Introduction
Clostridium perfringens is a Gram-positive, anaerobic, spore-forming bacterium found in various environments, especially in the gastrointestinal tract (GIT) of humans and animals (Songer, 1996; Van Immerseel et al., 2009). It is not a foodborne pathogen causing food poisoning, but also increases the risk of enterotoxaemia, gangrenous dermatitis and necrotic enteritis, especially in swine and poultry (Grass et al., 2013; Songer, 1996; Van Immerseel et al., 2009). C. perfringens produces various toxins (α, β, β2, ε, ι and the enterotoxin CPE) associated with the disease outbreaks (Caly et al., 2015; Timbermont et al., 2014; Uzal et al., 2014).
With increased limitations on antibiotic use, the efficacy of alternatives such as bacteriocins and bacteriophages has been evaluated (Cotter et al., 2013; Joerger, 2003).
Bacteriocins are ribosomally synthesized peptides or proteins, which display a broad or narrow spectrum of antimicrobial activity. They show bactericidal action against several pathogenic bacteria and are widely regarded as natural bio-preservatives in food and feed industries (Gálvez et al., 2007; Greer, 2005).
Bacteriophages (phages) can also be used as effective alternatives to antibiotics for pathogen control (Sabah and Richard, 2014). Bacteriophages are viruses that infect and replicate within the bacteria, leading to host lysis and cell death. Phages and their derivatives have emerged as natural bio-preservatives for the prevention, treatment, and eradication of pathogenic bacteria in a wide range of processing environments (Enderson et al., 2014; Martinez, et al., 2008).
Therefore, in this study, we isolated a bacteriocin-producing strain and two bacteriophages from the feces of domestic animals and characterized their antimicrobial activities against C. perfringens. We determined their antimicrobial spectrum, conditions of activity and stability. In addition, the isolated bacteriocin and bacteriophages were evaluated for combined effects against C. perfringens. We investigated the probiotic effects of bacteriocin-producing bacteria and bacteriophages in vitro to develop potential candidates for probiotic use in domestic animals.
Materials and Methods
Indicators used in this study were obtained from the Korean Collection for Type Culture (KCTC). Bacteriocin-producing bacteria were isolated from the feces of chickens and maintained at -80℃ in stock solution (skim milk and glycerol mixture). Streptococcus hyointestinalis strain was cultured in GM17 (M17 medium supplemented with 0.5% glucose). We isolated phages against C. perfringens from the feces and cecal contents of chickens and stored in saline magnesium buffer (SM buffer; 50 mM Tris-Cl, 100 mM NaCl, 8 mM MgSO4·7H4O, and gelatin 0.002% (w/v), pH 7.5).
The fecal samples of chickens were collected from a poultry farm at Chung-Ang University. The pig fecal samples were collected from a local farm in Anseong, Gyeonggi-do, Republic of Korea. Fecal samples were isolated from 35-day-old Ross 308 broiler chickens, 38-week-old Hyline Brown laying hens, and 22-week-old Landrace pigs. Samples were used immediately for the screening of bacteriocin-producing bacteria and lytic bacteriophages.
Fresh fecal samples were serially diluted ten-fold with sterile anaerobic dilute solution (Na2HPO4 6.0 g, KH2PO4 4.5 g, L-cysteine 0.5 g, Tween 80 0.5 g per L) and plated on brain heart infusion (BHI) or lactobacilli MRS agar and incubated at 37℃ for 24 h under anaerobic conditions (Bryant, 1972).
After incubation, 40 colonies (20 per medium) in each sample were randomly selected and inoculated into 5 mL of BHI or MRS broth (Han et al., 2014). The isolates were incubated at 37℃, and centrifuged at 10,000 g for 10 min at 4℃. The supernatants were filtered with a 0.45 μm filter (Pall Life Sciences, USA) followed by testing for antagonistic activity against C. perfringens according to the spot-on-the-lawn method (Mayr-Harting et al., 1972). The supernatants were serially diluted two-fold, and 10 μL of samples were spotted onto the surface of 0.7% BHI soft agar (1.25 mM CaCl2) inoculated with an overnight culture of C. perfringens KCTC 3269T . After incubation at 37℃ for 24 h, the plates were checked for the inhibition zones.
