance method, heating menstruum, strain type and number and stationary phase cultures.
The initial microbial counts and their after heat treatments of samples non-inoculated L. monocytogenes are shown in Table 1. The raw steak samples of showed counts for aerobic and anaerobic mesophilic and aerobic psychrotrophilic bacteria, Enterobacteriaceae and LAB higher than 3 Log CFU/g; while for anaerobic psychrotrophic bacteria, yeast and mold and B. thermosphacta counts ranged from 1.51 to 2.17 Log CFU/g. For all microbial groups, the prevalence of microbial in the raw goat meats was almost 100% (data not shown).
After sous-vided by different processes, all microbial counts were not detectable, were lower than 1 Log CFU/g or lower than 3 MPN/g, except for the aerobic and anaerobic mesophilie and LAB. These microbial may be Bacillus and LAB forms which can grow in a nutrient-rich culture and non-selective media such as PCA under aerobic and anaerobic conditions, respectively (Moerman et al., 2001). The variance data analysis demonstrated a no significant effect amongst the sous-vide processes. The mean counts ranged from 1.72 to 1.88, 1.13 to 1.22 and 1.03 to 1.14 Log CFU/g, respectively. According to these results, aerobic mesophilic bacteria count in turkey cutlet of 2.05 Log CFU/g was found after cooking at 65°C for 40 min using sous-vide machine (Akoglu et al., 2018). For study of the microbiological quality of beef steaks sous-vided at a core temperature of 70°C for 2 min, total plate counts of 2.48 Log CFU/g were found after cooking (Bolton et al., 2000). Nevertheless, pathogens as L. monocytogenes, Salmonella spp., Bacillus cereus and Clostridium perfringens were not found, and as Enterobacteriaceae, LAB, mold and yeast counts were lower than 1 Log CFU/g after cooking. These microorganisms absence could be commonly because of the combination of sous-vide cooking and vacuum packaging has resulted in these microbial destruction (Nyati, 2000). Thermal pasteurization times for sous-vided meat were provided, confirming that even the difference sous-vide processes considered in this study (60°C for 47 min, 65°C for 12 min and 70°C for 3 min) were far enough to pasteurize meat. It describes the different depletion in the estimated microbial groups between experiments (Baldwin, 2012).
The influence of sous-vide processes on sous-vide loss was described in Table 2. The sous-vide losses were affected by sous-vide process (p=0.001). There was an increase in sous-vide loss as temperature rise. The samples sous-vided at 60°C for 47 min showed lower sous-vide loss than those sous-vided at 65°C for 12 min and 70°C for 3 min. In cooked meat, water losses are caused by three major actions. First, risen temperature and/or reduced pressure causes water to evaporate (Baldwin, 2012). Second, most of the water absorbed within the muscle were maintained by myofibrillar proteins. The myofibrillar proteins can be shrunk due to increased temperatures during cooking, a process starts at 40°C and evolves into more acute with risen temperatures. A parallel decrease in the interfibrillar volume may be due to this shrinking action. It leads to a decrease maintain water ability of myofibril. Therefore, part of the water held by capillarity is lost during cooking (Pathare and Roskilly, 2016). Finally, a shrinkage of the perimysial connective tissue at 56°C to 62°C may be, caused a muscle fiber bundles compression. It in turn encourages water to be released from the meat cut (Baldwin, 2012). Practically, risen temperatures of sous-vided lamb at least 70°C would reach to higher cooking losses than holding longer times at 60°C (Roldan et al., 2013). In present study, the sous-vide loss had a positive correlation with surface shrinkage (r=0.874, p<0.01, Table 3).
Surface shrinkage is essential in retaining quality standards of restructured goat steak. The effect of different sous-vide processes must be estimated in the connection. The samples surface shrank after sous-vided by between 13.28% and 22.16% (Table 2). The surface shrinkage of sample increased as temperature was increased (p<0.001). The surface shrinkage in cooked meat is caused by structure meat changes during cooking. The connective tissue network and the muscle fibers co-operatively shrink longitudinally at 60°C–70°C, the contraction increasing with rise temperature. Commonly, the intra-muscular collagen (largely perimysial) shrinks longitudinally at 64°C (Tornberg, 2005).
The sample sous-vided at 60°C for 47 min showed lower CIE L* (lightness) and h° with higher CIE a* (redness) than those at 65°C for 12 min and 70°C for 3 min (p<0.05) (Table 2). Accordingly, pork cheek samples heated at 60°C showed higher values of CIE L* than those at 80°C. This result of CIE L* may be related to the greater amount of exuded water, which persists saturating the water surface. This appearance has been identified as a cause of higher CIE L* values (del Pulgar et al., 2012). Moreover, CIE L* had a positive correlation with sous-vide loss (r=0.704, p<0.05, Table 3). The intensity of ClE a* (redness) in cooked meat is conversely related to the denatured myoglobin degree, which a denaturing process is become at 60°C (Geileskey et al., 1998). In this investigation, the h° had a negative correlation with CIE a* (r=–0.782, p<0.05, Table 3). Hence, sample sous-vided at 60°C for 47 min exposed higher values of CIE a* (more intense red color) than those other sous-vide processes (p<0.05). Hue angle (h°) is influenced by the myoglobin chemical state and is conversely related to value of CIE a*. Hence, samples sous-vided at 60°C for 47 min indicated the lowest Hue values than those sous-vided at 70°C for 3 min (p<0.01), may due to the lesser degree of myoglobin denaturation mentioned above.
