Introduction
There are many methods to quantitatively and qualitatively analyze flavor components of feeds to determine their quality, including high pressure liquid chromatography, gas chromatography, and Mass spectrometry. However, instruments for these methods require some expensive reagents. In addition, experienced operators are needed for sample preparation and instrument operating. Moreover, these methods are extremely selective. Furthermore, only limited targets can be detected (Lehotay and Hajslova, 2002; Muller and Steinhart, 2007; Nollet, 2000).
Electronic tongue is mostly used in liquid analysis using sensory arrays and pattern recognition system (Vlasov et al., 2002). Basically, single taste buds composed of 50 to 100 taste cells can sense five tastes such as sweetness, sourness, saltiness, bitterness, and umami (Deisingh et al., 2004). Harper (2001) has reported that results of sensory evaluation using an electronic sensory machine are positively correlated with results of using human sensory panelists. In case of human sensory evaluation, experiment not only needs trained sensory panelists, but also needs time for training panelists with high cost. Lyon and Lyon (2001) have indicated that human sensory evaluation can be affected by individual sensory experience. For these reasons, Legin et al. (2003) have found that it is possible to use an electronic tongue to replace human sensory evaluation for wine.
Research on consumers purchasing characteristics and satisfaction for Hanwoo beef has revealed that there are differences in consumer preference depending on beef muscle types: loin, 43.5%; rib, 22.9%; tenderloin, 10.5%; brisket, 9.9%; and the sum of top round and shank, 4.7% (Hwang et al., 2010). Acceptability for shank and round muscles has also found to be less than that for other muscles (Jeremiah et al., 2003). Chemical characteristics of meat such as collagen contents, nucleotide acids, free amino acids, and fatty acid compositions are also dependent on muscle types such as chuck roll, strip loin, top round, and brisket (Cho et al., 2008; Guillemin et al., 2009; Hiner and Hankins, 1950; Lee et al. 2010). Most round muscles with high quality are used for steaks while some round muscles with high amounts of connective tissues are used for stew meat or ground beef similar to shank muscles which has highly tough parts (Ramsbottom and Strandine, 1947).
Meat aging technologies such as dry aging and wet aging have been utilized to improve meat quality for a long time. In wet aging, meat is aged in a vacuum-sealed pack to preserve its moisture. In dry aging, meat is dried without packing for a few weeks. Effect of dry aging on enzyme activities such as protein degradation has been determined. It has been found that dry aging contributes to intense flavor and taste of meat (Perry, 2012). Sitz et al. (2004) have demonstrated that some consumers prefer dry-aged meat more than wet-aged meat. In addition, they are willing to pay more for dry aged meat. Kim et al. (2016) have reported that drying aging process can increase the tenderness of top round muscles and consumer acceptability.
Since no study has demonstrated how wet-aging and dry-aging might affect sensory characteristics of shank and round muscles of beef during aging using an electronic tongue, the objective of this study was to determine the effect of aging method) and time on physical, chemical, and sensory properties of two muscles. This study was accomplished by proving the physicochemical, textural and sensory properties of wet-aged and dry aged beef according to different aging periods and muscles. Besides, meat sensory evaluation by using an electronic tongue can provide unique information of aging beef in useful way.