After identification of inhibition zones against C. perfringens, the supernatants were adjusted to neutral pH and treated with proteinase K and catalase at a final concentration of 1 mg/mL (Sigma Chemical CO., USA) at 37℃ for 1h, followed by heating at 80℃ for 10 min to inactivate the enzymes and tested again for bacteriocin levels (Todorov and Dicks, 2005).
To isolate the bacteriophages, the fecal samples were mixed with BHI broth supplemented with 2 X CaCl2 and 2 X MgSO4 in the ratio 1 : 2. The mixture was inoculated with C. perfringens KCTC 3269T and incubated at 37℃ for 48 h for preenrichment. After incubation, the sample was centrifuged at 10,000 rpm for 10 min at 4℃, and the supernatant was filtered.
The pre-enriched samples were processed using the double-layer method for phage propagation (Zinno et al., 2010). Each double-layered plate contained 100 μL of samples and 100 μL of overnight incubated C. perfringens KCTC 3269T and 4 mL of BHI soft agar (0.7%) on BHI agar. After incubation at 37℃ for 24 h, a single plaque was isolated from the plate and placed in 1 mL of SM buffer in an Eppendorf tube, and laid for 12 h at 4℃ to spread out. The buffer was centrifuged at 10,000 g for 10 min and the supernatant was filtered to eliminate the indicator. The filtered supernatant was propagated by the double-layer method (Carvalho et al., 2010; Salama et al., 1989).
Isolated phage titer was determined by spot-on-the-lawn method as described above. The propagated phages were serially diluted in SM buffer followed by the addition of 10 μL on the surface of soft BHI agar containing C. perfringens, and the titer was expressed as plaque forming units (PFU/mL).
To identify the bacteriocin-producing bacteria, their DNA was isolated using a Powerfecal DNA isolation kit (MOBIO., USA) and the 16S rRNA gene was PCR-amplified using universal primers (27F and 1492R) followed by purification and sequencing (Solgent, Republic of Korea). The identified strains were confirmed and compared with BLAST in NCBI (Basic Local Aliments Search Tools in National Center for Biotechnology Information, USA).
The bacteriocin-producing strain was incubated in BHI broth (1% v/v) at 37℃, and the samples were removed at 2-h intervals to measure the antagonistic activity of bacteriocin. The bacteriocin activities were conducted by the spot-on-the-lawn method (Teo and Tan, 2005). Bacteriocin activity was expressed in terms of arbitrary units per mL (AU/mL), defined as the highest two-fold dilution showing definite inhibition of the indicator (Han et al., 2014).
To identify the inhibitory effects of bacteriocin against other pathogenic species, the bacteriocin-producing bacteria was serially diluted ten-fold with sterile 1X phosphate buffered saline (PBS; NaCl 8.0 g, KCl 0.2 g, Na2HPO4 1.44 g, KH2PO4 0.24 g per L), and spread onto GM17 agar plates. After incubation at 37°C for 24 h, they were overlaid on the soft agar (0.7%) with indicator organisms. After further incubation at 37°C for 18 h, we determined the inhibition zone around the colonies of bacteriocin-producing bacteria.
The bacteriocin-producing S. hyointestinalis B19 strain was inoculated (1% v/v) in 1 L of GM17 broth at 37℃ for 15 h. The bacteria were removed by centrifugation at 10,000 g for 10 min at 4℃ followed by 0.45 μm filtration. The culture supernatant was precipitated at 40% saturation by the addition of fine-ground ammonium sulfate and stirred for 1 h at 4°C. After centrifugation at 10,000 g for 15 min at 4℃, the precipitate was resuspended in a low volume of 100 mM sodium phosphate buffer pH 6.5 (Villani et al., 1995). The precipitate solutions were dialyzed and concentrated by Amicon centrifugal filter (3 kDa molecular cut off, Merck Millipore Ltd, Germany). Each step was expressed in terms of AU/mL of inhibition zones of C. perfringens KCTC 3269T (Lee et al., 2015; Villani et al., 1995).
To test for heat stability, the partially purified bacteriocin was heated at 25℃, 50℃, 90℃ for 1 h, or 121℃ for 15 min. To identify the effects of pH stability of bacteriocin, equal amounts of 2 M pH 2 (glycine-HCl), pH 4 (acetate), pH 6 (sodium phosphate), pH 8 (Tris), and pH 10 (glycine-NaOH) buffer were mixed, and incubated at 4℃ for 24 h (Lee et al., 2015). Any residual activity of the bacteriocin against C. perfringens KCTC 3269T was determined by the spot-on-the-lawn method (Teo and Tan, 2005).
