Introduction
Curing meat products is a time-honored practice dating back centuries, aimed at enhancing flavor, color, texture, and shelf-life (Jia et al., 2023). Central to this process is the use of nitrite salts, which play a pivotal role in ensuring food safety by inhibiting bacterial growth (Jo et al., 2020). However, concerns have been raised regarding the potential health risks associated with excessive nitrite consumption, including the formation of carcinogenic compounds, such as nitrosamines (Yong et al., 2021). Consequently, there has been a growing interest in exploring alternative curing methods that maintain the safety and quality of cured meats while reducing reliance on synthetic nitrite additives (Shakil et al., 2022; Zhang et al., 2023b).
One such avenue involves utilizing natural sources of nitrite, including those derived from the microbial conversion of nitrate present in certain food ingredients (Kim et al., 2019a; Yong et al., 2021). These naturally occurring nitrites offer promising alternatives to their synthetic counterparts, potentially mitigating concerns regarding the health effects of nitrosamines while maintaining the desirable attributes of cured meat (Choi et al., 2017; Kim et al., 2019b). By systematically analyzing the impact of naturally converted nitrite and ultrasound marination on both the curing process and residual nitrite levels, we aim to provide valuable insights into developing safer and more sustainable methods for producing cured meat products.
Additionally, advancements in food processing technologies, such as ultrasound, have led to the exploration of novel techniques to enhance the efficiency of curing processes (Kang et al., 2016). Ultrasound, with its ability to facilitate mass transfer and enhance the penetration of marinades into meat matrices, is an intriguing approach to improve the uniformity and effectiveness of curing treatments (Inguglia et al., 2018).
Thus, this study aims to contribute to the ongoing discourse surrounding the optimization of curing processes in the meat industry. By leveraging natural sources of nitrite and innovative marination techniques, we aspire to offer pragmatic solutions that prioritize food safety and consumer health without compromising the quality of cured pork loin.
Materials and Methods
The natural source of nitrate, Angelica keiskei, was purchased from Garurang (Seoul, Korea) in powdered form. A. keiskei (50 g) was mixed with distilled water (500 mL), extracted for 2 h at 100°C, and cooled to 25°C. Subsequently, Staphylococcus carnosus (Bactoferm S-B-61, Chr. Hansen, Gainesville, FL, USA) was inoculated into the extract and incubated in a shaking incubator (JSSI-100C, JSR, Gongju, Korea) for 24 h at 37°C and 180 rpm to convert nitrate into nitrite. The fermented A. keiskei extract was centrifuged for 10 min at 3,000×g using a centrifuge (Supra R22, Hanil, Gimpo, Korea), and the supernatant was filtered through a Whatman No. 1 filter paper. The nitrite content of the fermented A. keiskei extract was diluted with a small amount of water to achieve a concentration of 180 ppm.
Fresh pork loins (longissimus thoracis et lumborum) were purchased from a local butcher shop (Wanju, Korea) at 48 h postmortem. Six loins from pigs (Yorkshire×Landrace×Durok) were cut to a thickness of 3 cm, and 36 loin chops were randomly selected for use in the experiments for the six treatments. Considering the residual nitrite content identified in a previous research, a concentration of nitrite added was selected as 180 ppm of curing solution (Honikel, 2008). The six treatments were as follows: NC, pork loin cured with 8% sodium chloride in distilled water; PC, pork loin cured with 8% sodium chloride+180 ppm sodium nitrite in distilled water; PA, pork loin cured with 8% sodium chloride in a pre-converted A. keiskei extract (180 ppm of nitrite); PA+A, pork loin cured with 8% sodium chloride+0.125% ascorbic acid in a pre-converted A. keiskei extract (180 ppm of nitrite); PA+U, pork loin cured with 8% sodium chloride in a pre-converted A. keiskei extract (180 ppm of nitrite) and subjected to ultrasound treatment after curing; PA+AU, pork loin cured with 8% sodium chloride+0.125% ascorbic acid in a pre-converted A. keiskei extract (180 ppm of nitrite) and subjected to ultrasound treatment after curing. Six loin chops from each treatment group were brined by immersion in 40% brine by weight and the treatments were cured for 4 days at 4°C. Ultrasound treatment was conducted on PA+U and PA+AU in an ultrasonic bath (CPX5800H-E, Emerson, St Louis, MO, USA) for 30 min at a temperature below 15°C, according to the previous research with slight modification (Leães et al., 2023). Then, all the treatments were cooked at 80°C for 1 h and cooled for 2 h at 20°C to conduct the following analysis.