Materials and Methods
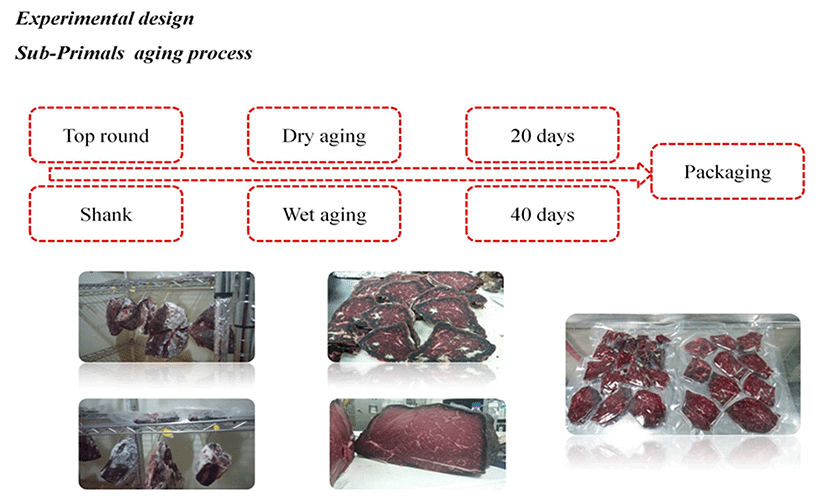
Top round and shank meats at 12 d postmortem from a total of 12 Holstein steers (age of about 26 mon) were purchased from a commercial slaughter house. Average carcass weight was 293 kg. Carcass yield grade was C according to Korean carcass grading procedure (National Livestock Cooperatives Federation, NLCF 1998). In the laboratory of Konkuk University, whole samples were cut into pieces (1.5-2.0 kg per piece). All samples (top round and shank) were randomly allocated to wet aging (top round, WTR; shank, WS) and dry aging (top round, DTR; shank, DS) groups with identical digit number for each group. For wet aging, muscles were aged in sealed vacuum-packed bags. For dry aging, muscles were hung in refrigerated room with air circulation system. Aging conditions in the refrigerated room were controlled at temperature of 1.0±0.5°C and relative humidity (RH) of 80-85% with air flow of 0.5-1.5 m/s. Samples were collected on day 20 and day 40 during the aging period. Experimental design and samples are shown in Fig. 1.
Moisture, crude fat, crude protein, and ash contents of samples were analyzed according to the procedure of AOAC (2002). After different aging time (20 d or 40 d), weight of each sample was compared to the initial weights. Weight loss percentage (%) was calculated using the following equation:
Weight loss (%) = {(initial weight of sample – weight after aging)/ (initial weight of sample)} × 100
Cooking loss and shear force were evaluated using published method (Kim and Lee, 2003). Briefly, steak samples (about 100 g) with thicknesses of 1.5 cm were prepared in vacuum package. These samples were cooked in a water bath at 75°C to reach internal core temperate of 70°C. They were then placed at room temperature for 30 min. Cooking loss was calculated by comparing steak weights before cooking and after cooking. To measure shear force, sample was collected from each steak parallel to muscle fiber with six replicated samples after cooling. Measurement of shear force was conducted by attaching Warner-Bratzler shear force onto an Instron universal testing machine (Instron Corporation, USA). The condition of this machine was set at cell load of 50 kg and cross-head speed of 200 mm/min.
Color measurement of all samples was conducted using a colorimeter (NR-300, Nippon Denshoku, Japan). Before measurement, all samples were placed on a bloom at room temperature for 30 min. The colorimeter was calibrated with a white plate (L*, lightness; a*, redness; b*, yellowness).
Each sample (2 g) was homogenized in 18 mL of distilled water. Homogenized sample was then subjected to pH measurement using a pH meter (pH 900, Precisa Co., Switzerland).
Fatty acid composition was analyzed using the method of AOAC (1995). First, lipid was extracted from sample according to method of Folch et al. (1957) with slightly modification. Briefly, the sample was treated with 10% boron trifluoride (BF3) in methanol for methylation. The sample was then heated at 60°C for 40 min in a water bath. After cooling to room temperature, hexane and distilled water were added into the sample followed by centrifugation at 2,000 rpm for 15 min. The supernatant was used to analyze fatty acid compositions. Chromatography conditions were: initial oven temperature, 100°C, held for 4 min; ramping at 3°C/min to 240°C, held for 15 min. Temperatures of injector and detector were maintained at 225°C and 285°C, respectively. Flow rate of helium was set at 0.75 mL/min. Then 1 μL of solution was injected into the machine in split mode (200:1). Nonadecanoic acid methyl ester (0.3 mg/mL) was used as internal standard. It was added to each sample prior to fat extraction and methylation. The isooctane layer was dehydrated with anhydrous sodium sulfate and subjected to analysis using gas chromatography (5,890, Agilent Technologies, USA) equipped with a flame ionization detector using an SP-2560 column (100 m × 0.25 mm × 0.2 μm).
Analysis of free amino acid was conducted using the method of Nishimura et al. (1988). Briefly, 10 g of meat was homogenized in 25 mL of distilled water for 10 min. The sample was then centrifuged at 11,500 g for 10 min at 4°C. The supernatant was filtered through a filter paper. After adding 5% trichloroacetic acid into the filtrate, the sample was centrifuged again (11,500 g for 10 min at 4°C). The supernatant was filtered with a 0.45 μm membrane filter and subjected to analysis for free amino acids using an amino acid analyzer (Jasco, Japan LC-NETII/ADC Analyzer, Japan).