Lytic activities of the phages were evaluated by measuring the optical density (O.D.) during incubation of the mixtures containing C. perfringens and the phage. We incubated 3 mL of BHI broth with 1% (v/v) overnight cultured C. perfringens KCTC 3269T (108 CFU/mL) and 1% diluted phages at multiplicity of infection (MOI) values of 1.0 (108 PFU/mL) and 0.1 (107 PFU/mL). The mixtures were incubated at 37℃ under anaerobic conditions for 12 h and 0.2 mL samples were obtained in triplicate at 2 h intervals to measure O.D. values using a 96-well cell culture plate and a microplate reader (Molecular Devices LLC., USA) (Albino et al., 2014).
The combined effects of the isolated bacteriocin and phages were evaluated. The partially purified bacteriocin (1/2 diluted of desalted solutions) and P4, A3 phages (diluted to 108 PFU/mL) were used in the combined effect test. We inoculated 5 mL of BHI broth with 50 μL (1%) of 108 CFU/mL C. perfringens KCTC 3269T followed by supplemental treatments. First, a single supplement was added including 150 μL (3%) of the bacteriocin, P4 phage, and A3 phage. Second, the mixture of bacteriocin and phages was supplemented with B19 (1%) bacteriocin and a cocktail of P4 (1%) and A3 (1%) phages. The total percentage of the added supplements was similar for all the treatments. The mixtures were incubated at 37℃ for 12 h under anaerobic conditions. Before and after incubation, 200 μL of the samples were extracted in triplicate for O.D. measurement at 600 nm. The viable cell counts of the residual C. perfringens were identified by diluting all the samples ten-fold with a 1 X PBS buffer, and spread on BHI agar followed by incubation at 37℃ for 24 h under anaerobic conditions (Leverentz et al., 2003; Martinez et al., 2008).
To identify the antagonistic effect of the bacteriocin-producing bacteria and the combination of bacteria and lytic phages, a 5 mL of BHI broth was inoculated with 50 μL (1%) of 108 CFU/mL overnight cultured C. perfringens KCTC 3269T . We first added 50 μL (1%) of 107 CFU/mL diluted S. hyointestinalis B19 to the BHI broth. We then added 50 μL (1%) of 107 CFU/mL of overnight-cultured S. hyointestinalis B19 and 50 μL (1%) of each dilution to 108 PFU/mL P4, A3 cocktail of the phages. The mixtures were incubated at 37℃ for 12 h under anaerobic conditions. The survival of C. perfringens was determined by plating decimal dilutions on plates of C. perfringens selective agar (Oxoid Ltd., UK.) and BHI agar (Martinez et al., 2008).
To test acid tolerance, the overnight culture of S. hyointestinalis B19 was centrifuged at 10,000 rpm for 10 min at 4℃ and washed with 1 X PBS buffer. It was harvested again and resuspended in 5 mL of the pH adjusted buffer to a final pH 2.0, 2.3, 2.5, 3.0, 4.0, 5.0 and 7.0 (control). The suspensions were incubated at 37℃ for 1 h, and the survival of S. hyointestinalis B19 was determined by the spread plate count on GM17 agar.
The bile tolerance test was conducted by spreading the overnight cultures of S. hyointestinalis B19 on GM17 agar containing bovine bile (Sigma, USA) at 0%, 0.5%, 1%, 2%, 3%, 4%, and 5%, respectively. The plates were incubated at 37℃ for 48 h (Dunne et al., 2001; Han et al., 2014).
Results
Among the 1,160 strains isolated from the fecal samples of chickens and pigs, 31 strains showed inhibitory activity against the indicator strains. The supernatants of these strains were neutralized with 1 M NaOH, and treated with enzymes such as catalase and proteinase. Three catalase-negative and proteinase-positive strains were selected, and one bacteriocin-producing strain against C. perfringens was finally selected.
The strain was identified by 16S rDNA sequencing and compared with BLAST in NCBI. The 16S rDNA sequence showed 99.09% similarity with that of Streptococcus hyointestinalis ATCC 49169T . Therefore this strain was identified as Streptococcus hyointestinalis B19.