The cooked pork loin chops were cut to a thickness of 2 cm, and the color of the cross-section was measured immediately using a colorimeter (CR-400, Minolta, Tokyo, Japan). The aperture size was 8 mm, the illuminant was D65, and the angle of observation was 2°. Color values are presented as CIE L*a*b*, and the number of measurements was seven.
NO-heme (curing pigment) and total haem pigments (total pigment) in the samples were determined using the method described by Hornsey (1956). To measure the curing pigment concentration, 10 g of ground sample was mixed with 80% (v/v) acetone for 5 min under reduced light conditions. The mixture was then filtered through Whatman No. 1 filter paper (Whatman International, Maidstone, UK), and the absorbance of the filtrate was measured at 540 nm using a spectrophotometer (Optizen 2120 UV plus, Mecasys, Daejeon, Korea). NO-heme concentration was calculated by multiplying the absorbance by 290. For the total pigment concentration, a 10 g minced sample was mixed with acidified 80% acetone and left to react for 1 h. The resulting solution was filtered through a Whatman No. 1 filter paper, and the absorbance of the filtrate was measured at 640 nm using a spectrophotometer. The total pigment concentration was determined by multiplying the absorbance at 640 nm by a constant factor of 680. The curing efficiency was calculated as the percentage of total pigment converted to a curing pigment, serving as an index of the degree of cured color stability.
To analyze pork loins treated with naturally converted nitrite, ascorbic acid, and ultrasound marination, a sample weighing 10 g was homogenized in 150 mL of heated distilled water. The mixture was then combined with 10 mL of 0.5 N NaOH and 10 mL of 12% ammonium thiosulfate and heated at 80°C for 20 min. After heating, 20 mL of ammonium acetate buffer was added to the cooled mixture, and the volume was adjusted to 200 mL with distilled water. The solution was then incubated at 20°C for 10 min. Following incubation, the mixture was filtered through Whatman No. 1 filter paper. To 20 mL of the filtered sample, 1 mL of sulfanilamide solution, 1 mL of N-(1-naphthyl)ethylenediamine dihydrochloride reagent, and 3 mL of distilled water were added. This final mixture was incubated at 20°C for 20 min, and the absorbance was measured at 540 nm, following the method described by Korea Food and Drug Administration (KFDA, 2016).
The pH of raw pork loins treated with naturally converted nitrite, ascorbic acid, or ultrasound marination was determined using a pH meter (AB15, Thermo Fisher Scientific, Waltham, MA, USA). Five grams of each sample was homogenized with 20 mL of distilled water, and the pH value of each homogenate was measured using pH/ATC electrodes after calibration with buffers of pH 4, 7, and 10.
The curing yield of pork loins treated with naturally converted nitrite, ascorbic acid, or ultrasound marination was determined by measuring the weight differences before and after the curing process. Similarly, the cooking yield was calculated by assessing the difference in weight before and after cooking.
The water holding capacity (WHC) of raw pork loins subjected to naturally converted nitrite, ascorbic acid, and ultrasound marination was determined using the press method described by Trout (1988). The water-holding force was assessed by positioning the sample between two Plexiglas plates and applying a pressure of 40 kg for 3 min. The meat area and released moisture were quantified using a planimeter (Planix 7, Tamaya Technics, Tokyo, Japan). The WHC was calculated using the following formula:
As per AOAC (2000), the moisture content (method 950.46B) of the cooked samples was determined by measuring the weight loss after drying the samples at 105°C for 12 h in a drying oven (SW-90D, Sang Woo Scientific, Bucheon, Korea).
The thermal properties of raw pork loins were analyzed using a differential scanning calorimeter (DSC-4000, PerkinElmer, Waltham, MA, USA). Approximately 30 mg of the pork sample was placed in a sealed aluminum pan, with an empty aluminum pan serving as a reference. The heat flow differential between the sample and reference pans was measured. The analysis was conducted over a temperature range of 20°C to 100°C, with the heating rate set to 10°C per minute.
