Introduction
The use of synthetic nitrite in the manufacturing of meat products is normally expressed as curing (Honikel, 2008; Jeong, 2016). To prevent meat and meat products from spoilage during curing, nitrite is usually added to inhibit the growth of spoiling microorganisms (Sebranek et al., 2012). According to Weiss et al. (2010), synthetic nitrite can impart a notable reddish pink color to the meat, add a characteristic flavor to meat products, inhibit lipid oxidation, and control the growth of several pathogenic and spoilage organisms. However, In the meat-processing industry, the major health concerns are the addition of synthetic nitrite (Jeong et al., 2010) becuase the addition of synthetic nitrite in meat products has been doubted due to the potential conversion of nitrite to nitrosamine, a possible carcinogen (Bedale et al., 2016). Consumer’s preference for natural and organic food has an important influence on the meat-processing industry (Lorenzo et al., 2014). Consumer’s demand for healthier natural and organic food products had led to a noteworthy increase in their production (Bedale et al., 2016; Choi et al., 2016). Sebranek et al. (2012) reported that one way to avoid direct addition of synthetic nitrite to meat products is by adding ingredients that have natural nitrate contents with a starter culture to convert nitrate to nitrite. Djeri and Williams (2014) reported that pre-converted nitrite from celery juice is similar to synthetic nitrite. However, celery contains allergenic substance. For this reason, meat-processing industries are actively seeking new sources of natural nitrite to replace synthetic nitrite.
Green vegetables are known as major sources of nitrate and nitrite in human diet (Chang et al., 2013; Djeri and Williams, 2014). Nitrate levels can be significantly high in many green vegetables such as spinach. According to Walker (1990), nitrate level in spinach is as high as 2,470 ppm. Djeri and Williams (2014) reported that vegetables containing nitrates are commonly utilized in combination with lactic acid starter cultures to produce naturally cured meat products. Krause et al. (2011) indicated that preconverted nitrite from natural sources can maintain the pink color of meat products if used at sufficient levels. Nitrate can be converted to nitrite by microorganisms prior to formulating meat products in the pre-generation process (Choi et al., 2015).
Therefore, the aim of this study was to evaluate the effect of fermented spinach as source of pre-converted nitrite on color development, residual nitrite content, and physicochemical properties of cured pork loin.
Materials and Methods
Pre-converted nitrite from spinach was prepared as followed. Commercial samples of spinach were purchased from a local market. All raw samples were freeze-dried and crushed into powder form. Ten grams of each powder was mixed with 100 mL of distilled water for 30 min. Next, 0.1% active nitrate reductase culture containing Lactobacillus farciminis (KCTC 3681) was added to the mixture and placed in a shaker incubator at 30°C for 36 h. Each mixture was filtered through Whatman No. 1 filter paper and evaporated with a rotary evaporator (EYELA N-1000, Rikakikai, Japan) at less than 50°C. Concentrated products of pre-converted nitrite sources from fermented spinach were then stored in amber flasks in the dark at 4°C until used within 24 h. The pre-converted nitrite sources from spinach had the following characteristics: pH = 5.24, L*-value = 52.43, a*-value = −0.26, b*-value = 7.07, nitrite content = 4916.82 ppm. Nitrite contents in fermented solution was measured by diazo coupling method (KFDA, 2016).
Fresh pork loin (castrated boars, Landrace × Yorkshire × Duroc; approximately 110 kg, M. longissimus) were purchased from a local processor at 48 h postmortem. All subcutaneous and intramuscular fat as well as visible connective tissue were removed from the muscle with a knife. The loin was sliced at a thickness of 2.0 cm with a meat slicer and stored at 0°C before curing. The sliced meat samples with 30% curing solution (8.0% of salt) in proportion to the pork loin were cured by tumbling for 24 h at 4°C. They were then held at 4°C for 48 h to allow the curing solution to equilibrate throughout. Cured meats without the addition of nitrite served as negative controls (−) and those with 0.015% sodium nitrite in proportion to the pork loin added served as positive controls (+). The remaining four groups of cured meats were treated with pre-converted nitrite sources from fermented spinach as follows: 10% of pre-converted nitrite sources from fermented spinach + 20% of water (T1), 20% of pre-converted nitrite sources from fermented spinach + 10% of water (T2), and 30% of pre-converted nitrite sources from fermented spinach (T3). The cured meats were heated at 80°C for 60 min in a chamber (MAXi3501, Kerres, Germany) and cooled at 21°C for 3 h. This procedure was replicated three times.