Achieved D-values for shear force and the TPA analysis of sous-vided samples are shown in Table 2. The samples sous-vided at 60°C for 47 min showed lower shear force, hardness, gumminess and chewiness than those sous-vided at 65°C for 12 min and 70°C for 3 min (p<0.01). During cooking, changes in meat tenderness are connected with heat-induced alteration of myofibrillar proteins and connective tissue, since heat solubilizes the connective tissue and this reaches to meat tenderization. The meat toughening is caused by myofibrillar protein denaturation. Water losses from the muscle tissue upon heating also conduce to this meat strengthening. However, the change from a viscoelastic to an elastic material affects the changes in texture during heating (Baldwin, 2012). The meat becomes firmer because the elastic modulus increases and desires larger tensile stress to extend fractures after cooking over 65°C and up to 80°C (Tornberg, 2005). Moreover, collagen solubilization presented greater with slow cooking time. But myofibrillar shrinkage would have reached its maximum even with the shortest cooking time (del Pulgar et al., 2012). In this investigetion, sous-vide loss and surface shrinkage were correlated with improved shear force and texture profile, which significant correlation between sous-vide loss or surface shrinkage and shear force, hardness, gumminess or chewiness among samples were observed (r=0.803–0.937, p<0.01, Table 3).
After grilling at core temperature 70°C for 2 min, the grilled samples sous-vided at 60°C for 47 min showed significant greater grill loss (p<0.05) than those sous-vided at 65°C for 12 min and 70°C for 3 min (Table 4). However, the grilled samples sous-vided at 60°C for 47 min indicated lower total cooking losses than those sous-vided at 65°C for 12 min and 70°C for 3 min (p<0.05) which there were 28.03%, 30.18%, and 34.66%, respectively. Basically, the sous-vide process affect to restructured goat steak samples, which the higher the temperature, the greater the water losses. Thus, one of the benefits required by sous-vide meat cooking chefs at low temperature, a greater juiciness due to a more concentrated juice keeping (Becker et al., 2016).
For surface shrinkage of grilled sous-vided samples, there was an increase in surface shrinkage loss as rise temperature. Additionally, the grilled samples sous-vided at 60°C for 47 min exhibited lower total surface shrinkages than those at 65°C for 12 min and 70°C for 3 min (p<0.001) which there were 25.13%, 33.42%, and 36.74%, respectively. According to TPA analysis of grilled sous-vided samples, the grilled samples sous-vided at 60°C for 47 min showed significant lower shear force, hardness, gumminess, springiness and chewiness (p<0.01) than those at 65°C for 12 min and 70°C for 3 min (Table 4). Additionally, there was a significant positive correlation between total surface shrinkage and shear force, hardness, gumminess, springiness or chewiness at all sous-vide processes, which shown coefficient values (r) range from 0.716–0.913 (Table 5). As the result to increase in total surface shrinkages and total cooking losses of grilled sample as sous-vide temperature was increased. Previous study reported that increased tenderness was generated by cooking at 60°C. Lower cooking loss and toughness were appeared at temperature close to 60°C (del Pulgar et al., 2012). Cooking loss is correlated with juiciness. Some studied demonstrated that the approach of tenderness was enhanced a juicier feel (Becker et al., 2016).
In this study, the grilled samples sous-vided at 60°C for 47 min demonstrated lower CIE L* and h° values than those at 65°C for 12 min and 70°C for 3 min (p<0.05) (Table 4). Doneness of cooked meat is estimated by color and visual appearance. Most color determination agreed with color and visual evaluations of low temperature meat’s color stability. Redness values of meat reduced with rise temperature from 50°C to 65°C (Becker et al., 2016).
Conclusions
D-values of L. monocytogenes were affected by different sous-vide temperatures for inoculated restructured goat steak. There was a decrease in its D-value as temperature rose and its z-value was 8.20°C. For safety of sous-vided product, 6D-values at 60, 65, and 70°C were applied to cook non-inoculated restructured goat steak. After cooking by different sous-vide processes, the microbial counts of all samples were reduced and pathogens were not detected. While different sous-vide processes appeared to play a main role on the qualities of sous-vided and grilled sous-vided restructured goat steak. It actively affects water losses, surface shrinkage, instrumental color and texture profiles. The samples sous-vided at 60°C for 47 min showed greater the physicohemical quality compared with those at 65°C for 12 min and 70°C for 3 min.













