Introduction
Eggs are a representative perfect food and widely used as raw material of processed foods. There are various processed forms of liquid eggs – whole egg, egg yolk, and egg white. Egg yolk is used for mayonnaise flavoring and additives, and egg white is used for processed meat products, marine products and liquefied beverages. Mass liquid eggs are used as raw materials in various food industries and the demand for liquid eggs is expected to increase further with its convenience and economic efficiency (Kim et al., 2014). However, pasteurization of liquid eggs is not simple since proteins of egg white are denatured at a temperature of 57°C or higher (Kang et al., 2011). In the removal processing of the egg shells, unpasteurized eggs are exposed to pathogenic microorganisms at high risk. Accordingly, caution needs to be taken when unpasteurized liquid eggs are used for processed products. In Sakha et al. (2012), S. Enteritidis proliferated more rapidly in unpasteurized liquid eggs than in pasteurized liquid eggs.
Salmonellosis continues to be a major food-borne disease in food industry, especially, poultry related products. In Korea, the criteria for microbes in unpasteurized liquid eggs are defined as less than 500,000 CFU per gram of bacteria, less than 100 CFU per gram of Escherichia coli and negative for Salmonella (MFDS, 2015). It should also be cooled to below 5°C after egg breaking and should not be stored for more than 72 hours (MFDS, 2015). However, secondary contamination by Salmonella may occur by various factors such as fecal matter, dust, and so on during the process of manufacturing liquid eggs. Actually, three types of Salmonella serotypes were isolated as a result of monitoring liquid eggs distributed domestically (Kim et al., 2015). Salmonella growth is affected by internal factors such as the moisture activity of food, pH, initial microorganisms and external factors such as the storage temperature, packaging method, and storage humidity. Therefore, proper management in the production, storage, and distribution processes is necessary to control Salmonella.
In order to establish the appropriate temperature and time for food storage and distribution, models for predicting the growth of Salmonella in egg products were developed (Elias et al., 2016; Na, 2014; Sakha et al., 2012; Singh et al., 2011). But most studied mainly on S. Enteritidis growth and did not deal with other serotypes of Salmonella. Recent study on the growth and survival of Salmonella in unpasteurized egg yolk and egg white (Moon et al., 2016) showed that there is a slight difference in Salmonella growth between S. Enteritidis and other serotypes of Salmonella. In the study of Moon et al. (2016), S. Enteritidis proliferated more rapidly than other serotypes, which was accordance with the Salmonella growth predictive model of homemade mayonnaise (Malheiros et al., 2007). Accordingly, it is necessary to conduct a study on the development of growth predictive model in liquid eggs for various Salmonella types. In particular, in Korea, the expiration dates of unpasteurized liquid egg are the same regardless of egg yolk, egg white, and liquid whole egg. Therefore, it is necessary to compare and analyze the changes in Salmonella depending on the storage time in various temperature conditions considering the manufacturing environments, distribution processes of liquid eggs.
In this study, we predicted the growth of Salmonella in unpasteurized liquid whole egg and egg yolk stored at 10–40°C, where Salmonella mainly proliferate, using a growth predictive model. The purpose of this study was to establish a proper storage temperature and the expiration dates of unpasteurized liquid eggs using the model predicting the growth of Salmonella.
Materials and Methods
The 10 liter of unpasteurized liquid whole eggs used in this experiment was purchased from an egg processing plant in Anseong, Gyeonggi-do in 2012. At the same time, 400 shell eggs were prepared and separated into egg yolk and egg white. The conditions of eggs used in this study were as follows: the microbial levels of unpasteurized liquid whole egg, egg yolk, and egg white used in this study were less than 500,000/g for general bacterial count, less than 100/g for E. coli count, Salmonella negative. The acidity (pH) was 6.39±0.11 in egg yolk, 9.23±0.02 in the egg white, and 7.49±0.05 in liquid whole egg, which all satisfying the quality control standards of South Korea.
