Introduction
Tomato is a vegetable that is consumed fresh and in processed products. The antioxidant activity of tomatoes has been recognized in several epidemiological studies; regular consumption of fruits and vegetables including tomatoes was implicated as being important in preventing cancer and cardiovascular disorders (Giovannucci, 1999; Heber, 2000; Rao and Agarwal, 2000). Tomato antioxidants, such as vitamins C and E, phenolics, flavonoids, and lycopene are the major sources which exhibit antioxidant activity of raw and processed tomatoes (Beutner et al., 2001; Leonardi et al., 2000; Stewart et al., 2000).
Antioxidants can be classified in two groups on the basis of their solubility. Vitamin C and other water-soluble polyphenolic compounds are hydrophilic antioxidants. Lipophilic compounds include carotenoids, especially lycopene, and vitamin E and chlorophylls. Polyphenol compounds and carotenoids have different solubility. The solubility of phenolic compounds varies depending on their molecular weight and glycosylation degree, acylation or esterification. In particular, increased glycosylation increases water solubility. In case of lipophilic compounds, chlorophylls are more water soluble than carotenoids.
Studies have conducted to improve the functional and antioxidant properties of meat products using tomato (Candogan, 2002; Deda et al., 2007; Garcia et al., 2009; Kim et al., 2008; Østerlie and Lerfall, 2005). However, no studies have evaluated the antioxidant activity of tomato extracts depending on the concentration of ethanol as a solvent. The objectives of this study were to evaluate the antioxidant activity of tomato powder according to ethanol concentrations of 0, 25, 50, 75, and 100%, and to evaluate the antioxidant and antimicrobial activities of pork patties prepared with ethanol-extracted tomato (EET) powders.
Materials and Methods
Fresh tomatoes (Lycopersicon esculentum) were purchased in a local wholesale market. Tween 20, butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), Folin-Ciocalteu reagent, linoleic acid, ethylene-diaminetetraacetic acid (EDTA), 2-thiobarbituric acid (TBA), and 1,1-diphenyl-2-pycrylhydrazyl (DPPH)-radical were purchased from Sigma-Aldrich (Germany). Ascorbic acid, trichloroacetic acid (TCA), gallic acid, petroleum ether, ferric chloride, and ferrous chloride were purchased from Junsei Chemical (Japan). Plate count agar and violet red bile agar were obtained from Difco (USA). Potassium ferricyanide was purchased from Avocado Research Chemicals (UK).
Fresh tomatoes were thoroughly washed, cut and homogenized prior to drying at 60°C using a hot air oven (LDO-250F, Labtech, Ltd., Korea) as described previously (Kim and Chin, 2016). The collected dried powder was mixed with the various aforementioned concentrations of ethanol (0-100%) at a dried powder-to-ethanol ratio of 1:20 as previously described (Kim and Chin, 2016). Ethanol extracts from tomato powder were obtained by stirring of the mixture at 4°C for 24 h and filtering through Whatman #41 filter paper. This extraction step was repeated twice and both filtrates were collected prior to evaporation. After evaporation, each of the concentrates was frozen at −70°C prior to lyophilization using freeze dryer (FT5505, IlShin Co., Korea). Each preparation was stored at −70°C until utilized.
The total phenolic compounds (TPC) of EET were measured by the modified Folin-Ciocalteu method as previously described (Lin and Tang, 2007). Each EET powder (0.1 g) was dissolved in 10 mL of ethanol. Then, 0.1 mL of the EET solution was mixed with 2.8 mL of distilled deionized-water (dd-water), 2 mL of 2% Na2CO3, and 0.1 mL of 50% Folin-Ciocalteau reagent. The absorbance of the mixture was measured at 750 nm using a UV-1601 spectrophotometer (Shimadzu, Japan) after incubating the mixture at room temperature for 30 min. TPC was expressed as gallic acid equivalents (GAE) g/100 g dry matter.