The myofibril fraction index (MFI) of pork loin samples treated with naturally converted nitrite, ascorbic acid, and ultrasound marination was measured before heating, following the method described by Culler et al. (1978). Briefly, a 4 g sample was homogenized with 40 mL of MFI buffer (comprising 100 mM potassium chloride, 20 mM potassium phosphate, 1 mM ethylenediaminetetraacetic acid, 1 mM magnesium chloride, and 1 mM sodium azide), and then centrifuged at 1,000×g for 15 min at 4°C. The resulting pellet was resuspended in 40 mL of MFI buffer and centrifuged again under the same conditions. After dissolving the pellet in 10 mL of MFI buffer, the solution was filtered through a 10-mesh steel strainer. The protein concentration was adjusted to 0.5 mg/mL, and its absorbance was measured at 540 nm. To amplify the absorbance unit, a factor of 200 was applied, where a higher value indicated an increased proteolytic level of the myofibril structure.
The protein solubility of raw pork loin samples treated with naturally converted nitrite, ascorbic acid, and ultrasound marination was assessed using BCA protein assay kits (Thermo Fisher Scientific). Buffer A was used to extract total proteins (myofibril and sarcoplasmic), whereas Buffer B was used to extract sarcoplasmic proteins, following the method of Joo et al. (1999). The difference in protein solubility between Buffer A and Buffer B was considered the myofibril solubility. Buffer A consisted of 1.1 M potassium iodide in 0.1 M potassium phosphate at pH 7.4, and Buffer B contained 0.025 M potassium phosphate at pH 7.4.
Shear force measurements were performed on pork loin samples treated with naturally converted nitrite, ascorbic acid, or ultrasonic marination. Cylindrical samples with diameters of 10 mm were extracted using a hole puncher oriented vertically relative to the muscle fibers. A texture analyzer (TA-XT Plus, Stable Micro Systems, Surrey, UK) equipped with a Warner-Bratzler blade was used for measurements at a test speed of 2 mm/s.
To estimate lipid oxidation in cooked pork loins, a thiobarbituric acid reactive substances (TBARS) test was performed following the method described by Tarladgis et al. (1960). A 10 g sample was homogenized with 100 mL of 0.1 M HCl. Then, the homogenate was heated to 100°C, and the distillate was subsequently collected. The distillate was reacted with 0.02 M TBA reagent at 100°C for 35 min. After cooling the solution in chilled water, the absorbance was measured at 538 nm to determine the malondialdehyde content using a standard curve provided by Sigma-Aldrich (St. Louis, MO, USA).