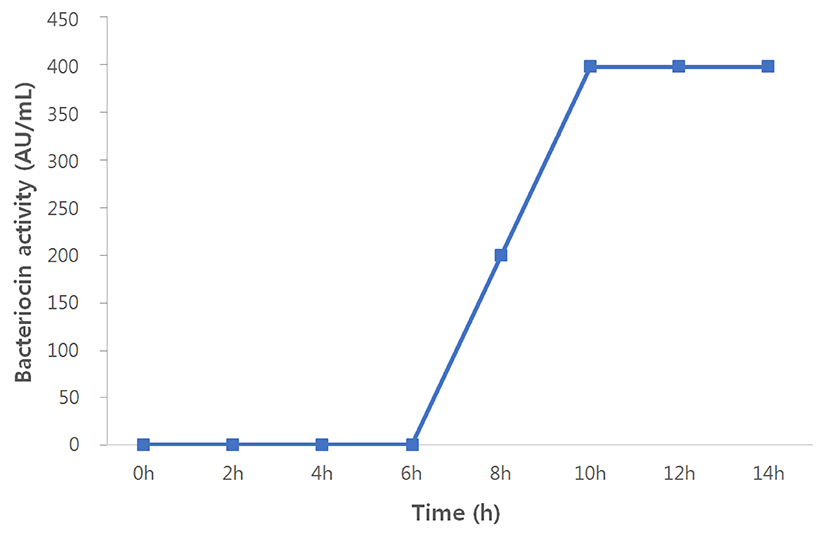
Bacteriocin production by S. hyointestinalis B19 in the BHI medium was detected at the beginning of 8 h growth time (200 AU/mL), which reached the maximum levels at 10 h (400 AU/mL; Fig. 1).
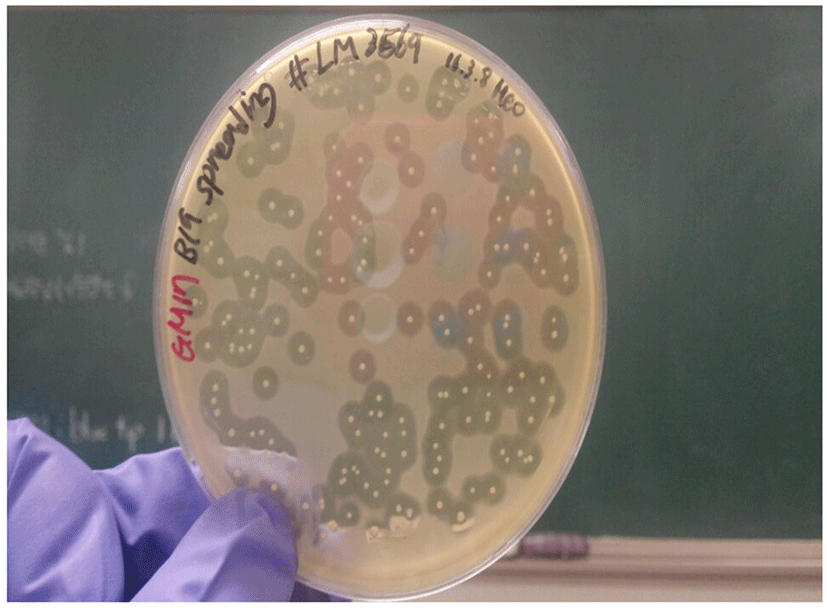
The colonies of bacteriocin-producing bacteria were tested for their antimicrobial activities against a few Gram-positive and Gram-negative bacteria. Most of the selected C. perfringens and Listeria monocytogenes strains showed a strong inhibition zone around the colonies of bacteriocin-producing bacteria (Fig. 2). However, the bacteriocins failed to inhibit all the tested Gram-negative strains (Escherichia coli, Salmonella, Campylobacter spp.) and Gram-positive Staphylococcus aureus strains (Table 1).
The bacteriocin activity of the cell-free supernatant (1 L) obtained after culturing bacteriocin-producing bacteria was 400 AU/mL. Ammonium sulfate precipitation enhanced the bacteriocin activity to 12,800 AU/mL, and finally Amicon desalting and concentration resulted in 25,600 AU/mL activity (Table 2).