The predominant polyphenols of EET were identified as previously described (Vallverdú-Queralt et al., 2014) with slight modification. Five polyphenol standards were selected including gallic acid, catechin, vanilic acid, rutin and quercetin. Approximately, 0.04 g of sample was homogenized with 4 mL of 80% ethanol. The homogenate was sonicated for 5 min and centrifuged at 300 × g for 15 min. The supernatant was collected in a flask and the extraction was repeated. Both supernatants were combined, evaporated, and reconstituted with Milli-Q water (0.5% formic acid) up to 2 mL. This extract was filtered through a 0.45 μm PTFE filter into an insert-amber vial for high-performance liquid chromatography (HPLC) analysis. The analysis condition is summarized in Table 1. A 20 uL volume of EET was injected in a HPLC instrument (LC-10Avp, Shimadzu, Japan) and the flow rate was adjusted to 0.8 mL/min. Mobile phases consisted of acetonitrile (A) and 0.5% formic acid (B). Separation was carried out in 45 min under the following conditions: 5 min, 85% B; 20 min, 80% B; 30 min, 20% B; 31 min, 85% B; and 45 min, 85% B. The column was equilibrated for 5 min prior to each analysis.
EET radical scavenging activity was determined as described previously (Huang et al., 2006). EET solution (2 mL, 1-10 mg/mL in ethanol) was mixed with 0.2 mM methanolic DPPH radical solution (0.5 mL). After the mixtures were maintained at room temperature for 30 min in darkness the absorbance of the treatment was measured at 517 nm using L-ascorbic acid as the reference.
The ferrous iron chelating ability of EETs was measured using a modification of a previously described method (Le et al., 2007). Sample solution (0.5 mL, 1-10 mg/mL in ethanol), 0.1 mL of 0.6 mM ferrous chloride, and 0.9 mL of methanol were mixed and allowed to stand for 5 min at room temperature for the chemical interaction. Then, 0.1 mL of ferrozine (5 mM in methanol) was added and the mixture left for 10 min at room temperature. The ferrous chelating ability (%) was obtained by measuring the absorbance of the Fe2+-ferrozine complex at 562 nm and was calculated as [(ΔA562 of control − ΔA562 of sample) ÷ ΔA562 of control] × 100. EDTA was used as the positive control.
The reducing power of EETs was measured as described previously (Huang et al., 2006). EET solution (2.5 mL; 1-10 mg/mL), 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6), and 2.5 mL of potassium ferricyanide (10 mg/mL) were mixed and incubated at 50°C for 20 min. After incubation, 2.5 mL of TCA (100 mg/mL) was added and the mixture was centrifuged at 200 × g for 5 min. Then, the 1.25 mL of upper layer was mixed with 1.25 mL of dd-water and 250 μL of ferric chloride (1 mg/mL). The absorbance of the mixture was measured at 700 nm.
The antioxidant activity of EETs in linoleic acid emulsion was measured as described previously (Yen and Hsieh, 1998). A 2.5 mL volume of linoleic acid emulsion mixture comprised with linoleic acid, Tween 20, and phosphate buffer (0.2 M, pH 7.0) were homogenized with a 0.5 mL of sample solution (1 and 5 mg/mL). Then the mixture was mixed with phosphate buffer (2 mL, 0.2 M, pH 7.0) and incubated at 37°C. A 0.1 mL volume of each incubated sample was collected every 24 h and was mixed with 4.7 mL of ethanol (75%), 0.1 mL of ammonium thiocyanate (30%), and 0.1 mL of ferrous chloride (0.02 M in 3.5% HCl). After the mixture was incubated for 3 min at room temperature, the peroxide value was determined. A control was prepared in the same procedure without the extracts. Additionally, butylated hydroxytoluene (BHT) was used as a reference. A high optical density at 500 nm indicates low antioxidant activity.
The whole experiment was performed in triplicate. Results were expressed as mean and standard error of the results. Two-way analysis of variance (ANOVA) was performed using SPSS 21.0 software (SPSS, USA) as factors for treatments (reference, E0, E25, E50, E75, and E100) and concentration (0, 1, 2.5, 5, and 10 mg/mL). Duncan’s multiple range test was used to determine significant differences at the 5% level.