Data analyses were performed using SPSS Statistics version 20 (IBM, Armonk, NY, USA). Three replicates were performed for each experiment, with at least three technical replicates. To identify significant differences among treatments, a one-way analysis of variance was employed, followed by Duncan’s multiple range test to determine data with significant differences (p<0.05).
Results and Discussion
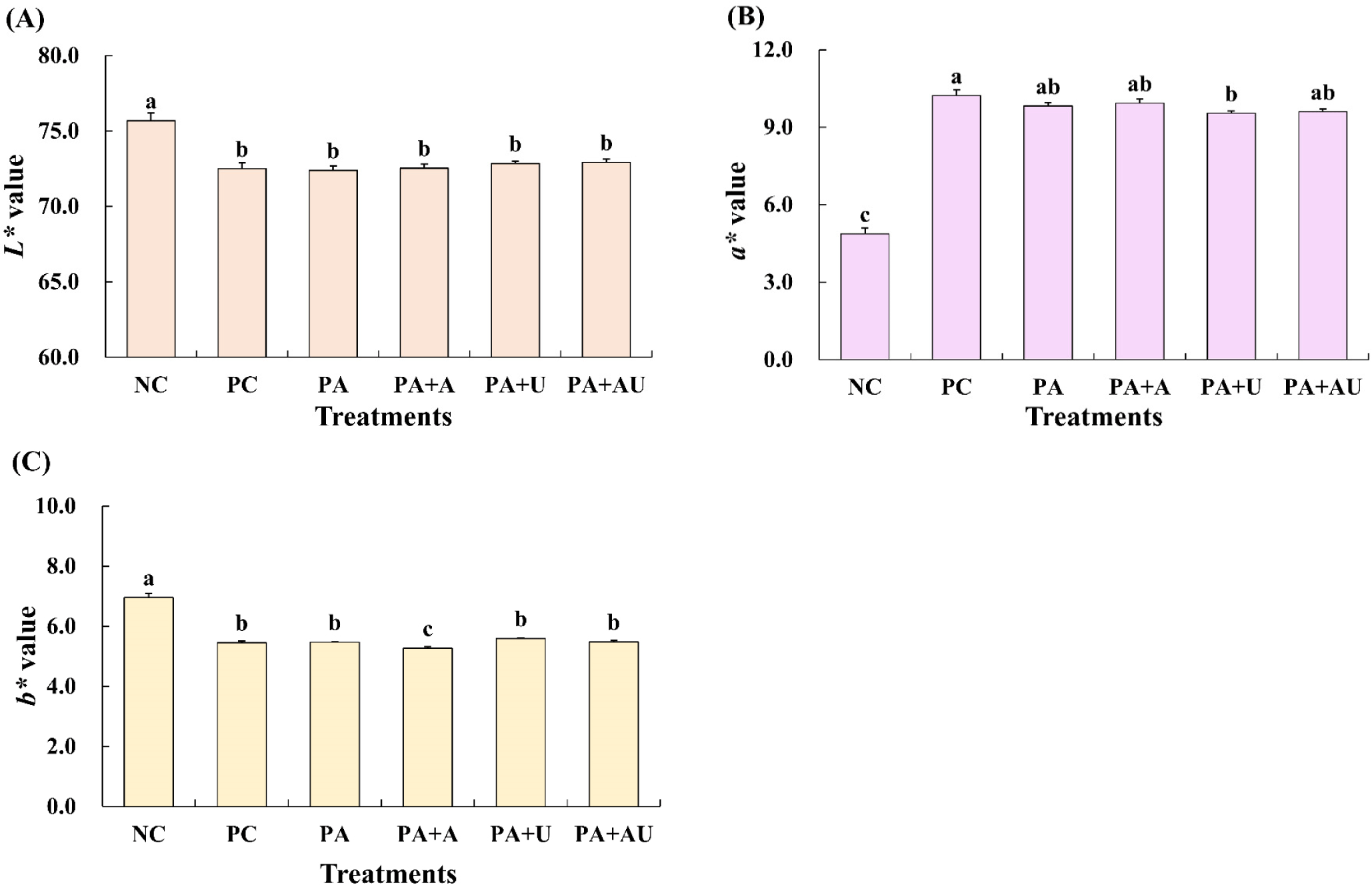
Fig. 1 illustrates the effects of naturally converted nitrite, ascorbic acid, and ultrasound on the color of marinated pork loin. The CIE L* and CIE b* values were highest for the NC treatment, and treatments with naturally converted nitrite, ascorbic acid, and ultrasound resulted in lower CIE L* and CIE b* values than those of NC. The CIE a* values for NC-treated pork loin were the lowest. Among the treatments, there was no significant difference between PC with nitrite and treatment with pre-converted A. keiskei, except for treatments with naturally converted nitrite and ultrasound (PA+U). Choi et al. (2017) reported that CIE a* and CIE b* of cooked meat emulsions increased with increasing fermented red beef extract, and CIE a* increased with the addition of ascorbic acid due to its acceleration effect on NO-myoglobin. Hwang et al. (2018) found that, among pre-converted natural products, celery and spinach extracts increased the CIE L*, CIE a*, and CIE b* of raw meat batter. Kim et al. (2017) observed that the CIE L* and CIE b* values of raw meat cured with fermented spinach extract were higher than those of a positive control with 0.015% nitrite. The comparable color values of meat cured with naturally converted nitrite and sodium nitrite can positively influence color acceptance of consumer (Serdaroğlu et al., 2023). As inferred from the color value results, there does not seem to be a significant difference between nitrite and naturally converted nitrite and ascorbic acid, and ultrasound treatment does not appear to have a significant effect on chromaticity.
