Introduction
There is a rapid increase in the elderly population of Korea, and it is predicted that Korea will be a super-aged society by 2026 (Bae and Kim, 2015). With ageing arises the limitation of digestibility that results in decreased protein absorption, thereby contributing to sarcopenia (Ahn and Kang, 1999). Several studies on digestibility using in vitro digestion models have increased the understanding about the bioavailability and bioaccessibility of nutrients (Van Loo-Bouwman et al., 2014; Verwei et al., 2003). However, there have been failed attempts to increase the digestibility of meat and meat products, despite the rapid development in food processing and technology.
Food consumption in Korea has changed over the last decade mainly due to an increase in the per capita income (Lee and Cho, 2012). The Korean beef, Hanwoo, is preferred over the imported beef, owing to the perception that it is superior in palatability and quality (Hwang et al., 2010a; Moon, 2012). However, the Korean Hanwoo beef industry is experiencing different challenges due to the liberalization of the better quality imported beef that is cheaper as compared to Hanwoo (Cho et al., 2011). Thus, development of innovative and more nutritional Korean Hanwoo beef cuts and products, in particular for the elderly population, is required to face this competition (Hwang et al., 2010b).
One of the ways to examine the nutritional value of a food product is by testing the digestibility and breakdown of nutrients. Recent nutritional studies have adopted in vitro digestion models to examine the nutritional profile and digestibility of a variety of food products (Liu et al., 2016; Nimalaratne et al., 2015). In comparison to the in vivo trials with humans and animals, these models may save time, ethical constraints as well as the cost, and produce reproducible and robust data (Yoo and Chen, 2006). The in vitro physicochemical upper gastrointestinal system (IPUGS) used in this study is one of the in vitro digestion models designed to simulate the upper gastrointestinal tract of humans. Its unique features include anatomical and geometrical resemblance to humans, flexible material-made reactor, secretion channels for even distribution in the stomach reactor, and controlled gastric peristalsis under temperature-controlled conditions. The aim of this study was to investigate the digestive behavior of the four different Korean Hanwoo beef cuts using IPUGS. We evaluated the digestion pattern of each Korean Hanwoo beef cut using IPUGS through the analysis of the pH profile, proteolysis pattern based on the measurement of the molecular weight (MW) of peptides, free amino acid profile, and surface of the digested sample using a scanning electron microscope (SEM).
Materials and Methods
Tenderloin, sirloin, brisket and flank, and bottom round cuts of the Korean Hanwoo beef (quality grade 1+) were purchased from a local market in Korea. Each sample (75 g) was boiled in hot water for 15 min and ground by a domestic mixer for 15 s to mimic chewing immediately before the initiation of in vitro digestion experiment. Simulated salivary fluid (SSF) and simulated gastric fluid (SGF) were prepared according to the methods described by Minekus et al. (2014) and Minekus et al. (1999), respectively (Table 1). Each of the four beef cuts was analyzed in triplicate.
| Concentration (mM) | ||
|---|---|---|
|
|
||
| Component | SSF (pH 7) | SGF (pH 2) |
| KCl | 15.1 | 29.5 |
| KH2PO4 | 3.7 | - |
| NaHCO3 | 13.6 | 17.9 |
| NaCl | - | 85.5 |
| MgCl2(H2O)6 | 0.15 | - |
| (NH4)2CO3 | 0.06 | - |
| CaCl2 | - | 2.0 |
| HCl | - | 150 |
SSF, simulated salivary fluid; SGF, simulated gastric fluid.
We designed IPUGS to simulate the digestion process of humans following a modification of the method described by Yoo (2008). IPUGS (Fig. 1) and the secretions (SSF and SGF) were pre-heated to 37°C in a chamber prior to the initiation of digestion experiment. The homogenized beef sample (75 g) was put into the mouth reactor and mixed to form bolus with SSF including alpha amylase (75 kU/L, Sigma Cat. No. 10065). SSF with alpha amylase was pumped with a peristaltic pump at a rate of 7.5 mL/min for 15 mins into the mouth reactor, which was kept at 37°C. The bolus sample was transferred to the esophagus every 30 s to allow its ingestion, followed by its transfer to the stomach reactor. SGF was delivered at a speed of 3.5 mL/min for 10 min before the initiation of digestion simulation in order to mimic the cephalic phase. SGF containing pepsin (500 kU/L, Sigma Cat. No. P7000) and lipase (500 kU/L, Sigma Cat. No. L3126) was delivered to the stomach reactor via a peristaltic pump at a rate of 3.5 mL/min for the first 90 min of digestion, followed by a reduction in the speed to 2 mL/min from 90 to 240 min. Peristalsis was mimicked at a rate of 3 cycles/min for the stomach reactor using compressed air-controlled rings. A 20 mL digested sample was collected from the pylorus of IPUGS every 15 min during the 4 h duration of digestion and placed in a boiling water bath to halt enzymatic reactions. The sample was centrifuged at 4,000×g for 30 min and stored at −80°C until further analysis.