Pork patties were manufactured with pork ham (cross-bred, Landrace × Large Yorkshire) and back fat containing 1% of water extracted tomato (WET) and 0.5 and 1.0% of EET as described previously (Kim and Chin, 2016) (Table 2). Approximately 70-80 g of the mixture was formed into one patty, which was placed on a polystyrene plate and kept at 4°C until analyzed. Patties were analyzed immediately and after refrigerated storage for 3, 7, and 14 d for the physicochemical and antioxidant activities described below.
1)Treatments: Control= patty without tomato extract; REF= patty containing 0.01% of BHT; WET1.0= patty containing 1% of WET; EET 0.5 and 1.0= patties containing 0.5 and 1.0% of EET, respectively.
pH values of patty samples were measured using a pH-meter (MP-120, Mettler-Toledo, Switzerland) at five different points of each pork patty with a properly calibrated pH/temperature. The surface color values of patties were measured using a color reader (CR-10, Minolta, Japan) equipped with illuminant D65, 8 mm aperture, and 10° standard observer. The data for color values were expressed by L* (lightness), a* (redness) and b* (yellowness) after calibration with white plate standard (L=91.0, a=1.20, b=0.30).
TBARS of pork patties was measured and the results expressed as mg of malondialdehyde (MDA)/kg sample (Shinnhuber and Yu, 1977). Homogenized patty samples (2 g) were mixed with 0.5 mL of antioxidant solution (mixture of propylene glycol, BHT, BHA, and Tween 20), 3 mL of TBA solution (1%), and 17 mL of TCA solution (2.5%). The mixture was heated at 100°C for 30 min. Then, 5 mL of supernatant and 5 mL of chloroform were combined and centrifuged (200 × g for 5 min). After centrifugation, 2 mL of the upper layer was mixed with 2 mL of petroleum ether. The mixture was centrifuged again at 200 × g for 10 min. The extent of lipid oxidation was measured at 532 nm using a spectrophotometer (UV-1601, Shimadzu, Japan). TBARS value was calculated by multiplying the optical density (O.D) of each sample at 532 nm and a factor of 9.48 from a standard curve of MDA.
Total bacterial counts and number of Enterobacteriaceae were determined using of total plate count (TPC) agar and violet red bile (VRB) agar, respectively. Ten grams of homogenized pork patties were diluted with 90 mL of sterilized dd-water and diluted. A 0.1 mL volume of each dilution was spread on TPC and VRB agars. Inoculated samples were incubated at 37°C for 2 d and the results were expressed as log colony forming units (CFU)/g.
The experiment was performed in triplicate. Results were expressed as mean and standard error of the results obtained from the three independent pork patties. Data were analyzed by two factors factorial analysis using SPSS 21.0 for Windows software. The two factors were the four storage times (0, 3, 7, and 14 d) and the five treatments (control, reference (BHT 0.01%), 1.0% of WET, 0.5% EET, and 1.0% EET). Means were compared using the Duncan’s multiple range test at a 5% significance level.
Results and Discussion
TPC of tomato powders extracted with different levels of ethanol were listed in Table 3. The recovery yield of oven-dried tomato powder at 60°C was 5.45% (data not shown). Moreover, ranges of extraction yield were from 9.45 to 70.9%. The lowest extraction yield (9.45%) was observed at 100% EET. Therefore, E100 showed the lowest total yield (0.52%) as compared to other treatments (p<0.05), whereas 0 to 75% concentrations of extraction solvents showed similar total yield (from 3.14 to 3.87%). TPCs of all concentrations quantified ranged from 1.57 to 2.02 g/100 g dry weight. Among the treatments, 100% EET powder showed the lowest content of TPCs (p<0.05). The contents of gallic acid and catechin were detected by HPLC. The contents of gallic acid and catechin ranged from 36.0 to 47.6 mg/100 g and from 49.3 to 61.7 mg/100 g, respectively. There were no significant differences among treatments (p>0.05). Although tomatoes at 60°C results in a decrease in antioxidants (i.e., vitamin C) (Gahler et al., 2003), bioaccessibility of phenolics increases due to the breakdown of the cell wall of tomatoes by heating (Tulipani et al., 2012). Therefore, increased polyphenols of tomatoes by heating is linked to the increases of antioxidant activity (Gahler et al., 2003). TPCs of vegetables and fruits were extracted according to the polarity of the extraction solvent (Moure et al., 2001). Pellegrini et al. (2007) reported that antioxidant components contained phenolic compounds of tomato based on different extraction solvents; the content of caffeic acid of water extract was higher than that of acetone extract. Thus, pure ethanol extract (100%), which has less polarity of tomato, showed the least TPCs among the treatments (p<0.05). Therefore, the polarity of extraction solvent might significantly affect the content of TPCs from tomato powder.