| Volume | 1 L | 40 mL | 4 mL |
|---|---|---|---|
| Strain / purification step | Culture supernatant | Ammonium sulfate | Amicon desalting |
| S. hyointestinalis B19 | 400 AU/mL | 12,800 AU/mL | 25,600 AU/mL |
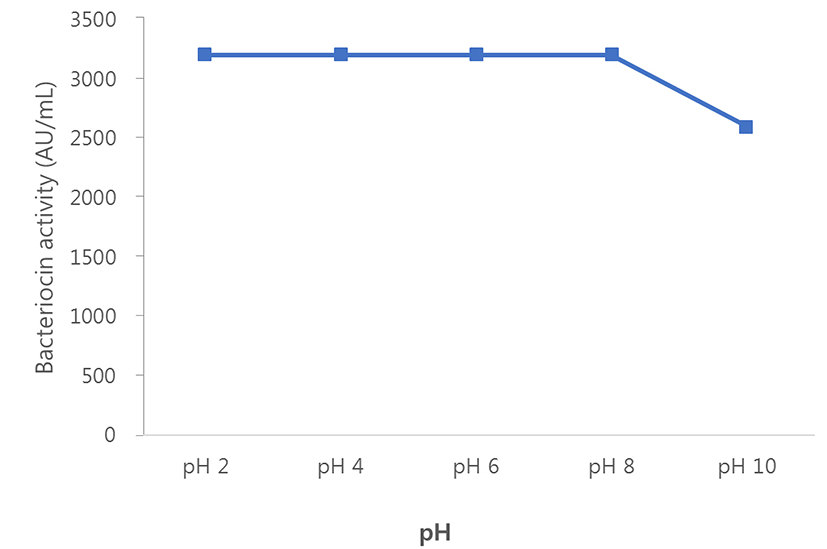
The effects of heat and pH on the stability of bacteriocin were investigated. The partially purified bacteriocin was a heat-stable and pH-stable protein. These activities remained fully active at buffer pH 2, 4, 6 and 8 (3,200 AU/mL). At pH 10, the bacteriocin activity of the strain was reduced by 20% approximately (2,600 AU/mL; Fig. 3).
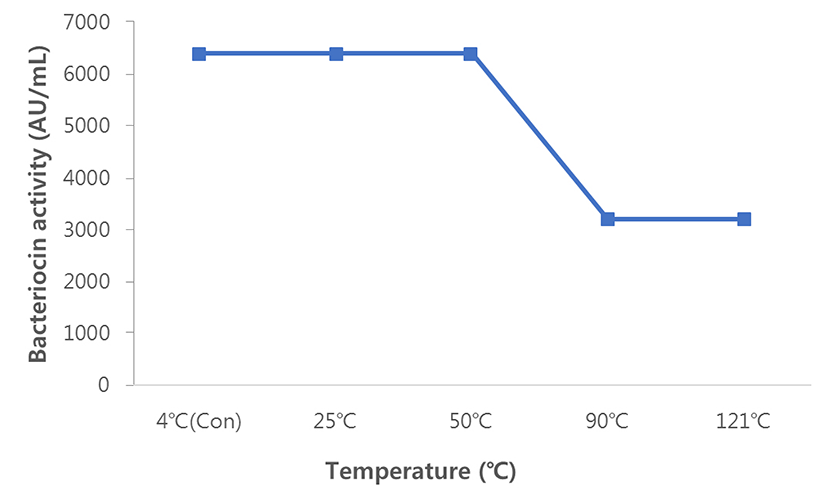
Bacteriocin (6,400 AU/mL) was fully stable during heat treatment at 4℃, 25℃, and 50℃. However, the activity was reduced by 50% (3,200 AU/mL; Fig. 4) after heating at 90℃ and autoclaving at 121℃.
We isolated 10 phages (A1 to A6 and P1 to P4) against C. perfringens KCTC 3269T . A1 to A6 phages were isolated from the feces of laying hens (Hyline Brown, 38-weeks-old) and P1 to P4 phages were isolated from the feces of pigs (Landrace, 22-weeks-old). All the isolated phages showed activities against C. perfringens KCTC 3269T alone. These isolated phages were propagated by double-layer methods to reach 109 PFU/mL. In this process, four phages, A1, A3, P2, and P4 were selected for further experiments (data not shown).