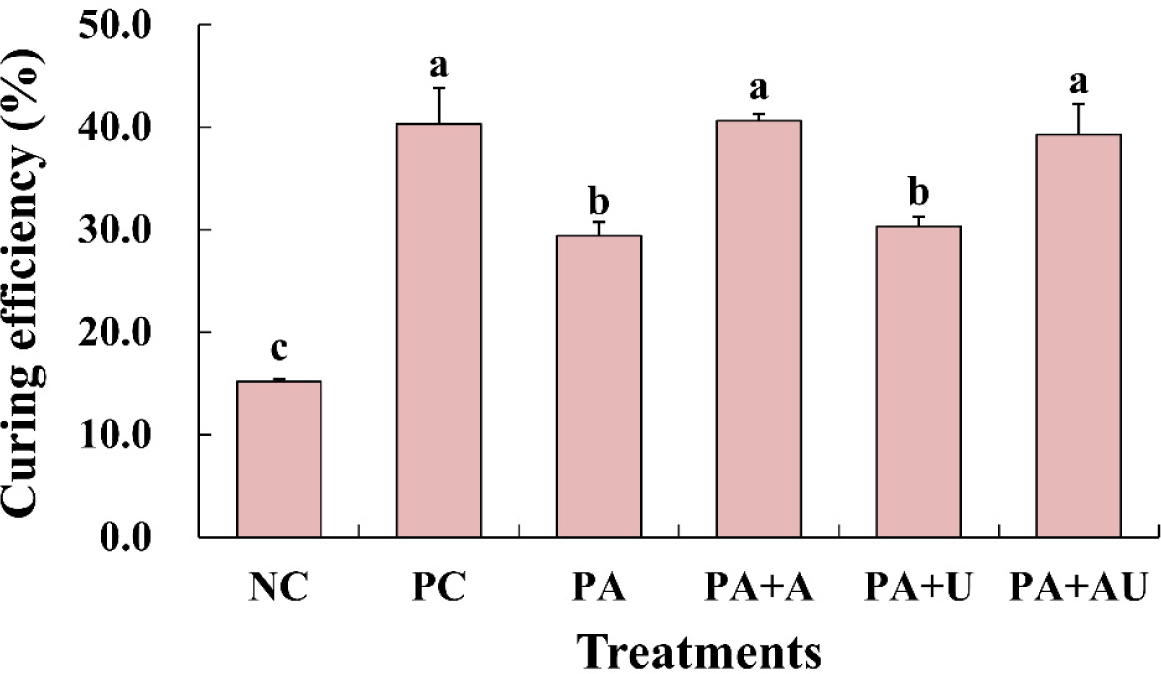
The effects of naturally converted nitrite, ascorbic acid, and ultrasound on the curing efficiency of marinated pork loin were significant (p<0.05; Fig. 2). The curing efficiency of marinated pork loin was the highest in the PC treatment group and the naturally converted nitrite and ascorbic acid treatment groups (PA+A, PA+AU). It was found that ultrasound was not effective in improving the curing efficiency of marinated pork loins. Choi et al. (2020) reported that the curing efficiency of meat products cured with white kimchi powder and starter culture was higher than that of the control cured with 0.01% nitrite and 0.05% ascorbic acid. Bae et al. (2020) reported that the curing efficiency of meat products cured with radish powder and starter culture, such as S. carnosus, was significantly increased. Jantapirak et al. (2023) reported that the curing efficiency of chicken sausages using normal sodium nitrite levels in the formulation was much higher than that of the low-sodium nitrite treatment. The results of this study suggest that a similar effect can be expected when nitrite is used in combination with naturally converted nitrite and ascorbic acid.