To artificially contaminate the unpasteurized liquid whole egg, egg yolk, and egg white described above, 5 kinds of Salmonella serovars — the two reference strains of S. Enteritidis ATCC 13076 and S. Gallinarum ATCC 9184, and S. Typhimurium monophasic, S. Bareilly, and S. Richmond which are the main issues in liquid eggs in South Korea (Kim et al., 2015) were used in the experiment.
An aliquot (about 8 mL) of the liquid whole egg, egg yolk, and egg white was put into a 10 mL tube and inoculated with the mixed Salmonella serovars with the initial concentration of 3 Log CFU/g. A total of 1,000 mL inoculated liquid eggs were stored at 5, 10, 15, 20, 25, 30, 35 and 40°C (n=15–20 samples per each temperature). Changes in the Salmonella growth in unpasteurized liquid egg were measured for up to 960 h. That is, each 10 mL sample was homogenized by adding 20 mL of sterilized 0.1% buffered peptone water (BPW, Oxoid, UK) to a test tube and using a vortexer for 30 s. One millitliter of the homogenized sample was diluted with 9 mL of sterilized 0.1% peptone water for each concentration level using the decimal dilution method and was uniformLy distributed to the selected medium, Xylose Lysine Deoxycholate Agar (Oxoid, UK). Each medium was incubated in a 37°C incubator for 24 h, and the number of cells was counted to calculate Log CFU/mL.
The development of a predictive model at each temperature was performed through first and second rounds. The first growth predictive model is based on the Baranyi function equation proposed by Baranyi and Robert (1994). The lag phase duration (LPD) and the maximum specific growth rate (μmax) of Salmonella depending on temperature and storage time were calculated with DMFit 3.5 Excel® Add-in (IFR, UK).
Logy0 : Initial populations (Log CFU/mL)
y : Population at any time (Log CFU/mL)
μmax : Maximum specific growth rate (Log CFU/h)
ymax : Maximum population (Log CFU/mL)
q0 : Measure of the initial physiological state of cell
LPD and μmax, the variables of a primary model calculated in each temperature, were used to develop a secondary growth predictive model. The secondary model was developed on the basis of a Davey model (Davey, 1989) and a square-root model (Ratkowsky et al., 1982), using Graph Pad Prism V4.0 (Graph Pad Software, USA).
To verify the suitability of the growth predictive model, growth curves of Salmonella in liquid whole egg and yolk were fitted to the Baranyi model. Measured values of Salmonella in the unpasteurized liquid whole egg and egg yolk stored at 20°C and 30°C, which were not used in the predictive model, were applied for validation. For assessment between the measured and predicted values, values of Bias factors (Bƒ), accuracy factors (Aƒ) and root mean square error (RMSE) proposed by Baranyi et al. (1999) were compared. If Bf is less than 0.7 or greater than 1.15, the model is not suitable. Af indicates the inaccuracy of the developed model as the Af value moves away from 1 (Baranyi et al., 1999). If Bƒ and Aƒ value is close to 1, and RMSE was close to 0, the developed secondary model is considered to be fit for predicting the growth of Salmonella.
Each experiment was repeated twice and the results were analyzed using SAS program version 9.3 (SAS Institute Inc., USA). A significant difference (p<0.05) between LPD and μmax was analyzed using a one-way ANOVA which utilizes the Duncan's multiple test method. In addition, t-test was used to analyze the significant difference in μmax and the LPD of liquid whole egg and egg yolk (p<0.05).