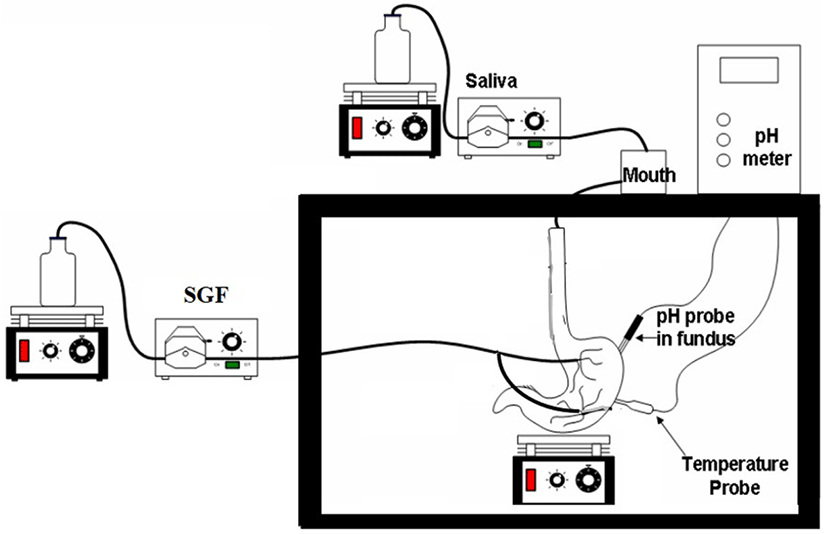
A pH meter was placed in the middle of the stomach compartment of IPUGS to record the changes in pH every 15 min for 4 h digestion period.
We performed SDS-PAGE using 12.5% acrylamide as described by Laemmli (1970) to evaluate the hydrolysis profile of beef samples. A 2× sample buffer (0.125 M Tris, 4% SDS, 20% glycerol, 2% β-mercaptoethanol, pH 6.8) was added to the supernatant of each sample in a 1:1 ratio, centrifuged for 5 min at 9,000×g, and heated at 95°C for 10 min. Electrophoresis was performed at 20 mA per gel for 1 h using PowerPacTM HV (BioRad) and Mini-Protean® Tetra System (BioRad). After electrophoresis, the gel was stained for 1 h with a coomassie blue solution (0.025% coomassie blue g-250, 40% methanol, and 7% glacial acetic acid) and analyzed using Molecular Imager® GelDocTM XR plus Imaging system (BioRad) and Image LabTM Software 5.1 (BioRad).
Cooked beef samples of the four Korean Hanwoo beef cuts and the solid part of the samples collected after the 4-h digestion simulation were freeze-dried. The freeze-dried samples were analyzed with an acceleration voltage of 0.8 kV using a SEM (Hitachi SU-70, Japan).
For the determination of general amino acids, 1 mg of each type of cooked beef sample was labeled with phenyl isothiocyanate (PITC) by a Pico tag method. PITC-labeled samples were dissolved in 400 µL of buffer and 10 µL of each sample was injected into a reverse-phase high performance liquid chromatography (RP-HPLC, Waters 510, USA). The same method was used for samples collected during the digestion period. The supernatant of the sample was filtered using a 0.45 µm filter and a 10 K Amicon tube. The filtered samples of approximately 100 µL were labeled with PITC. PITC-labeled samples were dissolved in 200 µL of buffer and 10 µL of each sample was injected into the RP-HPLC. Waters Pico-tag column (3.9×300 mm, 4.0 µm) was used with solvents A and B at a flow rate of 1 mL/min. The solvent A comprised 140 mM sodium acetate with 6% acetonitrile and solvent B comprised 60% acetonitrile in HPLC-grade water. The absorbance was measured at 254 nm wavelength using a Waters 2487 UV detector. The solvent gradient started at 14% of solvent B and was maintained for 9 min after sample injection, followed by an increase in the proportion of solvent B at 0.5%/s (for 12 s), 3.13%/min (for 8.3 min), and 4.5%/s (for 12 s), and then the proportion of solvent B was decreased to 0%.