Extraction of meat was conducted using published method (Escudero et al., 2012) with slight modification. Briefly, 5 g of meat was homogenized with 20 mL of 0.01 N HCl for 8 min using a bag mixer. The homogenized sample was centrifuged at 10,000 g for 20 min at 4°C. The extract was filtered through Whatman No. 1 (11 μm) filter paper to remove impurities such as fat and tissues. The extract was stored at -80°C until analysis. Four standard compounds (MgSO4 for bitterness, HCl for sourness, NaCl for saltiness, and MSG for umami) were prepared to check the cross-selectivity of sensors. These standard compounds were prepared at the same concentration (0.01 mol/L). Electronic tongue analysis was performed using an Electronic tongue machine (Taste sensing system SA 402B, Insent Intelligent Sensor Technology, Inc., Japan).
All treatments for this study had a split-split-plot design with three factors. The main factor was muscle type (top round and shank meats). Sub-plot treatment was aging method (wet-aging or dry-aging). Sub-sub plot was aging times (20 d or 40 d). General linear model (GLM) and Pearson correlation analyses were performed using SPSS version 18.0 (SPSS Inc., USA). Data were expressed as mean and standard error of mean. Statistical significance was considered when p-value was less than 0.05.
Results and Discussions
Data on proximate compositions of top round and shank muscles are shown in Table 1. At 20 d of dry aging or wet aging, moisture contents of top round and shank muscles ranged from 68.42% to 73.80% while fat contents of both muscles ranged from 1.09% to 2.88%. Proximate compositions of top round and shank muscles were similar to those reported in a previous study (Lee et al., 2010) showing that ratios of moisture contents to fat content in top round and shank muscles were lower than those in higher quality grade beef. Although moisture contents of both top round and shank muscles at 20 d were not significantly (p>0.05) different between wet aged meat and dry aged meat, dry-aged meat at 40 d had lower moisture contents than wet-aged meats in both top round and shank muscles. After aging for 20 d and 40 d, protein contents of all groups were increased (p<0.05) except for those in the wet aged top round group. Protein contents of dry-aged beef were increased more than those of wet-aged beef at 40 d (p<0.001). This result could be related to the fact that the reduction ratio of moisture content in the dry-aging process was relatively higher than that in the wet-aging process. Wet aged beef had lower (p<0.05) ash content compared to dry aged beef.
1)TR, top round; 2)SH, shank muscle
Results of weight loss, cooking loss, pH, and shear force of wet and dry aged beef are shown in Table 2. The pH values of both top round and shank with dry-aging process at 40 d were significantly increased compared to those of aged beef at 20 d after dry aging. Increased pH in dryaged beef has been observed (Obuz et al., 2014). Increasing pH in beef through the aging process can be occurred by the formation of nitrogen compounds from proteolysis (Aksu et al., 2005). However, the pH of wet aged beef was decreased (p<0.05) from 20 d to 40 d. The pH value of shank was higher than that of top round regardless of aging time or aging method. According to Lee et al. (2010), pH values of various muscles are different. Aging method and aging time showed significant (p<0.001) interaction effect on pH of meat. Wet aged beef showed lower pH value than dry-aged beef at both 20 d and 40 d (p<0.05). The decrease in pH of meat with vacuum package during aging might have contributed to accumulation of lactic acid originated from meat born lactic acid bacteria (Blixt and Borch, 2002). The increase in pH of meat during dry-aging process could be due to the formation of nitrogen compounds from proteins caused by proteolysis (Aksu et al., 2005). Aging process can influence pH and water-holding capacity of meat (Boakye and Mittal, 1993). Oiao et al. (2001) have explained that pH of meat has positive correlation with water holding capacity and expressible drip while water-holding capacity has negative correlation with moisture content.
1)TR, top round; 2)SH, shank muscle
Cooking loss of dry-aged muscles was significantly (p<0.001) lower than that of wet-aged muscles. However, there was no significant (p>0.05) difference in cooking loss between top round and shank. The decrease in cooking loss of dry-aged muscles might be due to less moisture content caused by evaporation (Juárez et al., 2011). Similarly, Obuz et al. (2014) have reported that wet-aged loin steak has higher cooking loss than dry-aged loin steak.
