Introduction
Sulfur is a major inorganic element and essential for the entire biological field because of its incorporation into amino acids, proteins, enzymes, vitamins, and other biomolecules, and unlike humans and monogastric animals, plants can use inorganic sulfur and can synthesize sulfur-containing amino acids such as methionine and cysteine (Lioudmila et al., 2003). Organic sulfur compound such as MSM (methylsufonylmethane) is the representative non-toxic sulfur compound and is extracted from plants involved in the formation of sulfur-containing amino acids, such as methionine, cysteine and taurine (Total Health, 1998). These sulfur compounds are quickly evacuated from the body and caused no problems in toxicity tests with rats over a period of 90 d, even at 1.5-2.0 g/kg (Magnuson et al., 2007).
Researchers that study meat science have often attempted to increase meat quality (Kim et al., 2015; Lee et al., 2009). One of the most important factors for improving meat quality is reducing lipid oxidation. Meat products manufactured with dietary sulfur fed animals are well known to possess significant antioxidant effects (Kim et al., 2015; Lee et al., 2009). Song et al. (2013) reported that pigs that ate feed containing a high concentration of sulfur had a lot of antioxidants in their body. Additionally, supplementation with dietary sulfur is known to decrease saturated fatty acid and increase polyunsaturated fatty acid (Danielle et al., 2014). Kim et al. (2015) reported that the ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA) is an important indicator for cardiovascular disease prevention.
Dry-cured sausages are some of the oldest and most famous meat products in southern Europe because of the dry climate in areas such as Spain, Italy and France. However, these meat products have high fat contents (20-40% lard in dry-cured sausage formulation). Thus, they are more susceptible to lipid oxidation than other meat products, and are not recommended for health management (Utrilla et al., 2014). Moreover, saturated fatty acid and cholesterol, which increase the rate of cardiovascular disease, are highly distributed in fermented sausages (USDA, 2000). Processed sulfur-fed pork can decrease saturated fatty acids and increase polyunsaturated fatty acids in meat products (Kim et al., 2015). Also, meat products manufactured from processed sulfur-fed pork have higher moisture contents and water-holding capacity than general meat products (Kim et al., 2015; Lee et al., 2009).
Meanwhile, electronic tongue (E-tongue), taste sensing system, is an instrument that evaluates the taste by electronic techniques (Latha and Lakshmi, 2012). Their sensing system could represent the specific taste to the figures. Recently, E-tongue is used for evaluating the quality of meat product. Especially, it is necessary for aging meat products because this system could measure richness and umami taste which are important to these products (Nodake et al., 2013). Thus, representing the taste quality of aging meat products using E-tongue might be increasingly valuable.
In our previous study, dry-cured ham by using 0.3% processed sulfur-fed pigs showed relatively a higher meat quality and shelf life extension than with the dry-cured ham made from pigs fed 0.1% processed sulfur (Kim et al., 2015). Thus, the object of this study was to find out the physicochemical and sensory properties of the fermented sausages made with processed sulfur-fed pigs.
Material and Methods
The sample used in the present study was obtained from a population of pigs fed the same basal diet composition described by Kim et al. (2015). A total of 60 three-way crossbred pigs (Duroc × Landrace × Yorkshire) from Yang-Ju Livestock Federation in the Republic of Korea were raised for 174 d. The animal experiment was managed by the animal care committee of Konkuk University of Seoul, Republic of Korea. Non-processed sulfur-fed pigs (NP) were fed a basal diet; 0.3% processed sulfur-fed pigs (SP) were fed a mixed diet until 121.01±1.26 kg Body weight without a significant difference between NP and SP. The elemental sulfur concentration was 97.9% processed sulfur according to the Korea Feed Ingredients Association. The feeding system and watering processes were managed by automatic circulation. Slaughter was performed at slaughter house in Pocheon Nongchucksan by knocking, the carcasses were chilled at 1℃ for 24 h and loin muscles were obtained from the carcasses. The samples were transported to Konkuk University for this experiment as soon as they were obtained.
The fermented sausages were produced according to a slight modification of the recipe described by Kim et al. (2014). Pork meat was trimmed to remove visible fats and connective tissue. Pork meat and pork back fat were ground separately using a grinder with a 5 mm plate. After grinding, basic ingredients were added to the meat and fat. The formulation of fermented sausage is presented in Table 1. Also, starter culture, Staphylococcus carnosus and Lactobacillus plantarum (107 CFU/g), was added at the same time as the basic ingredients. The ingredients were mixed manually for 5 min. Finally, the mixtures were filled with collagen casing and hung in a room for 30 d with a humidifier and air conditioner capable of adjusting the temperature. The ripening conditions on the first day consisted of a respective temperature and RH of 20±2℃ and 90±5%. The drying conditions on subsequent days, the temperature and RH were 12±2℃ and 75±5%. The pH and water activity of the fermented sausage were measured during the drying and ripening processes. After the manufacturing process, the proximate composition, physicochemical and microbiological experiments were analyzed for meat quality.