Results
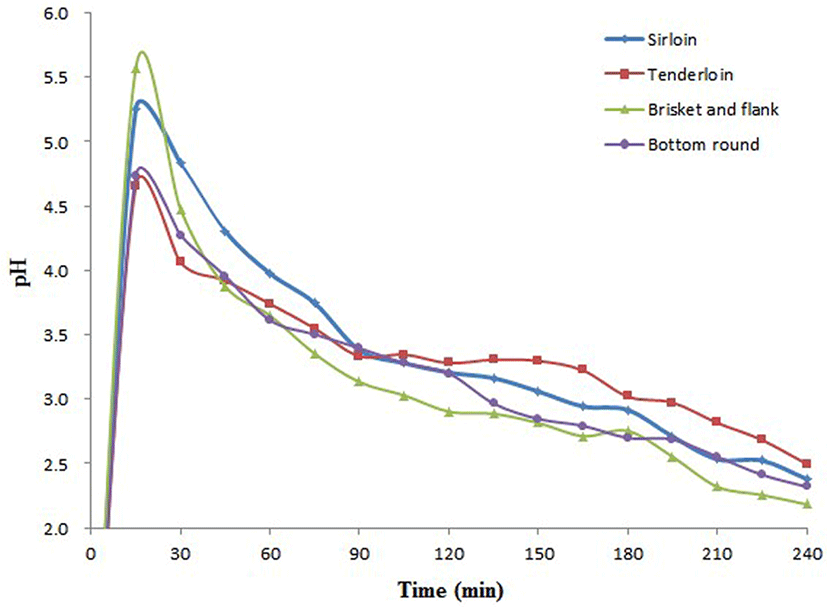
The pH of the digestive fluid in the stomach reactor of IPUGS was approximately 1.30. Addition of each sample with SSF resulted in a rapid increase in the pH to 4.65-5.57. However, the steady secretion of SGF resulted in a gradual decrease in the pH of the chymes to 2.18-2.65 after 4 h digestion (Fig. 2).
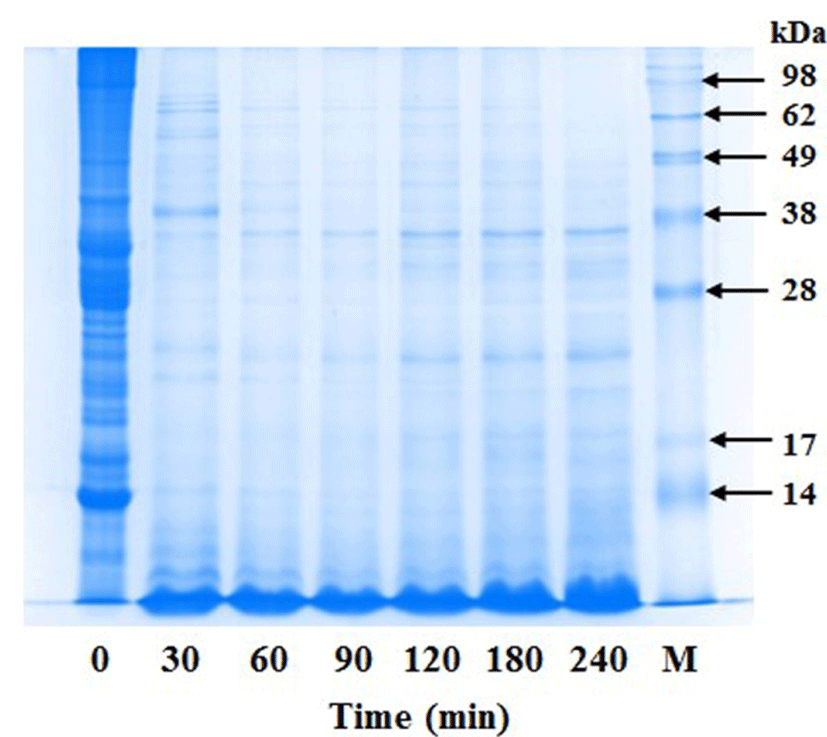
The digestibility of Korean Hanwoo beef cuts was studied using SDS-PAGE. The representative SDS-PAGE gel of brisket and flank cut during 4 h digestion is shown in Fig. 3. It was evident that an increase in the digestion time resulted in a gradual decrease in proteins and/or peptides of higher MW in all samples with a corresponding increase in proteins and/or peptides of smaller MW (Fig. 3). Proteolysis of the digested Korean Hanwoo beef cuts was confirmed by RP-HPLC (data not shown). Many proteins of large MW were detected in the sample at 0-h digestion. However, the 4-h digestion in IPUGS resulted in a significant decrease in proteins of high MW, as observed with SDS-PAGE analysis.