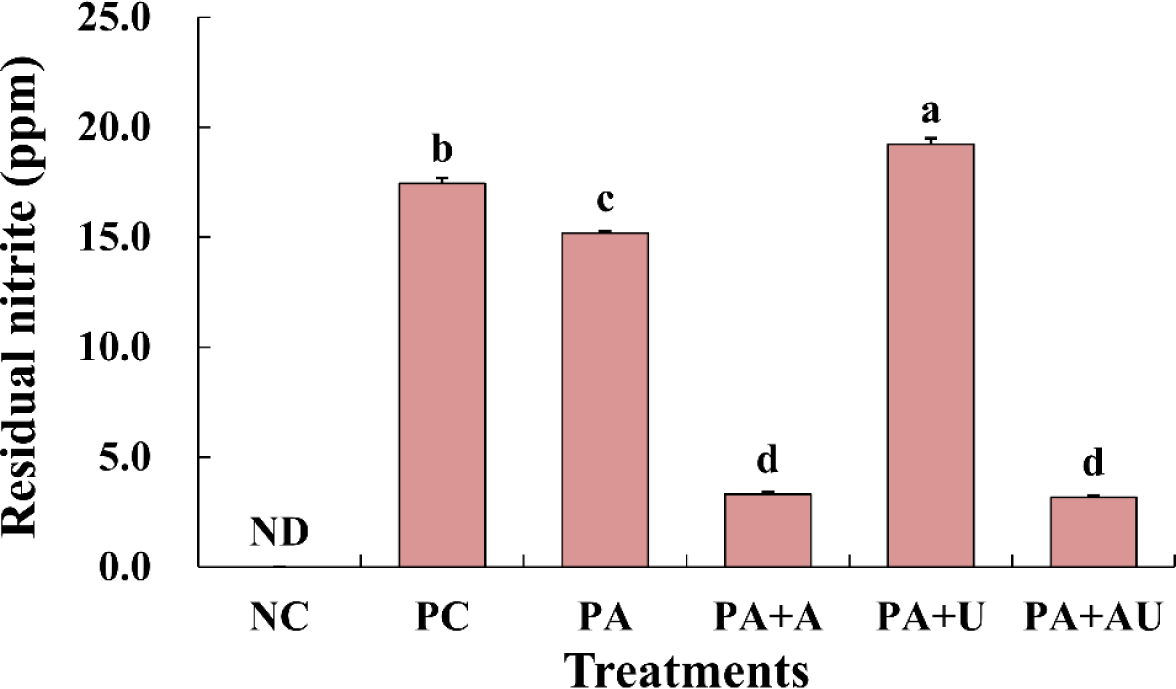
The effects of naturally converted nitrite, ascorbic acid, and ultrasound on the residual nitrite in marinated pork loin are shown in Fig. 3. In the NC group, no nitrite was detected in the residual nitrite of the marinated pork loin. The residual nitrite content of the PC with nitrite was higher than that of the other treatments with pre-converted A. keiskei and ascorbic acid (p<0.05). The residual nitrite in the marinated pork loin was the highest for converted nitrite and ultrasound (PA+U). The results of this study indicated that ultrasound treatment did not have a greater effect on residual nitrite than expected. According to Choi et al. (2017), the residual nitrite content decreases when ascorbic acid is added to cured pork loin containing fermented red beet extract. Kim et al. (2019a) reported that pork loin cured with organic acids had a lower residual nitrite content than that cured without organic acids, and malic acid and citric acid showed the highest residual nitrite scavenging ability. King et al. (2016) reported that, in cured sausage pork, the higher the level of ascorbic acid added, the higher the nitrite depletion rate. Therefore, it is considered that adding ascorbic acid alone is effective in reducing the residual nitrous acid content even without ultrasound treatment.
The pH values of naturally converted nitrite, ascorbic acid, and ultrasound-marinated pork loins are shown in Table 1. The pH of nitrite in sample (PC) was the highest, and the pH of NC and PC were higher than those of the other treatments with pre-conversion and ultrasound (p<0.05). These results are similar to those of Choi et al. (2017), who showed that the pH after treatment with fermented red beet extract was lower than that of the control without nitrite and with nitrite. Hwang et al. (2018) reported that the pH of pork sausages treated with nitrite was higher than that of sausages treated with natural pre-converted nitrite sources. These results, along with the pH of pork loin, appear to be influenced by natural nitrite fermentation products.
a–e Different letters within the same row indicate significant differences between treatments (p<0.05).
NC, cured without sodium nitrite; PC, cured with sodium nitrite (180 ppm); PA, cured with a pre-converted Angelica keiskei extract (180 ppm of nitrite); PA+A, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid; PA+U, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and marinated using ultrasound; PA+AU, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid, and marinated using ultrasound.
Table 1 shows the curing yield, cooking yield, WHC, and moisture content of naturally converted nitrite, ascorbic acid, and ultrasound-marinated pork loin. The curing yield, cooking yield, and WHC of naturally converted nitrite and ultrasound on marinated pork loin did not differ significantly among all treatments (p>0.05). Krause et al. (2011) reported that the cured meat with natural nitrite did not show a significant difference in the curing yield and cooking loss. Kim et al. (2019a) indicated that the curing yield and cooking loss of cured pork loin with green nitrite showed no significant difference between the control and the treatment groups. Similar to several studies, this study also confirmed that there was no difference in the curing yield and cooking yield of meat products depending on the addition of nitrite and pre-converted natural nitrite. Similar results with Kim et al. (2019b) for the curing were observed with no significant differences in cooking loss. The moisture content of the pork loin was highest when treated with sodium nitrite (Table 1). This might be because the moisture content is affected by the pH of the marinated pork loin.