a-dMeans with different superscripts in the same row are different (p<0.05).
1)Treatments: E0=0% ethanol extracted tomato (EET); E25=25% EET; E50=50% EET; E75=75% EET; E100=100% EET.
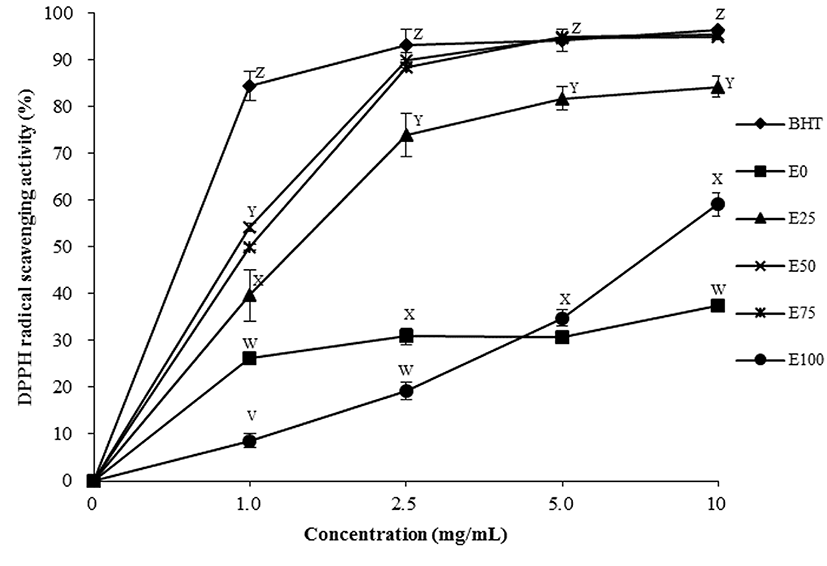
Since interactions between treatment and ethanol concentration were observed in the DPPH radical scavenging activity (p<0.05), data were separated and the treatments were assessed by a treatment or concentration. As shown in Fig. 1, DPPH radical scavenging activities of all treatments increased with increased levels of ethanol for the extraction. The most effective antioxidant activities were observed in tomato powders extracted with 50 and 75% ethanol which displayed the highest antioxidant activity, regardless of any other ethanol levels (p<0.05). A high correlation between DPPH and TPC has been described (Dudonné et al., 2009). This could be partially due to the inclusion of one or more aromatic rings containing one or more hydroxyl groups, which can scavenge free radicals by forming phenoxyl radicals (Bors and Michel, 2002; Rice-Evans et al., 1996). Phenolic compounds are important constituents of plants due to their antioxidant activity via inactivation of free radicals or restricted decomposition of hydroperoxides into free radicals (Pokorny, 2001). In this study, 50 and 75% EET had high free-radical scavenging activity (p<0.05). Thus, combined antioxidants from water and ethanol solvent of these tomato extracts might increase the antioxidant activity, resulting in higher DPPH radical scavenging activity than other treatments (p<0.05).