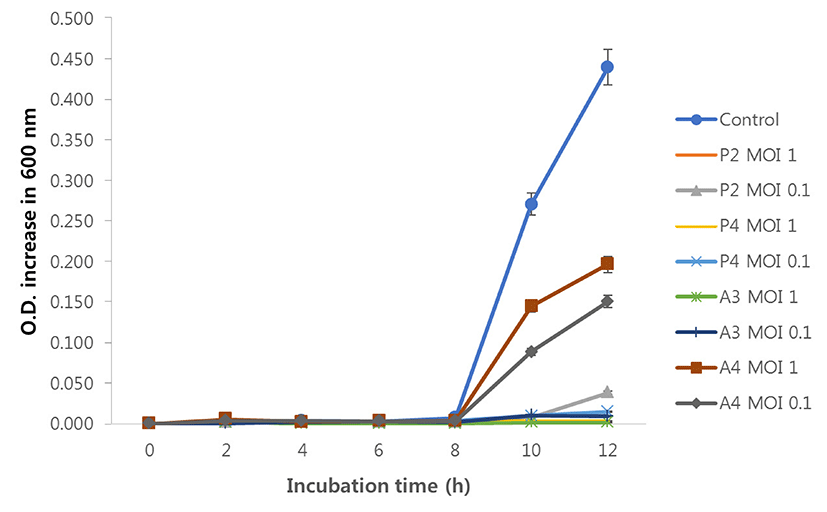
Lytic activities of the four phages were evaluated by measuring the increase in optical density (O.D.) of C. perfringens KCTC 3269T using a 96-well microplate reader. All the treatments showed inhibition of Clostridium perfringens KCTC 3269T growth compared with control (indicator only), however, the degrees of inhibition varied. After 12 h of incubation, P2 at MOI 1, P4 at MOI 1 and 0.1, and A3 at MOI 1 and 0.1 treatments completely (rate of O.D. increasing values less than 5%) inhibited the growth of C. perfringens compared with control. The P2 at MOI 0.1 treatment inhibited minimally (8.7% increase), while A4 phage treatments showed the lowest inhibition (MOI 1; 44.7%, MOI 0.1; 34.3% increase) (Fig. 5).
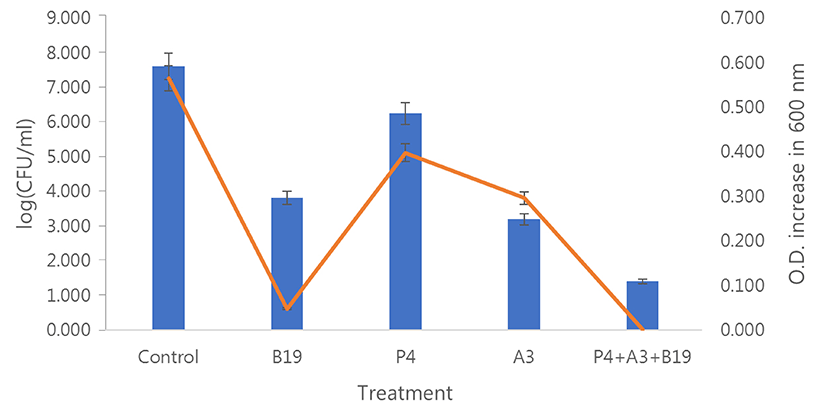
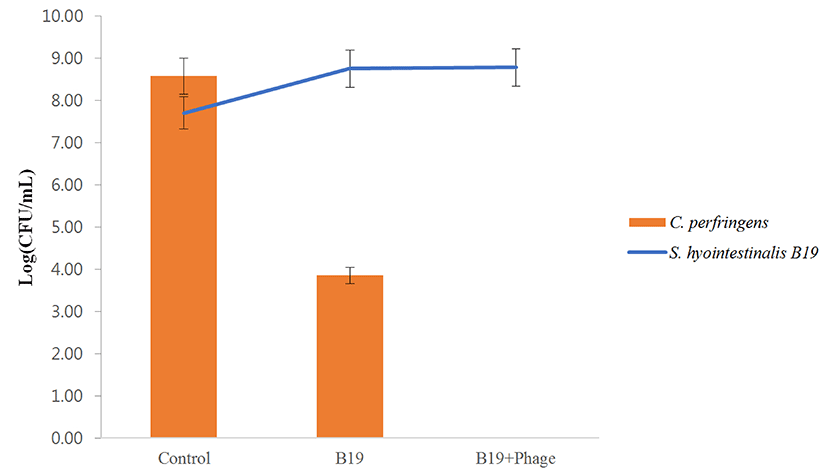
The combined effect of the isolated bacteriocin and phages was evaluated using the P4 and A3 phages in this experiment. The viable cell counts and O.D. measurements of the treated samples at 600 nm were evaluated simultaneously. After incubation for 12 h, the treatments with phages combined with bacteriocin, or phage and bacteriocin alone reduced the bacterial populations compared with control (indicator only). Treatments with the phage or bacteriocin alone reduced the bacterial populations by 3.80 (B19 bacteriocin alone) 1.36 (P4 phage alone) to 4.41 (A3 phage alone) log units. In comparison, the addition of phages and bacteriocin together reduced the bacterial populations by 6.20 (B19+P4+A3 treatment) log units. As the data reveal, combining the phages with bacteriocin resulted in higher inhibition compared with the phage or bacteriocin alone. The O.D. variation was almost similar across the bacterial populations (Fig. 6). The antagonistic effect of S. hyointestinalis B19 strain alone and in combination with the bacteria and the lytic phages was evaluated. Treatment with S. hyointestinalis B19 reduced C. perfringens population from 8.58 to 3.85 log units. A second treatment with S. hyointestinalis B19 combined with P4 and A3 phages eradicated the bacterial populations completely. Further, the population of S. hyointestinalis B19 in the combined treatment with C. perfringens and S. hyointestinalis B19 was higher than in the treatment with S. hyointestinalis B19 strain alone (Fig. 7)
The bacteriocin-producing S. hyointestinalis B19 was stable until pH 4.0 (at pH 7, average 8.64 log unit; at pH 5, 8.48 and at Ph 4, 8.55 log units, however, the survival of the strain was not detected below pH 3.
S. hyointestinalis B19 was stable at a bile salt concentration up to 1% (containing 0%, average 9.63 log unit; 0.5%, 8.80 and 1%, 8.84 log units). At 2% and 3% bile concentration, CFU of S. hyointestinalis B19 decreased (containing 2%, 7.19 and 3%, 5.40 log units). Finally at 4 and 5 %, the cell counts were not detected.