Changes in the thermal properties of pork loins treated with naturally converted nitrite, ascorbic acid, or ultrasound marination were measured using differential scanning calorimetry, and the results are presented in Table 2. The analysis revealed endothermic transitions at peak temperatures of approximately 57.78°C–58.56°C (peak 1) and 75.18°C–77.02°C (peak 2). According to Kim et al. (2023), peak 1 corresponds to the temperature at which myosin denaturation occurs, whereas peak 2 corresponds to the denaturation of sarcoplasmic proteins and actin. Additionally, Kim et al. (2021) noted that an increase in peak temperature indicates enhanced thermal stability of proteins prone to denaturation. Delta H represents the enthalpy change, reflecting the heat energy required for protein denaturation; a lower Delta H suggests that the protein is relatively stable before being altered by heat. We hypothesized that ultrasound treatment would influence the thermal properties of pork loins; however, the results indicated no significant effects. This lack of impact is likely due to the short duration of the ultrasound treatment, which was insufficient to alter protein structures.
a–c Different letters within the same row indicate significant differences between treatments (p<0.05).
NC, cured without sodium nitrite; PC, cured with sodium nitrite (180 ppm); PA, cured with a pre-converted Angelica keiskei extract (180 ppm of nitrite); PA+A, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid; PA+U, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and marinated using ultrasound; PA+AU, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid, and marinated using ultrasound.
The MFI, protein solubility, and shear force of marinated pork loin samples with naturally converted nitrite, ascorbic acid, and ultrasound are shown in Table 3. MFI is a good indicator of the degree of decomposition of meat proteins and can be used to determine the degree of tenderization in meat. The MFI of the marinated pork loin samples did not differ significantly among the treatments (p>0.05). Total protein solubility and myofibrillar protein solubility of pork loin samples were higher in treatments with ultrasound (PA+U and PA+AU) than in treatments without ultrasound. This shows that ultrasound affects myofibrillar protein solubility but does not affect muscle fragmentation. As mentioned previously, this is believed to be due to the short ultrasound processing time. The shear force of PC with sodium nitrite was the highest, whereas the shear force of PA+U was the lowest (p<0.05). Kim et al. (2019b) reported that the shear force of cured pork loin did not significantly differ between nitrite and natural nitrite from fermented Swiss chard; however, the shear force may be affected by pH and moisture content. Shear force is regarded as the most effective indicator of sensorial tenderness (Holman et al., 2020), and lower shear force can be positively associated with consumer acceptance of meat (Warner et al., 2021). Looking at the shear force results, softness tended to decrease when nitrite or naturally converted nitrite was added, and when ascorbic acid was combined, shear force of cured loin was increased. However, it appears that ultrasound treatment can improve the softness. This improvement in tenderness appears to be due to myofibrillar protein solubility.
a–d Different letters within the same row indicate significant differences between treatments (p<0.05).
NC, cured without sodium nitrite; PC, cured with sodium nitrite (180 ppm); PA, cured with a pre-converted Angelica keiskei extract (180 ppm of nitrite); PA+A, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid; PA+U, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and marinated using ultrasound; PA+AU, cured with a pre-converted A. keiskei extract (180 ppm of nitrite) and ascorbic acid, and marinated using ultrasound.