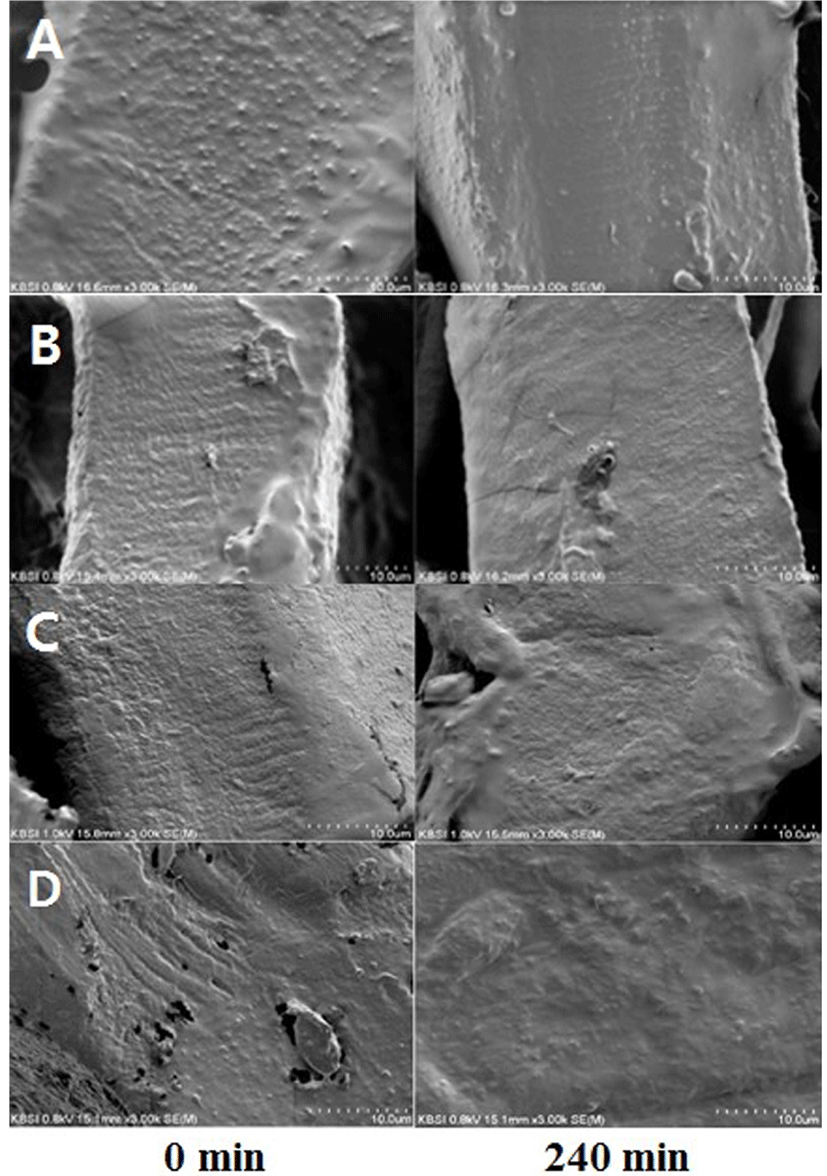
The surface of each beef cut was observed by SEM (Fig. 4). There was a significant difference in the smoothness of the surface between the initial undigested stage and post 4-h digestion stage for all the four cuts. The smoothness of the surface had visibly increased over the course of digestion. However, no significant difference was observed in the smoothness among the four cuts studied.
The total quantity of indispensable and dispensable amino acids in cooked Korean Hanwoo beef cuts before digestion was the highest in the bottom round cut and lowest in the brisket and flank cut (Tables 2 and 3). Analysis of the free amino acids released after the 4-h digestion revealed that the total amount of indispensable amino acids from the tenderloin cut was the highest (Table 4). In addition, tenderloin released higher amount of dispensable amino acids, although the difference was not significant as compared to the other cuts (Table 5). From these results, it is evident that tenderloin was the most digestible cut from the four beef cuts studied.
All values are means±SD, n=3.
a-dMeans in the same row differ significantly (p<0.01).
All values are means±SD, n=3.
a-dMeans in the same row differ significantly (p<0.01).
All values are means±SD, n=3.
a-dMeans in the same row differ significantly (p<0.05).
All values are means±SD, n=3.
a-dMeans in the same row differ significantly (p<0.05).