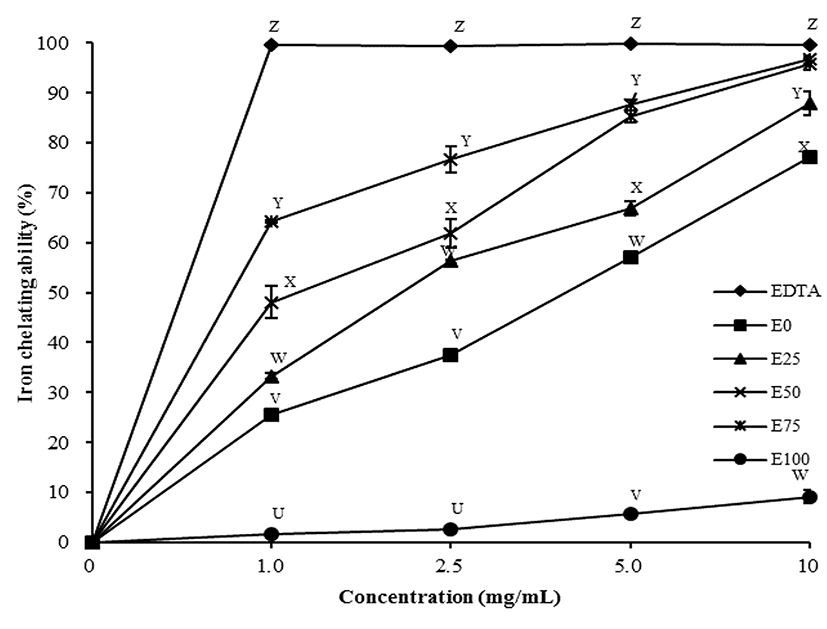
The iron-chelating ability of all extracts increased with increasing concentration of ethanol up to 75% concentration (p<0.05). Iron chelation activity of the 75% ethanol extract was higher than that of 50% at all ethanol concentrations (Fig. 2). EET, 50 and 75% EET, which had similar activities at 10 mg/mL were similar to the EDTA reference, and displayed higher iron chelating activity than other treatments (p<0.05). These results indicated that the 50 and 75% EET possessed higher antioxidant activity than the other concentrations. Similar to the DPPH results, iron chelating ability of tomato extracts was highly correlated with the content of polyphenols, which can act as radical scavengers (Lodovici et al., 2001), singlet oxygen quenchers (Foley et al., 1999), and metal chelators (Brown et al., 1998). Phenolic acids have a metal binding site – the 3’,4’-dihydroxy group on the ß-ring – and this group has potential iron-chelating ability due to its electron-donating abilities (Andjelković et al., 2006). Since the various combinations of water and ethanol soluble antioxidant affected the iron-chelating ability, 50 and 75% EET showed higher activity than the other concentrations (p<0.05).
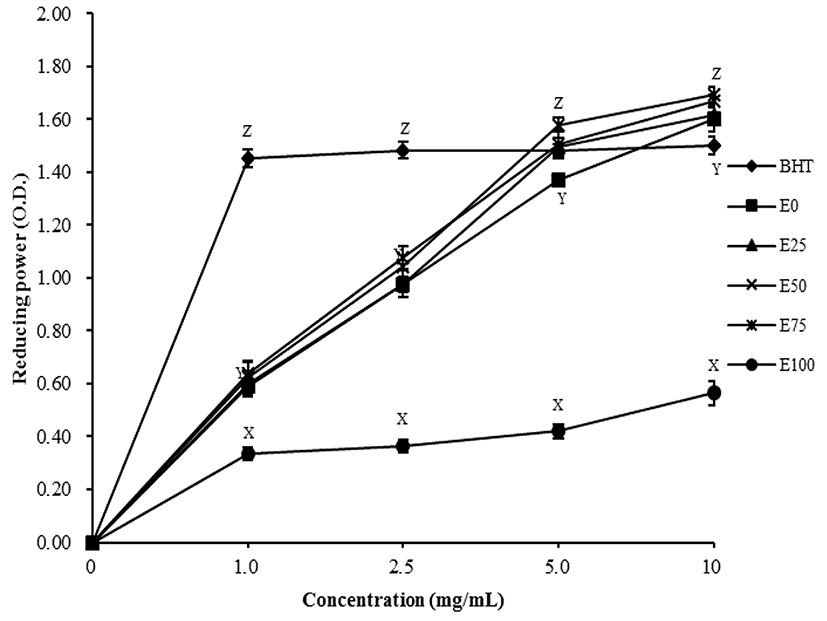
The reducing power of ethanol extracted powders was measured as the absorbance at 700 nm. All values of reducing power were higher than optical density values of 0.5 from concentrations of 1-10 mg/mL, which was considered as an acceptable range (Lin et al., 2009), except for the E100 treatment (Fig. 3). The reducing power of all treatments increased with increasing concentrations (p<0.05). Although the reducing powers of 0 to 75% EET powder were similar (p>0.05) at lower concentrations (1-2.5 mg/mL), those of 50 and 75% treatments were higher (1.67 and 1.69, respectively) than those of the reference (BHT, 1.50) at the highest concentration (10 mg/mL) (p<0.05). The reducing ability of antioxidant compounds generally depends on the presence of reductones (Duh, 1998), which have antioxidant activity by donating a hydrogen atom to break the free radical chain (Gordon, 1990). Since polyphenols in plants are strong antioxidant agents by acting as electron donors, increased reducing power may correspond with increasing concentrations of phenolic compounds (Senevirathne et al., 2006). TPC of treatments from 0 to 75% concentrations were similar (p>0.05) and higher than that of 100% ethanol treatment (p<0.05). These results suggested that the TPC from tomato extracts affect the reducing power, depending on the level of ethanol used for the extraction (p<0.05).