Weight loss of both muscles treated with dry-aging process was significantly higher than that of wet aged muscles at 20 d and 40 d. The ratio of weight loss in both muscles treated with wet and dry aging constituted to increase (p<0.001) during aging. In addition, aging method and muscle type showed significant (p<0.001) interaction effect on weight loss of beef. Although weight loss of wet aged shank showed lower tendency than that of wet-aged top round, the shank muscle treated with dry aging process showed higher weight loss compared to top round treated with dry aging.
No significant difference in shear force was found between muscle types, although the shear force of dry aged meat was lower (p<0.05) compared to that of wet aged meat. Aging method and aging time showed significant (p<0.001) effect on shear force. Dry aging process decreased shear force as expected. Although there was numerically difference of the shear force between 20 d and 40 d, the significant difference of shear force in wet-aged beef was not detected (p>0.05). However, wet aging process did not decrease the shear force at 40 d. According to previous studies, both shear force and tenderness with aged flavor in beef loin improved with increasing the aging time from 14 d to 49 d (Lepper-Blilie et al., 2016) in agreement with Obuz et al. (2014) who demonstrated shear force is decreased with increasing aging time. Campbell et al. (2001) have indicated that dry aging process can improve sensory characteristics and decrease shear force after 14 d and 21 d.
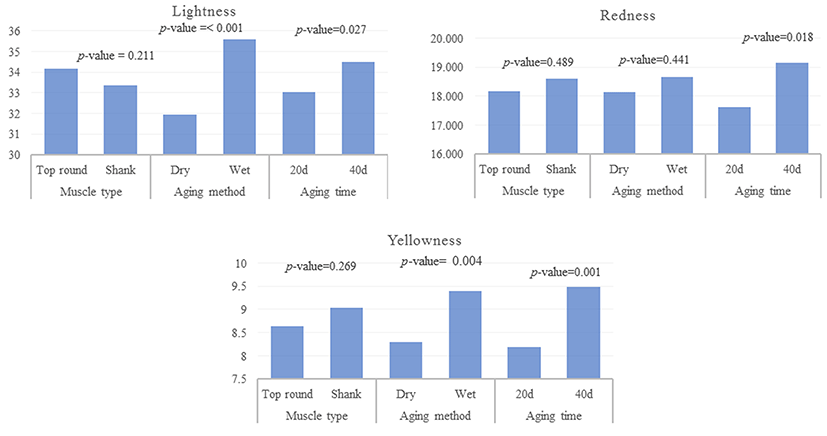
Muscle type did not affect L* (lightness), a* (redness), or b* (yellowness). An interaction between muscle type and aging method was not found for color values (Fig. 2). Lightness and yellowness values of dry aged beef were decreased compared to those of wet aged beefs. Lightness and yellowness values of aged beef at 20 d were higher (p<0.05) than those of wet aged beef. The redness value of aged beef was increased (p<0.05) during aging. Qioa et al. (2001) have reported correlations of lightness with pH (r=-0.9632), moisture (r=0.6633), and water-holding capacity (r=-0.8929). Therefore, the low lightness value in dry aged beef observed in the present study might be due to high pH and higher water-holding capacity of meat. Although aging method did not influence redness of meat, aging time did affect the redness value. Redness of shank muscle of wet aged beef was slightly but significantly (p<0.05) increased during aging. Similar result has been reported by Ismail et al. (2008) showing that aging time has effect on the color of ground beef. Color stability can be different according to muscle type since each muscle had different oxygen consumption rate and metmyoglobin reductase activity (Madhavi and Carpenter, 1993).