The pH values of samples were measured with a pH meter (Model 340, Mettler-Toledo GmbH, Switzerland). Briefly, 5 g of sample and distilled water (20 mL) were used to prepared sample homogenate (UI-tra-Turrax T25, Janke & Kunkel, Germany) and used for pH measurements. All samples were performed in triplicates.
The internal surface color of samples were monitored with a colorimeter (Chroma meter CR-210, Minolta, Japan) and the measuring area had a diameter of 8-mm. L* (100 = white, 0 = black), a* (positive = redness, negative = greenness), and b* (positive = yellowness, negative = blueness) values were used to indicate color. Color readings were measured for ten randomly chosen spots on the cured pork loins. They were utilized as estimate of discoloration. Hue angle (H°), color difference (ΔE*), and chroma difference (ΔC*) were calculated using the following equations: H° = arctan (b*/a*), ΔE = [(L*−Lcon(−)*)2+(a*−acon(−)*)2+(b*−bcon(−)*)2]1/2, and ΔC* = (Δa*2+Δb*2)1/2 (Grigelmo-Miguel et al., 1999) for measuring the difference of curing color developing among samples. American Meat Science Association guidelines for color measurements were followed (Hunt et al., 1991).
Lipid oxidation was assessed in triplicates using TBARS method of Tarladgis et al. (1960) with minor modifications. It was expressed as milligrams of malondialdehyde (MD) per kilogram of cured pork loin. Briefly, 10 g sample was blended with 50 mL distilled water for 2 min using a homogenizer (AM-7, Nihonseiki, Kaisha Ltd., Japan) and transferred to a distillation tube. The cup used for blending was washed with an additional 47.5 mL of distilled water and added to the same distillation flask along with 2.5 mL of 4 N HCl and a few drops of antifoaming agent (KMK-73, Shin-Etsu Silicone Co., Ltd., Korea). The mixture was distilled and 50 mL of the distillate was collected. Five milliliter of 0.02 M TBA in 90% acetic acid (TBA reagent) was added to each test tube containing 5 mL of the distillate and mixed well. The tubes were capped and heated in a boiling water bath for 30 min to develop chromogen and cooled to room temperature. Absorbance was measured at wavelength of 538 nm against a blank (prepared with 5 mL distilled water and 5 mL TBA reagent) using a UV/VIS spectrophotometer (Optizen 2120 UV plus, Mecasys Co., Ltd., Korea).
Volatile basic nitrogen (mg%) test was performed to determine the extent of protein deterioration during refrigerated storage. VBN was measured by the modified micro diffusion assay according to the method of Pearson (1968). A 5 g of sample was blended with distilled water and then fitered with Whatman No.1 (Whatman International, UK). On the outer section of Conway micro diffusion cell, 1 mL of the filtered sample solution and 1 mL of 50% K2CO3 solution were placed. On the inner section, 1 mL of 0.01 N H3BO3 and 50 μL of indicator were placed. After 90 min at 37°C, Inner section were titrated with 0.02 N H2SO4 solution.
Where a was the titer for the sample, b was the titer for the blank, f was the factor of reagent, N was normality, and S was sample weight (g).
To determine the total viable count (TVC), E. coli, coliform bacteria for each sample, 25 g of sample was aseptically transferred into a sterile stomacher bag containing 225 mL of 0.1% peptone water followed by pummeling samples into a stomacher (Masticater-Paddle-Blender, IUL Instrument, Spain) for 3 min. The homogenates were serially diluted with 0.1% peptone water. Serially diluted samples (1 mL) were then plated onto Petri dishes. A total of 20 mL of plate count agar (PCA; Difco, USA) was then poured over the plates containing serially diluted samples. After the medium was solidified, plates were incubated at 37°C for 48 h. Colonies developed on these plates were manually counted. Petrifilm (3M, Korea) was used to cultivate the diluted sample. Colonies with blue bubbles were counted as E. coli and those with purple bubbles and blue bubbles were counted as coliform bacteria.