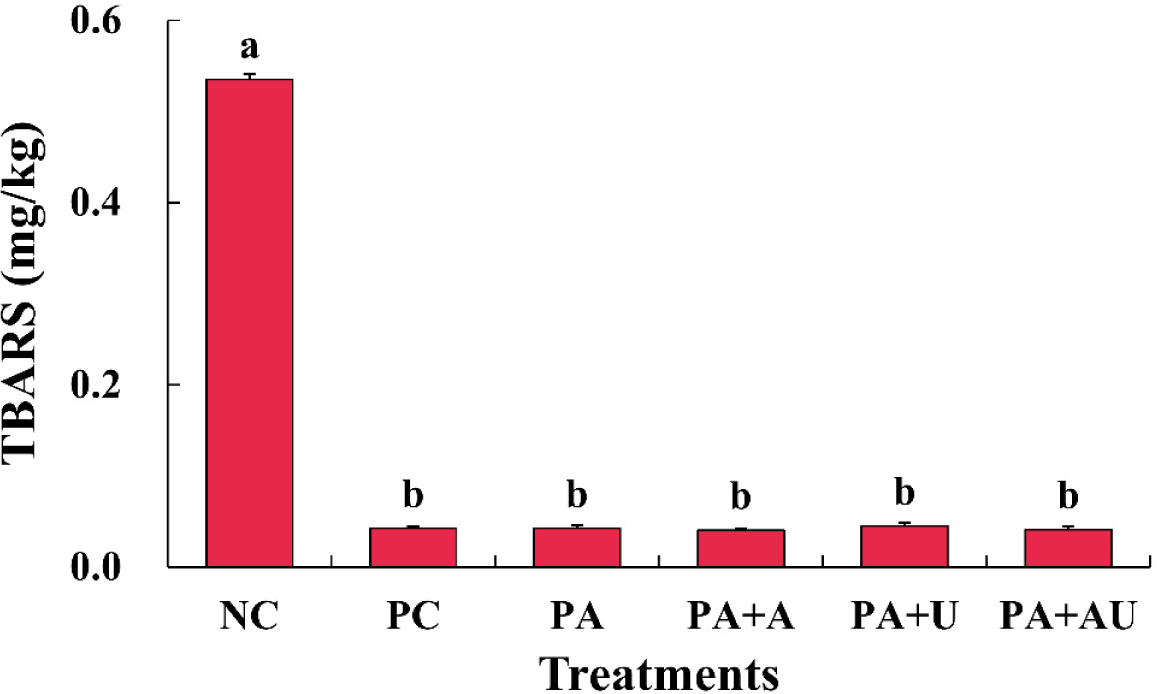
The effects of naturally converted nitrite and ultrasound on the TBARS of the cured pork loin were significant (Fig. 4). The NC had the highest TBARS value compared with that of the other treatments with nitrite and converted nitrite (p<0.05), and the TBARS values did not show a significant difference among the treatments with nitrite and converted nitrite (p>0.05). According to Kim et al. (2019c), pork loin cured with nitrite from fermented spinach and organic acids had a lower TBARS than that of the control cured without nitrite. Choi et al. (2017) reported that meat emulsions with pre-converted nitrite from red beets and ascorbic acid had higher TBARS values than that those of the control. Kim et al. (2019a) reported that meat products containing organic acids, including ascorbic acid, were effective against lipid rancidity. Similar to previous results, nitrite or pre-converted nitrite produced through fermentation is believed to effectively inhibit the rancidity of meat products through lipid oxidation. Ultrasound treatment appears to have no effect on rancidity or has little effect on lipid oxidation when nitrite was added. Meanwhile, difference of TBARS values among treatments can be occurred during storage, as a previous research using black tea as a nitrite replacer (Zhang et al., 2023a). Regarding ascorbic acid, it can retard the lipid oxidation during storage, by reducing heme iron and hindering free radical formation (Li et al., 2013). However, ultrasound-treated meat may be more susceptible to the lipid oxidation after storage, due to the exposure of fats to free radicals formed by ultrasound treatment (Peña-González et al., 2017). Therefore, the naturally converted nitrite of the marinated pork loin used alone can effectively suppress lipid oxidation by replacing sodium nitrite, and it was observed that ultrasound does not have a significant effect on lipid oxidation of freshly cooked meat. In further studies, the effect of these additives and treatment on the cured meat during long-term storage should be determined, although previous studies have provided insights into the changes that occur during storage.
Conclusion
When curing meat, pre-converted natural nitrite sources can be used as an alternative to synthetic nitrites. We used ultrasound and ascorbic acid to improve the quality of meat cured using naturally pre-converted nitrite raw materials. Ultrasound had a positive effect on myofibrillar protein solubility and shear force but had no effect on color development, curing efficiency, or lipid acidity. However, it appears to have a negative effect on residual nitrite. Therefore, when combining a pre-converted natural nitrite source with ultrasound, ascorbic acid should be added to help improve the quality of cured meat.