Results of free amino acids (FAA) analysis for different muscles treated with wet or dry aging process are shown in Table 3. Contents of most free amino acids were significantly (p<0.001) increased in both wet aged and dry aged beef after aging. Contents eof total free amino acids (TFA) and some free amino acids such as Asp and Glu related to umami taste in shank muscles (SH) were higher (p<0.001) than those in top round muscles (TR). Moreover, muscle type and aging method showed significant (p<0.001) effect on contents of TFA, Asp, and Glu. Shank muscle were influenced by aging more than top round muscle. Glutamic acid and aspartic acid could enhance the umami taste of meat. Contents of both Glu and Asp in dry aged beef were higher (p<0.001) than those in wet aged beef. Approximately 6-fold of increase (p<0.001) of glutamate content was found for wet aged beef after aging. Aging method and aging time showed significant (p<0.001) effect on glutamate content. Dry aging process resulted in lower glutamate content compared to the wet aging process. This might be due to moisture removal in the dry aging process since glutamate is a water-soluble component of umami taste (Sasaki et al., 2007). As expected, TFA content was increased (p<0.001) with increasing aging time. However, TFA content in dry aged muscle was lower (p<0.05) than that in wet aged muscle, similar to results of a previous report (Feidt et al., 1996) showing a positive relationship between FAA content and protein degradation in meat with increasing aging time. Therefore, the increase in free amino acids after aging might be due to proteolysis of muscles during the ageing process.
1)MT, Muscle type; 2)AM, Aging method; 3)AT, Aging time; 4)TFA, Total free amino acid
Results of fatty acid compositions of wet and dry aged beefs (top round and shank muscles) are shown in Table 4. It is known that fatty acid compositions in meat are associated with sensory flavor profiles (Wood et al., 2003). Fatty acid compositions were not affected by muscle types (p>0.05). However, compositions of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA) of wet aged beef were higher (p<0.05) than those of dry aged beef. Recommended ratio of PUFA to SFA (P:S) is 0.4 (Wood et al., 2003). The SFA (P:S) of beef after the dry aging process was more close to the recommended P:S ratio compared to that after the wet aging process. Dry aged beef had lower (p<0.05) C18: 3n3, which 3n3 has negative effect on flavor when C18 3n3 reacts with volatile compounds from the cooking process, than wet aged beef (Campo et al., 2003). Aging method and aging time showed significant (p<0.05) interaction effect on C18:3n3. This result might be due to the decrease of C18:3n3 during the dry aging process. MUFA (C14:1 and C16:1) at 20 d were lower compared to those at 40 d (p<0.05). However, some PUFA (C20:2 and C20: 3n6) were increased (p<0.05) after aging. Muscle types and aging method had significant (p<0.001) interaction effect on contents of C14:1 and C16:1. Madhavi and Carpenter (1993) reported that each muscle had different oxygen consumption and enzyme activities. Therefore, these high MUFAs in meat might be related with stearoyl-CoA desaturase activity (St.John et al., 1991).
1)MT, Muscle type; 2)AM, Aging method; 3)AT, Aging time
Results of electronic tongue analysis for aged beef are summarized in Table 5. Aged top round muscle showed significantly lower taste values compared to aged shank muscle except for bitterness. Dry-aged beef had lower sourness but higher bitterness, astringency, umami, and saltiness than wet-aged beef (p<0.001). Most tastes of aged beef might be affected by compositions of free amino acids (Watanabe et al., 2016). The sourness of wetaged beefs could be affected by accumulated lactic acid under anaerobic condition (Warren and Kastner, 1992). Taste enhancement of meat after the aging process is affected by free amino acids and nucleotides such as glutamate and aspartate affecting the umami taste (Liu et al., 2007). Umami compounds of beef are increased with aging time, with dry-aged beef having higher umami score compared to wet-aged beef (Li et al., 2014). Kawai, Okiyama, and Ueda (2002) have suggested that a combination of glutamate and 5’-nucleotides can enhance the umami taste of foods. Especially, umami taste can improve the flavor of meat (Lindemann, 2000; Maga, 1998).
MT, Muscle type; AM, Aging method; AT, Aging time; TR, top round; SH, shank muscle; Bitterness, Bitter; Astrin, Astringency After-b is aftertaste of bitterness; After-a is aftertaste of astringency; Rich is aftertaste of umami.
Conclusions
Physicochemical, texture, and sensory properties of top round and shank muscles are affected by aging methods (dry and wet) and aging time (20 d and 40 d). Shank muscles were more affected by the dry-aging process compared to top round muscles. Results of electronic tongue analysis showed that umami and saltiness tastes of muscles were enhanced by the dry-aging process. In addition, the aging process improved the taste of shank muscles. Instrumental tenderness of dry-aged beef regardless of muscle types was improved through aging time. However, the effect of aging on other flavor compounds such as fatty acids and nucleotides using ET and electronic nose for sensory evaluation needs to be determined in further studies. Comparison of different muscles treated with wet and dry-aging methods will provide information to control for aging time.