The residual nitrite content was expressed as ppm of cured pork loins. All residual nitrite assays were done in duplicates. All treatments were analyzed at the same time to minimize variation caused by time difference. Ten mL 0.5 N NaOH and 10 mL 12% (NH4)2SO4 solution were added into sample solution that was mixed with 10 g of sample and 150 mL distilled water and then, heated in a boiling water bath at 80°C. After cooling, 20 mL CH3 COONH4 buffer (pH 9.00) and 10 mL distilled water were added into the sample. After 10 min, the residual nitrite content in filtered Sample was measured by diazo coupling method. The residual nitrite content was calculated using a standard curve of nitrite solution (KFDA, 2016).
The effect of different concentration of pre-converted nitrite sources from fermented spinach on pH, color, TBARS, VBN, TVC, and residual nitrite contents was examined using a one way analysis of variance (ANOVA), where the measured variables were set as dependent variables and replicates were set as random effect. Differences among means were compared using Duncan's Multiple Range test. A significance level of p<0.05 was set for all evaluations. Data were analyzed using SAS 9.4 software (SAS Institute Inc.).
Results and Discussion
The pH values of raw cured meats formulated with fermented spinach extract are summarized in Table 1. The pH values of raw cured meats of negative controls (−) were higher (p<0.05) than those of raw cured meats treated with fermented spinach extract. However, the pH values of raw cured meats were not significantly different between negative control (−) and positive control (+). The pH values of raw cured meats treated with fermented spinach extract (pH 5.24) were decreased with increasing levels of fermented spinach extract added to the raw cured meat. The acidic pH of the fermented spinach extract is due to lactic acid produced by the starter culture (Krause et al., 2011). In this study, the pH of the raw cured meat was attributed to the pH of the fermented spinach extract (pH, 5.24) used. These results were in agreement with those of Krause et al. (2011) showing that the pH values of the starter culture, pre-converted vegetable juice powder, and control brines were all significantly different (with values of 4.24, 9.22, and 8.02, respectively). Similar results have been reported by Sindelar et al. (2007) showing significant difference in pH value of ham incubated with vegetable juice powders. Therefore, the potential survival and growth of lactic acid-producing bacteria (LAB) during product manufacturing might explain the decrease in pH. Djeri and Williams (2014) have reported that the decrease in pH of bologna with celery juice powder could be largely due to the increase in Log CFU/g of LAB. This is supported by the results of Pexara et al. (2002) showing a decrease in the pH value of meat depending on the availability of fermentable carbohydrates. Sebranek (1979) has reported that the importance of pH depending on nitrite level. A pH decrease during manufacture can double the rate of color formation and other curing reactions (Sebranek, 1979).
Color values of the raw cured meats formulated with fermented spinach extract are shown in Table 1. The lightness and yellowness values of raw cured meats formulated with fermented spinach extract were higher (p<0.05) than those of positive and negative control groups. The lightness and yellowness values of raw cured meats formulated with fermented spinach extract were decreased as the addition levels of fermented spinach extract were increased. Generally, the redness of raw cured meat can be improved because NO-myoglobin that has bright reddish color can be generated by nitric oxide from nitrite and myoglobin. With increasing levels of fermented spinach extract, nitrite contents in brine was increased. Therefore, the redness values of raw cured meats formulated with fermented spinach extract were increased with increasing levels of fermented spinach extract added. Similar results reported by Djeri and Williams (2014) showing that the lightness values of bologna treated with celery juice powder and cherry juice powder were lower than those of other treatments. However, the redness values of bologna treated with celery juice powder and cherry juice powder are higher than those of other treatments (Djeri and Williams, 2014). Krause et al. (2011) reported that the nitrite-cured control had significantly higher redness value than either vegetable juice powder and a starter culture treated sample or a pre-converted vegetable juice powder treated sample, while the latter two were not different from each other. According to Terns et al. (2011), the incubation conditions used for meat products added with vegetable juice powder are more important for the development of redness than the amount of starter culture used. Sindelar et al. (2007) have reported that redness might positively correspond to reflectance ratios while an increase in lightness might negatively affect pigment measurements as determined by reflectance ratio. The hue angles of raw cured meats formulated with fermented spinach extract were higher (p<0.05) than those of control groups (− and +). According to Djeri and Williams (2014), hue angle is indicative of red color. In their results, the hue angles of meats formulated with sufficient celery juice powder and cherry juice powder were similar to those of control meats added with sodium nitrite (Djeri and Williams, 2014). In the present study, the color difference of T1 was the highest (p<0.05), whereas that of the control (+) was the lowest (p<0.05). The color differences between raw cured meats were affected by the color of the fermented spinach extract. The chroma difference of the negative control (−) was the lowest (p<0.05), while that of T3 was the highest (p<0.05). Hunt et al. (1999) have reported that higher hue angle index indicates browner hues and that higher color differences indicate greater total color differences.