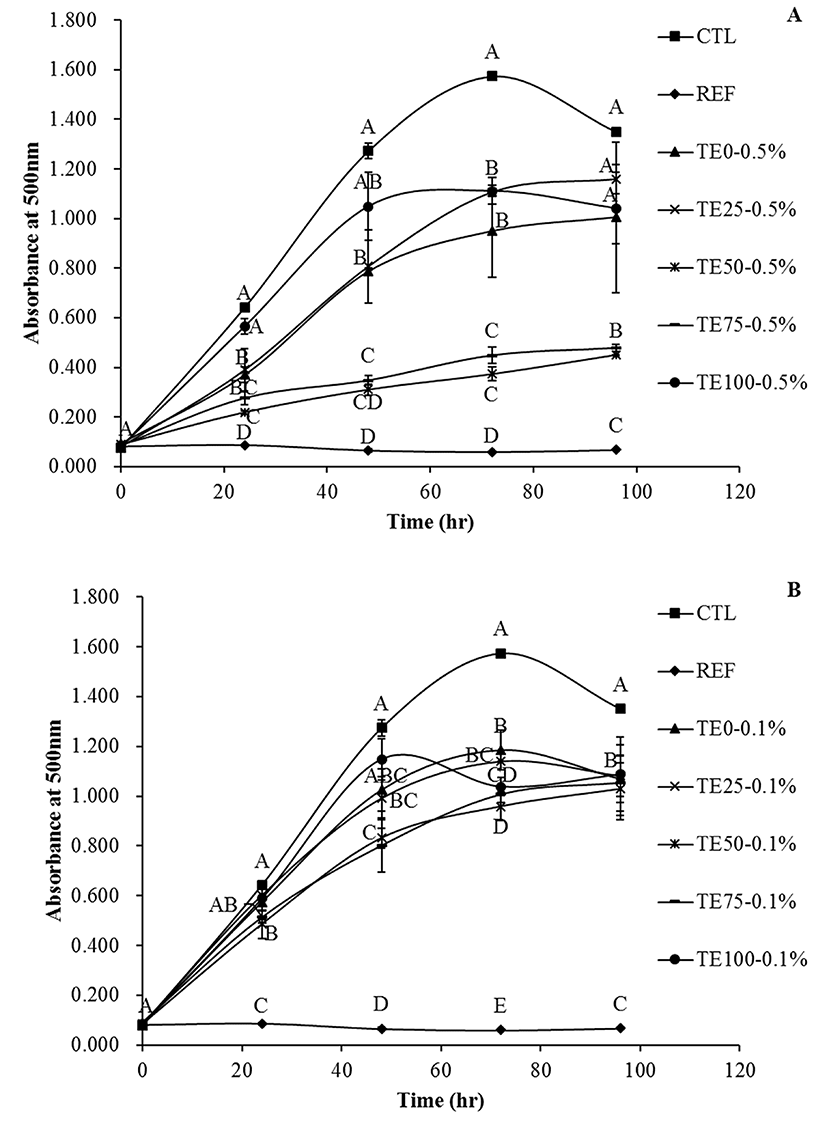
The results of linoleic acid peroxidation with various EETs are presented in Fig. 4. Antioxidant activity of EET at a concentration of 0.5% and 0.1% in linoleic acid emulsion was compared with control and 0.01% BHT and the results are shown in Fig. 4(A) and 4(B), respectively. The high absorbance at 500 nm (Abs500) was considered the high level of peroxide formation. The absorbance (Abs500) of control increased with the incubation at 37°C and the maximum value and the highest amount of lipid peroxides was observed at 72 h. Further incubation of the sample for up to 96 h resulted in a production of low molecular weight lipid peroxides, resulting in the formation of secondary oxidation products. Lipid peroxidation was suppressed by the addition of EETs during the incubation period. The maximum effective level was noted at a concentration of 0.5% EET from 50 and 75% ethanol (Fig. 4(A)). The reference, 0.01% BHT, effectively inhibited lipid peroxidation. This suggested that EET from 50 and 75% ethanol could be used effectively at a concentration of 0.5%. High content of antioxidants such as quercetin and kaempferol, prevent the peroxidation of linoleic acid (Jayaprakasha et al., 2001). Chlorogenic acid, 3,5-dicaffeolyquinic acid and dl-α-tocopherol are polyphenols that have a strong antioxidant activity by inhibiting the formation of conjugated diene from linoleic acid (Ohnighi et al., 1994). In addition, inhibitory effects of linoleic acid by antioxidants, such as the phenolic compounds are highly related to the structure of antioxidants. For example, hydroxyl groups of phenolic compounds could retard the lipid oxidation better than methoxy groups (Villares et al., 2012). Although TPC of all EET powders did not show significant differences, except for EET from 100% ethanol, 50% and 75% EET powders decreased the rate of linoleic acid peroxidation.