Discussion
In this study, the bacteriocin-producing strain, S. hyointestinalis B19 and two lytic phages, P4 and A3 phages, were isolated from feces of chickens and pigs, and were found to exhibit antagonistic effects against C. perfringens.
The S. hyointestinalis strain was first reported in 1988 from the intestines of pigs (Devriese et al., 1988). Recently, O’Conner et al. (2015) characterized a novel bacteriocin, Nisin H, produced by a strain of S. hyointestinalis DPC6484 isolated from the porcine intestine. However, the isolated S. hyointestinalis B19 strain was different from DPC6484 strain, and no nisin H genes were identified using PCR amplification (data not shown). The S. hyointestinalis strain generated broad-spectrum antimicrobial peptides and inhibited various Gram-positive strains (O’Shea et al., 2009). The isolated S. hyointestinalis B19 produced bacteriocin containing heat and pH-stable peptides showing activity at all pH and temperature variations experimentally and demonstrated a strong antimicrobial spectrum against a majority of Clostridium and Listeria species.
The isolated P4 and A3 phages exhibited lytic activity against C. perfringens KCTC 3269T. Lytic activities of the phages varied with the MOI of the phage and culture conditions. P4 and A3 phages showed the most effective inhibition against C. perfringens KCTC 3269T.
The combined effects of the isolated bacteriocin and phages were evaluated. Treatment with bacteriocin or phage alone resulted in instant bacteriocidal and bacteriostatic effects against C. perfringens, however, regeneration of C. perfringens was also observed (data not shown), which was in accordance with previous reports (Dykes and Moorhead, 2002). The most important finding in this study was that a synergistic effect was observed when the phages and bacteriocin were combined. In addition, co-culturing with the bacteriocin-producing S. hyointestinalis B19 reduced the population of C. perfringens significantly. In addition, the combination of S. hyointestinalis B19 and a cocktail of P4 and A3 phages eradicated C. perfringens completely. Interestingly, the population of S. hyointestinalis B19 in the combined treatment with C. perfringens and S. hyointestinalis B19 exceeded that of the S. hyointestinalis B19 strain inoculated alone.
Finally, the probiotic application of S. hyointestinalis B19, acid and bile resistance tests of the strain was evaluated. The S. hyointestinalis B19 strain showed tolerance to 3% bile salts and remained stable at pH 4.0.
In conclusion, the isolated bacteriocin and bacteriophages represent potential options for the biocontrol of C. perfringens. The bacteriocin-producing S. hyointestinalis B19 strain exhibits probiotic effects, with potential application in domestic animals.