All values are mean ± standard deviation of three replicates.
a-eMeans within a column with different letters are significantly different (p<0.05).
1)Control (−), curing meat with nitrite free; Control (+), curing meat with 0.015% nitrite; T1, curing meat with 10% pre-converted nitrite sources from spinach; T2, curing meat with 20% pre-converted nitrite sources from spinach; T3, curing meat with 30% pre-converted nitrite sources from spinach; 2)H°, arctan (b*/a*); 3)∆E*, (∆L*2 + ∆a*2 +∆b*2)1/2; 4)∆C*, (∆a*2 + ∆b*2)1/2.
The pH and color values of cooked meats formulated with fermented spinach extracts are shown in Table 2. The pH values of the control groups (− and +) were the highest (p<0.05). The pH values of treatment groups of cooked meats were decreased with increasing levels of fermented spinach extract added. Similar results were obtained for the pH values of raw cured meats. The pH values of cooked cured meats were also affected by the pH value of the fermented spinach extract. This finding was in agreement with the results of Zhang et al. (2007) showing that the pH values of sausages treated with Lactobacillus fermentum were lower than control sausages due to the production of lactic acid during fermentation.
All values are mean ± standard deviation of three replicates.
a-dMeans within a column with different letters are significantly different (p<0.05).
1)Control (−), curing meat with nitrite free; Control (+), curing meat with 0.015% nitrite; T1, curing meat with 10% pre-converted nitrite sources from spinach; T2, curing meat with 20% pre-converted nitrite sources from spinach; T3, curing meat with 30% pre-converted nitrite sources from spinach; 2)H°, arctan (b*/a*); 3)∆E*, (∆L*2 + ∆a*2 +∆b*2)1/2; 4)∆C*, (∆a*2 + ∆b*2)1/2.
The lightness value of the negative control (−) was the highest (p<0.05), and the redness value of the T3 was the highest (p<0.05). The lightness and the redness values of cooked cured meats added with fermented spinach extract were increased with increasing levels of fermented spinach extract. However, the yellowness values of cooked cured meats were decreased with increasing levels of fermented spinach extract. Similar results were obtained by Sindelar et al. (2007) showing that cured meat color faded based on reflectance ratios of combined means of vegetable juice powder. The hue angles of cooked cured meats formulated with fermented spinach extract were decreased with increasing levels of fermented spinach extract. The color difference of the positive control (+) was the highest (p<0.05). The chroma difference of cooked cured meat of T3 was the lowest (p<0.05). Changes in hug angle, color difference, and chroma difference of cooked cured meats showed trend similar to those of raw meat. Thus, color development of meat products could be affected by fermented spinach extract added.
The VBN value could be used as a significant indicator of deterioration of meat product freshness. It is affected by amino acid decarboxylase, enzymes, and microorganisms (Kohsaka, 1975). The VBN values of cured meats formulated with fermented spinach extract are shown in Table 3. The lowest VBN value was observed in the positive control (+) added with nitrite. The VBN value of T3 was lower (p<0.05) than that of the negative control (−) without the addition of any nitrite. According to Weiss et al. (2010), one of the most important functions of nitrite is its ability to inhibit the growth of food pathogens and enzymes in meat products, thus, decreasing VBN of meat products. These results are in agreement with the findings of Jay (1992) showing that the formation of volatile compounds is strongly associated with the growth of microorganisms, causing an increase in the VBN value of meat products. Some researchers have reported that inhibition of microbial growth due to nitrite content might explain the lower VBN values (Kohsaka, 1975, Weiss et al., 2010). Therefore, the lower VBN values of cured meats with addition of 30% fermented spinach extract compared to that of the negative control might be due to the nitrite content in the fermented spinach.