The changes of pH of pork patty samples with 1.0% WET and two levels of 50% EET (0.5 and 1.0%) are shown in Table 4. Addition of WET and EET (1.0%) decreased pH values of patty samples (p<0.05). However, 0.5% EET had a similar pH to those of control samples and the reference (p>0.05) pH values did not change during 14 d of storage (p>0.05). Candogan (2002) manufactured beef patties with 5, 10, and 15% tomato paste and evaluated the effect of tomato paste on sensory and physico-chemical characteristics of beef patties. Decreased pH on incorporation of tomato paste with beef patties was evident (p<0.05). Thus, the addition of WET and EET reduced pH values of pork patties.
Pork patty samples with 1% WET and EET were differently colored, as compared to the control (Table 4) (p<0.05). Although the lightness (L*) value was not changed by the addition of tomato extracts (p>0.05), redness (a*) and yellowness (b*) values increased in pork patties containing 1.0% of tomato extracted powder, regardless of the extraction solvent (p<0.05). Due to a relatively small amount, the patties containing EET at 0.5% showed similar redness value to the reference (BHT 0.01%) (p>0.05), and similar yellowness value to those of control and reference (p>0.05). However, increasing storage time decreased redness values due to the discoloration during storage (p<0.05). Carotenoid, the predominant color agent in tomato, affects tomato extracts (Garcia et al., 2009). Candogan (2002) reported that beef patties with tomato paste displayed increased redness and yellowness values, and decreased lightness. Thus, addition of EET to pork patties improved color of the product.
a-cMeans with different superscripts in the same column (treatment) are different (p<0.05).
a-dMeans with different superscripts in the same column (storage day) are different (p<0.05).
1)Treatment: Treatments are described in the legend of Table 2.
NS = not significant; * indicates p<0.05; ** indicates p<0.001.