Results and Discussion
Salmonella did not proliferate in all liquid egg products stored at 5°C even after artificially inoculating Salmonella (Fig. 1). These results coincided with the previous studies where cryopreservation minimizes the possibility of Salmonella proliferation (EFSA Panel on Biological Hazards, 2014) and the minimum growth temperature of S. Enteritidis is 6–8°C (Whiting et al., 1997). In contrast, Salmonella at a storage temperature of above 10°C proliferated in liquid whole egg and egg yolk over a time period. In liquid whole egg, the bacteria proliferated by more than 1 Log after 168 h (7 days) at 10°C and 72 h (3 days) at 15°C. In addition, Salmonella proliferated more than twice of the initial concentration after 12, 9, and 8 hours at 25, 35, and 40°C, respectively. In egg yolk, Salmonella proliferated by more than 1 Log after 96 h (4 days) at 10°C and after 33 h at 15°C, showing the proliferation about twice faster than in liquid whole egg. This study showed a similar result to Singh et al. (2011), where the mixure of four Salmonella serotypes including S. Enteritidis did not show proliferation in liquid whole egg stored below 7°C, but proliferated at 10–43°C. In the study of Gumudavelli et al. (2007), 5 types of S. Enteritidis proliferated in egg yolk at 10–43°C, but not at 7°C. Therefore, it is important to distribute the liquid whole egg and egg yolk below 10°C in order to lower the risk of Salmonella.
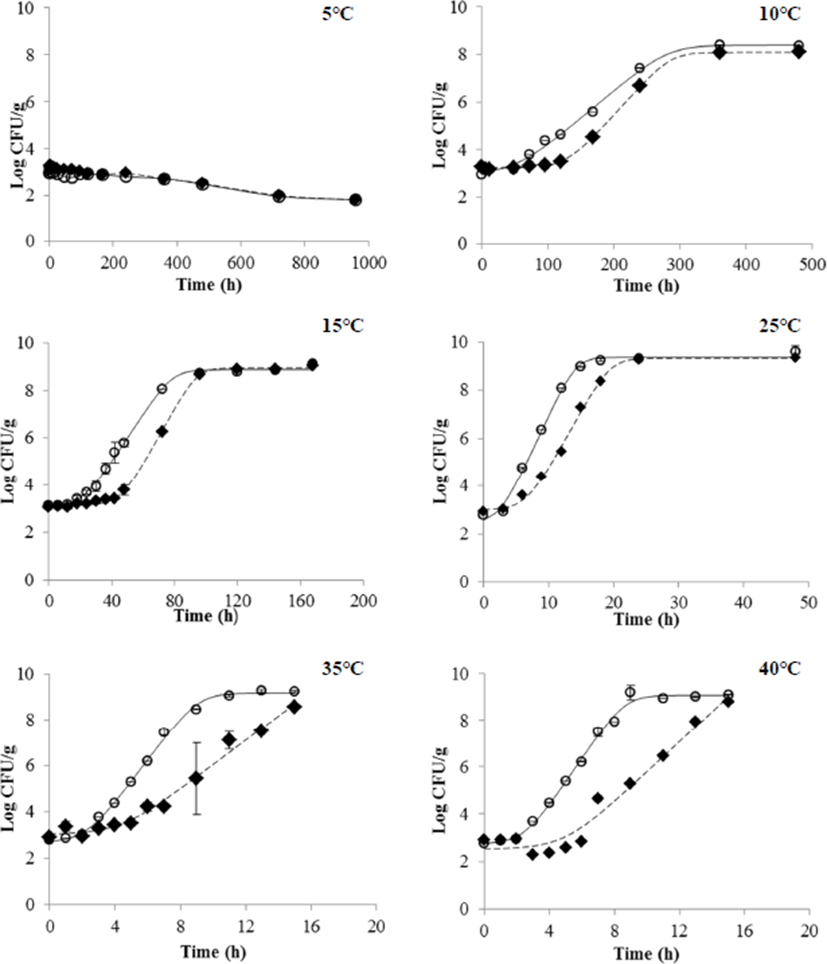