In general, lipid oxidation can potentially lead to rancidity development. Lipid oxidation in meat products may change their nutritive values, colors, and flavors (Kim et al., 2015). Sheard et al. (2000) suggested that TBARS at 0.5 mg/kg was a threshold value for rancidity perception by consumers. The effects of various fermented spinach extract levels on lipid oxidation of cured meats on day one are shown in Table 3. Analysis of variance indicated that the TBARS values of meats were significantly affected by the levels of fermented spinach extract added. The lowest TBARS value was observed in the positive control (+) with nitrite added. The TBARS values of cured meats were decreased (p<0.05) with increasing levels of fermented spinach extract. Therefore, pre-converted nitrite from the fermented spinach extract was the one that caused the decreasing of TBARS values of cured meat. According to Shahidi and Hong (1991), nitrite is generally an effective antioxidant against lipid oxidation of meat products. Some results have been showed that antioxidant activity of natural nitrite source, by Sinderar et al. (2007), showing no significant difference in lipid oxidation between control with nitrite and treatments with different levels of fermented vegetable juice powders. Djeri and Williams (2014) reported that the TBARS values of turkey bologna were similar among all treatments with celery juice powder and/or cherry juice powder. These results suggest that cured meats formulated with pre-converted nitrite from fermented spinach extract had comparable protective effect against lipid oxidation to that of positive control added with nitrite. Furthermore, the own antioxidant activity of spinach can inhibit lipid oxidation of cured meats. According to Bergman et al. (2001), aqueous spinach extract had considerable antioxidant capacity.
| Treatments1) | VBN (mg%) | TBARS (mg MA/kg) |
|---|---|---|
| Control (−) | 5.57±0.13a | 1.48±0.02a |
| Control (+) | 4.51±0.15c | 0.12±0.05e |
| T1 | 5.61±0.29a | 0.36±0.03b |
| T2 | 5.59±0.25a | 0.23±0.02c |
| T3 | 5.08±0.43b | 0.18±0.02d |
All values are mean ± standard deviation of three replicates.
a-eMeans within a column with different letters are significantly different (p<0.05).
1)Control (−), curing meat with nitrite free; Control (+), curing meat with 0.015% nitrite; T1, curing meat with 10% pre-converted nitrite sources from spinach; T2, curing meat with 20% pre-converted nitrite sources from spinach; T3, curing meat with 30% pre-converted nitrite sources from spinach.
The effects of fermented spinach extracts on total viable count and the number of E. coli and coliform bacteria of cured meats are shown in Table 4. The highest total viable count was observed in the negative control (−) without any nitrite added. The total viable counts of cured meats in treatment groups were decreased (p<0.05) with increasing levels of fermented spinach extract. Similar results was reported by Djeri and Williams (2014) showing that the anaerobic counts were lower in meat products treated with celery juice powder and cherry juice powder. These results might be due to the pre-converted nitrite from celery juice powder and cherry juice powder. In general, nitrite is known to be able to inhibit the growth of microorganism. According to Jackson et al. (2011), nitrite can significantly reduce microbial growth on inoculated meat products cured with pre-converted nitrite from natural sources. Lamkey et al. (1991) reported that spoilage meat during storage had more than 8 Log CFU/g of total viable counts. In this study, the control groups and treatment groups added with pre-converted nitrite from fermented spinach extract were expected to display lower levels of initial total viable counts. E. coli and coliform bacteria were not detected in control groups or treatment groups of cured meats added with fermented spinach extract. Choi et al. (2016) have reported that there was no E. coli or coliform bacteria in sausages of the control group and treatment groups at the early stage of curing. Jeong (2016) and Alahakoon et al. (2015) reported that nitrite could inhibit the growth of a number of aerobic and anaerobic microorganisms, especially Clostridium botulinum, Clostridium perfrigenes, Bacillus cereus, Staphyloccus aureus, and Listeria monocytogenes. According to Dubey et al. (2010), antimicrobial activity of spinach was most effective among some vegetable (pumkin, suran, ghuiya, and spinach). With increasing level of fermented spinach extract, antimicrobial activity was more improved than lower level of fermented spinach extract.
| Treatments1) | Total viable count | E. coli | Coliform bacteria |
|---|---|---|---|
| Control (−) | 1.65±0.11a | N.D | N.D |
| Control (+) | 0.46±0.06c | N.D | N.D |
| T1 | 1.01±0.12b | N.D | N.D |
| T2 | 0.44±0.15c | N.D | N.D |
| T3 | 0.34±0.12c | N.D | N.D |
All values are mean ± standard deviation of three replicates.
a-cMeans within a column with different letters are significantly different (p<0.05).