Discussion
In vitro digestion models have limitation in simulation of the human digestion process. This may be attributed to the use of rigid, glass-based reactor materials (Castelapapin et al., 1999; Sugawara et al., 2005;), delivery of secretions with a single pump (Macfarlane et al., 1998), and the use of magnetic stirrers or shaking water bath (Kapsokefalou et al., 2005; Molly et al., 1993) to simulate motility, which may create different fluid dynamics and affect the mixing of food materials with secretions. In this study, the digestibility of four Korean Hanwoo beef cuts was investigated using the newly developed IPUGS, which is composed of three sequential compartments simulating the conditions of the mouth, esophagus, and stomach of humans. IPUGS is made from non-glass material (platinum cure silicon rubber) to replicate the flexible, elastic, and stretchable nature of the human gastrointestinal tract (GIT) conditions as closely as possible. A large number of secretion channels implanted with Tygon Microbore tubings mimic the gastric secretion from the wall of the stomach reactor, while the peristaltic waves impart motility to overcome above problems.
The pH profile of the stomach reactor was monitored throughout the digestion simulation as a quality control aspect. The change in the pH is of extreme importance for the enzymatic activity. As shown in Fig. 2, the pH of the stomach reactor rapidly increased to approximately 5.0 upon entry of the beef bolus into the stomach reactor, demonstrating the buffering effect of the beef. The pH of the beef samples was about 5.8. The continuous secretion of SGF resulted in a steady decrease in the pH to approximately 2.0, thereby restoring the pH of the empty stomach. Therefore, IPUGS was efficient in the simulation of the acidic environment of the stomach, similar to that observed in human GIT.
Analysis of the SDS-PAGE results revealed that proteins and peptides with higher MW gradually decreased and those with smaller MW increased with an increase in the digestion period. Similar results were observed by Farouk et al., (2014). Furthermore, we compared the initial beef cut samples and digested samples over 4 h using RP-HPLC and found that the samples were digested efficiently, which decreased the amount of proteins and peptides with higher MW (data not shown).
We evaluated the surface of each sample using SEM. A significant increase in the smoothness of the surface was observed among the samples before and after the 4-h digestion (Fig. 4). Activation of pepsin during digestion resulted in the disruption of the peptide linkages. The protease action was more evident in the sample collected after digestion.
We observed a significant difference in the quantity of general amino acids, including indispensable and dispensable amino acids, among the four cuts (p<0.01) prior to digestion simulation (Tables 2 and 3). The bottom round cut showed the highest amount of both indispensable (18.66 mg/100 g) and dispensable amino acids (26.20 mg/100 g) as compared to the other cuts. However, tenderloin was the most efficiently digested cut following 4-h digestion and released the highest amount of indispensable amino acids (28.45 mg/100 g), indicative of its significantly higher digestibility as compared to the other cuts (Table 4). No statistical difference was detected in histidine, isoleucine, and tryptophan level. However, there was a statistical difference in the amount of lysine (3.7 mg/100 g), leucine (8.67 mg/100 g), and methionine (2.11 mg/100 g) between tenderloin and the other cuts (p<0.05). In addition, a statistical difference was observed in phenylalanine (4.06 mg/100 g), threonine (4.00 mg/100 g), and valine (2.23 mg/100 g) levels between tenderloin and other cuts. Lysine is absent in wheat proteins and leucine is one of the branched-chain amino acids essential for muscle synthesis. Thus, it is evident that tenderloin, one of the most expensive cuts sold in local markets, is supplemented with maximum amount of indispensable amino acids. On the other hand, indispensable and dispensable free amino acids found in sirloin and brisket and flank cuts were similar to those in the bottom round cut, but the price and palatability of the cuts may not necessarily reflect the nutritional value of the beef.
Conclusion
The digestibility of Korean Hanwoo beef cuts was investigated using IPUGS. The pH of the stomach reactor in IPUGS rapidly increased with the addition of beef bolus samples, followed by a gradual decrease due to the steady secretion of the acidic SGF. Toward the end of the digestion simulation, the gastric pH was restored. Proteins and/or peptides of higher MW markedly decreased over the course of 4-h digestion and the surface of the digested samples became smoother due to proteolysis. The total quantity of indispensable and dispensable amino acids before digestion was the highest in the bottom round cut. However after the 4-h digestion simulation, tenderloin produced the highest amount of indispensable free amino acids. These results indicate that IPUGS can be used for simulating the digestion of meat products under in vitro settings. Future studies should focus on the use of IPUGS for the examination of a variety of meat types, processing conditions, and meat cuts.