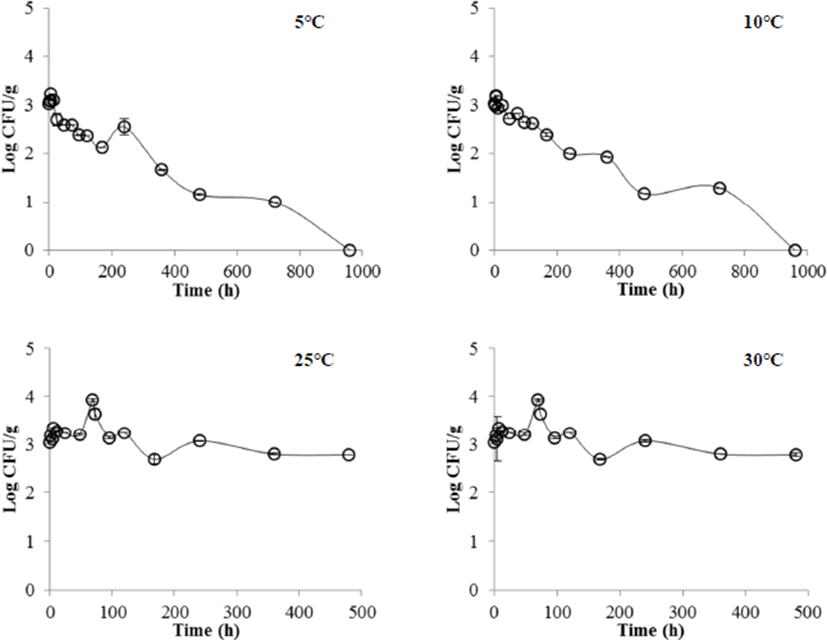
In the case of the liquid egg white (Fig. 2), Salmonella at 5 and 10°C was undetectable after 960 h (40 days). On the other hand, Salmonella at 25 and 30°C survived maintaining the initial concentration (3 Log CFU/g). These results are similar to the results of Moon et al. (2016), where S. Enteritidis and S. Typhimurium proliferated slightly in eggs stored at 25°C whereas they did not grow at 8, 10, and 15°C. Kang et al. (2011) reported that the growth of microorganisms in egg white was slower than that of liquid egg yolk and liquid whole egg.
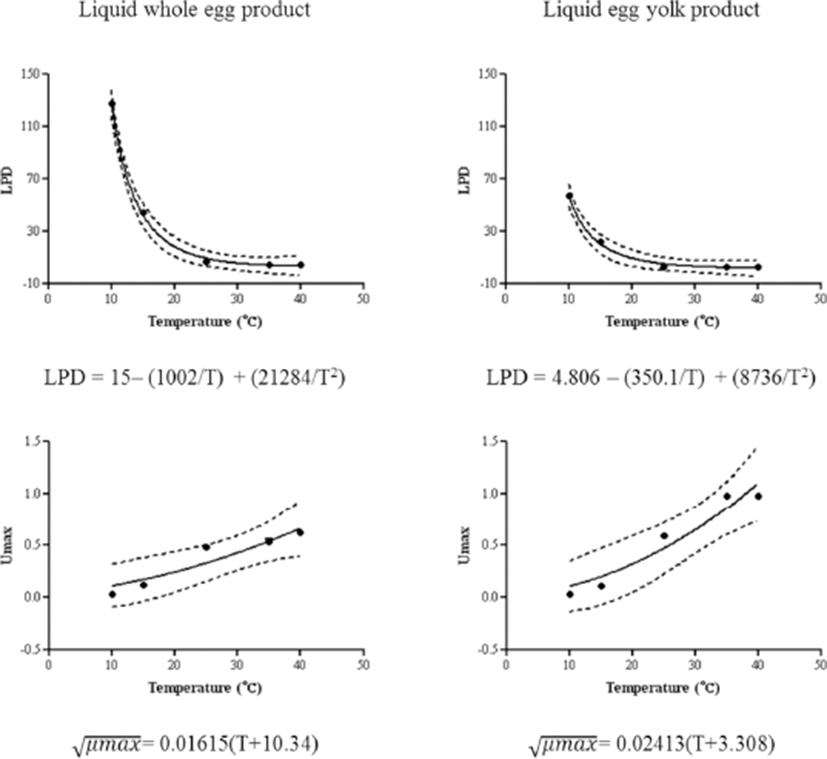
The Baranyi model was well fitted to the growth of Salmonella in liquid whole egg and egg yolk showing the r2 values of 0.98–0.99. Table 1 shows the growth parameters (LPD and μmax) for Salmonella, obtained using the Baranyi model, in liquid whole egg and egg yolk. As the storage temperature increased, LPD significantly (p<0.05) decreased and μmax significantly (p<0.05) increased in both liquid whole egg and egg yolk. At all temperatures, LPD was shorter and μmax was significantly higher (p<0.05), in egg yolk than in liquid whole egg, with the largest difference at 35°C. The difference in Salmonella spp. growth may be due to the albumen components such as lysozyme and ovotransferrin, which is present only in egg white. It is reported that the albumen can affect the degradation of bacterial cell walls, stunting the growth of Salmonella (Zhang et al., 2011).