1)Control (−), curing meat with nitrite free; Control (+), curing meat with 0.015% nitrite; T1, curing meat with 10% pre-converted nitrite sources from spinach; T2, curing meat with 20% pre-converted nitrite sources from spinach; T3, curing meat with 30% pre-converted nitrite sources from spinach.
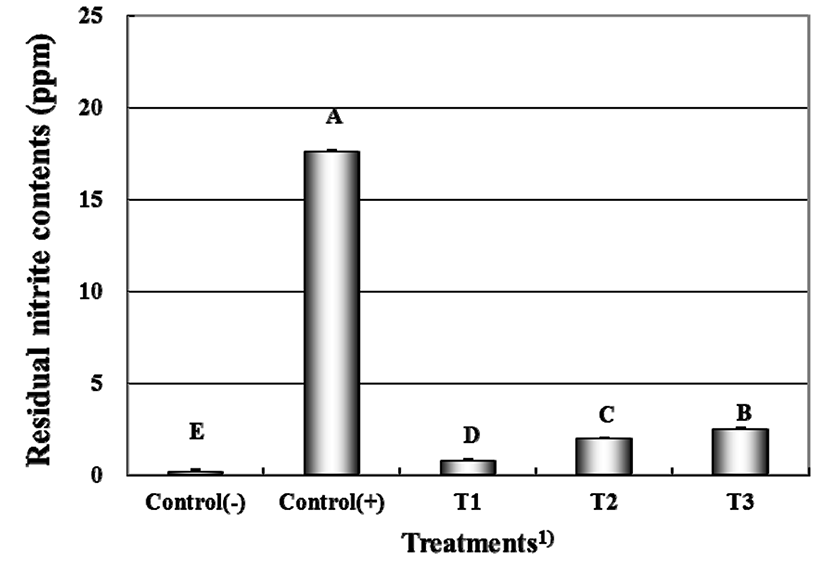
The residual nitrite contents of cured meats formulated with fermented spinach extract are shown in Fig. 1. The highest residual nitrite content was found in the positive control (+) added with nitrite. The lowest residual nitrite content was observed in the negative control (−) without adding any nitrite. The residual nitrite contents of in cured meats were increased with increasing levels of fermented spinach extract added (p<0.05). Therefore, the residual nitrite contents likely depend on pre-converted nitrite contents of the fermented spinach extract (pre-converted nitrite from fermented spinach extract: 4916.82 ppm) and curing solution (nitrite contents of 30, 20, and 10% fermented spinach curing solution: 147.50 ppm, 98.34 ppm, 49.17 ppm). Similar results were obtained by Sindelar et al. (2007) showing that the residual nitrite contents of meat products treated with vegetable juice powder were higher when greater amount of pre-converted nitrite was added to the meat products after incubation. These results were in agreement with those of Terns et al. (2011) showing that residual nitrite was observed in cured and cooked sausages treated with cherry powder and starter culture post-incubation. However, residual nitrite was not observed in cooked sausages treated with cherry powder and starter culture pre-incubation (Terns et al., 2011). Zhang et al. (2007) reported that residual nitrite contents of sausages with Lactobacillus fermentum fermentation were significantly lower than those of control sausages with 60 ppm nitrite added into the formulation.
Conclusion
We showed that fermented spinach extract contained pre-converted nitrite which could substitute synthetic nitrite to maintain the color development of cured meats. Therefore, pre-converted nitrite from fermented spinach extract might have significant potential for use in meat products. Development of meat products by replacing the synthetic nitrite with nitrite from natural source might provide a new growth to the meat processing industry.