Since there was an interaction (p<0.05) between pork patty treatment and storage day in the results of TBARS, data were separated out and assessed by treatment or storage day (Table 5). TBARS values of pork patties increased (p<0.05) with increasing storage days in all treatments. However, TBARS values showed differences among treatments during storage at 4°C (p<0.05). After storage day 3, control TBARS increased rapidly and was higher than other treatments (p<0.05). Pork patties containing WET and EET showed lower TBA values than those of the control and reference on day 7 (p<0.05). However, TBA values of patties containing WET and EET increased rapidly and were similar to those of control thereafter (p>0.05). This result indicated that WET and EET did not have distinctive antioxidant activity after 7 d of storage. Several researchers have reported that the addition of tomato products containing lycopene into meat products retards lipid oxidation (Candogan, 2002; Eyiler and Oztan, 2010; Garcia et al., 2009). Presently, application of water or EET powders to the pork patties delayed lipid oxidation at storage day 7. As previously reported, lycopene, a strong lipophilic antioxidant, had excellent antioxidant activity when applied to meat product. Dry fermented sausages with lycopene, which is a strong antioxidant from tomato peel, were manufactured and expressed high antioxidant compounds (Calvo et al., 2008). Candogan (2002) reported that addition of 5, 10, and 15% tomato paste significantly lowered TBARS values of beef patties, as compared to control patties (p<0.05) during 9 d of storage at 4°C due to lipid oxidation reducing effect of lycopene in the tomato paste (30-55 mg/100 g). In this experiment, although the effect of EET powder on antioxidant activity was not strong enough, both WET and EET of tomato exerted good antioxidant activity, owing to hydrophilic antioxidants, such as polyphenols. In addition, both of WET and EET extended three days of the chemical shelf-life by inhibition of lipid oxidation, as compared to the control patties (p<0.05).
a-cMeans with different superscripts in the same row are different (p<0.05).
A-CMeans with different superscripts in the same column are different (p<0.05).
1)Treatments: Treatments are described in the legend of Table 2.
Results of microbial counts of pork patties with water and EET powders during 14 d of storage under 4°C are presented in Table 5. Since there was an interaction between treatment and storage day (p<0.05), data were separated out and expressed as storage day in a treatment or treatment in a storage day. During refrigerated storage, total bacterial counts of pork patties rapidly increased after storage day 7 (p<0.05). Pork patties containing tomato extracts showed a 1 log reduction of total bacterial counts compared to control and reference on day 14 (p<0.05). Similarly, addition of WET and EET produced a 1-2 log reduction of Enterobacteriaceae in pork patties on day 14 (p<0.05). These results indicated that the tomato extract effectively retarded microbial growth, regardless of the extraction solvent and concentration. Sánchez-Escalante et al. (2003) manufactured beef patties containing lycopene-rich tomato pulp (LRTP) and packaged the product in a modified atmosphere. They evaluated storage stability during refrigerated storage by measuring redness, surface metmyoglobin, TBARS, microbial counts and sensory evaluation. The authors reported that incorporation of LRTP into beef patties did not show any microbial differences with the control sample (p>0.05), contrary to our results. Generally, at low pH, antimicrobial activity increase (Kim et al., 2013). A previous study proposed a microbial growth model in foods (Zwietering et al., 1993). These authors reported that, in sausages, minimum, maximum and optimum pH values of Lactobacillus growth were pH 2.8, 7.2, and 6.0, respectively. These results indicated that microbial growth can be inhibited, when the pH values of sausage were shift to acidic condition. Sánchez-Escalante et al. (2003) reported that the addition of LRTP did not affect the pH value (p>0.05). Our data, however, showed that addition of WET and EET to pork patties reduced the pH value resulting in lower microbial counts by antimicrobial activity of tomato extracts (p<0.05).
Conclusions
Among the polyphenols, gallic acid and catechin were predominant compound in EET powders, regardless of ethanol concentration. Among the various ethanol concentrations, 50 and 75% EET showed higher antioxidant activities than other concentrations (p<0.05) in DPPH radical scavenging and iron chelating activities, and linoleic acid lipid peroxidation. Pork patties containing WET (1.0%) and EET (0.5 and 1.0%) powders inhibited lipid oxidation by 7 d. Furthermore, the addition of EETs into pork patties inhibited antimicrobial activity by 1-2 log reduction during refrigerated storage. In conclusion, WET and EET powders may be potentially valuable as a natural antioxidant and antimicrobial agent in meat products.