The LPD and μmax calculated in the primary study was used to derive a secondary growth model by applying to a Davey model (Davey, 1989) and a square-root model (Ratkowsky et al., 1982), respectively (Fig. 3). The r2 values of the secondary models ranged from 0.92 to 0.99, indicating the suitability of the model applied. This is similar to trend of the previously developed LPD model for Salmonella in egg yolk solution and mayonnaise (Elias et al., 2016; Moon et al., 2016). In addition, μmax increased with increasing temperature and the difference between whole egg and egg yolk was larger. The previously developed μmax model of mayonnaise (Elias et al., 2016) was closer to the model of egg yolk than the whole egg. Therefore, it is likely that Salmonella may grow rapidly, when egg yolk solution and processed foods containing egg yolk are stored at room temperature.
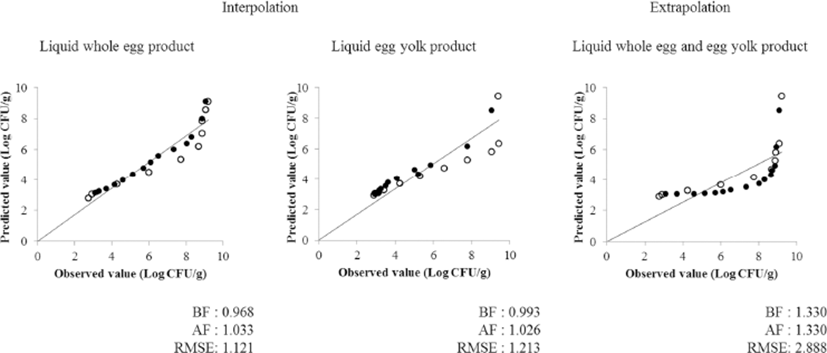
Further, the second growth predictive model was validated with experimental data at 20 and 30°C (Fig. 4). When comparing measured values with predictive values, Bf values of whole egg and egg yolk were 0.968 and 0.993, respectively. Af values were also close to 1, indicating that the model is fit for predicting the growth of Salmonella. Extrapolating the egg yolk model using the observed value of whole egg indicated that there is a difference in the proliferation pattern of Salmonella (Bf: 1.330). These data indicate that the models utilized in this study were suitable in predicting the growth of Salmonella spp. in the unpasteurized liquid whole egg and egg yolk under a variety of storage temperature and time conditions. Therefore, it is thought that the Salmonella growth predictive model can be used usefully in setting the storage temperature during the distribution process of unpasteurized liquid whole egg and egg yolk.
Conclusion
This study demonstrated there is a difference in the proliferation of Salmonella in unpasteurized liquid eggs depending on the types of liquid eggs (egg yolk, egg white, whole egg) and storage temperature, using Salmonella growth predictive model. Therefore, it is desirable to reset the expiration dates of liquid eggs taking into account of the product type, storage conditions (temperature and time), and microbiological factors.