Moisture, crude protein, crude fat and ash contents of fermented sausages were determined according to the AOAC (1995).
Water activity was measured at 25℃ using a water activity device (Aqua Lab CX-2, Decagon Device Inc., Germany).
pH was measured using a suspension homogenized 2 g sample and 18 mL distilled water for 1 min with a pH meter device (pH 900, Precisa Co., Switzerland).
Color was measured using Handy colorimeter 9 (NR-300, Nippon Denshoku, Japan). The machine was calibrated with a white square plate (CIE L*=+94.48, a*=−0.67, b*=+3.31). Values for CIE L*(lightness), CIE a* (redness), and CIE b* (yellowness) were determined at 30 d.
Lipid oxidation was determined according to the TBA (2-thiobarbituric acid) method from Witte et al. (1977). First, 2 g of sample was homogenized at 12,000 rpm for 1 min with 10 mL of 10% TCA (trichloroacetic acid) and 10 mL of distilled water. After homogenizing, the solution was filtered through a filter paper (Whatman No. 1, Whatman Inc., USA). 5 mL of the filtered solution was mixed with 5 mL of TBA (2-thiobarbituric acid 2.88 g/L) and then, the mixed solution was placed in a 90℃ water bath for 10 min. After cooling for 30 min, the absorbance of the solution was measured with a spectrophotometer (UV/Vis Spectrophotometer, Mecasys Co., Korea) at 532 nm. Thiobarbituric acid reactive substance (TBARS) values were determined from a standard curve of malondialdehyde.
2 g of sample was homogenized in 18 mL distilled water for 90 s and the supernatant was diluted for inoculation onto petrifilm and MRS agar. Total aerobes were inoculated onto petrifilm to count the total aerobic bacteria (3M Petrifilm, USA) for 24 h at 35℃. Lactic acid bacteria were cultured on MRS agar (Oxoid, England) at 30±3℃ for 72 h.
The fatty acid analysis was performed according to the AOAC (1995). Briefly, 25 mg of samples were mixed with potassium hydroxide (KOH) in methanol and heated. After cooling, 1 mL of isooctane solution and saturated sodium chloride (NaCl) were mixed with the solution. Chromatographic conditions were as follows: initial oven temperature, 100℃ (held for 4 min); ramping at 3℃/min to 240℃ (held for 15 min). The injector and detector were maintained at 225℃ and 285℃, respectively. The flow rate of helium was 0.75 mL/min, and 1 μL of solution was injected in split mode (200:1). Nonadecanoic acid methyl ester, as an internal standard, was added at 0.3 mg/mL to the samples prior to fat extraction and methylation. The isooctane layer was dehydrated with anhydrous sodium sulfate and analyzed by gas chromatography (GC) (5,890, Agilent Technologies, USA). An SP-2560 column (100 m × 0.25 mm × 0.2 um) (Sigma-Aldrich, USA) was used with a flame ionization detector.
Texture profile analysis (TPA) of the fermented sausages was performed at 4±1℃ with a Texture Analyzer (CT3-1000, Brookfield Engineering Laboratories, Inc., USA) to measure hardness, springiness, cohesiveness, gumminess and chewiness. TPA was performed following a slight modification of the method described by Claus (1995). A 1 cm sample was cut from the fermented sausages. The cut sample was compressed to 70% of its original height with a cylindrical probe of 10 cm diameter at a compression load of 15 kg and cross-head speed of 20 cm/min. Values for hardness, springiness, cohesiveness, gumminess, and chewiness were determined as described by Bourne (1978).
Extraction of meat was conducted by the modified method introduced by Escudero et al. (2012). 5 g of meat and 20 mL of 0.01 N HCl were homogenized using a bag mixer for 8 min. The homogenized sample was centrifuged at 10,000 g for 20 min at 4℃. The centrifuged sample was filtered through a whatman No. 1 filter paper to remove impurities. Then, the extracts were stored at −80℃ until analysis. The standard compounds, namely MgSO4 (bitterness), HCl (sourness), NaCl (saltiness), and MSG (umami), were prepared to check the cross-selectivity of the sensors. All standard compounds were the same concentration (0.01 mol/L). Electronic tongue analysis was performed using an Electronic tongue machine (Taste sensing system SA 402B, Insent Intelligent Sensor Technology, Inc., Japan). The chemical sensor of E-tongue machine has various taste receptors. The sensor immersed in the extract sample, then transferred the signal to the computer. The computer depicted the E-tongue value of bitterness, sourness, saltiness and Umami.
The free amino acid composition in fermented sausages was followed as described by Henderson et al. (2000). Briefly explaining the pretreatment of sample, samples were put into the 75% ethanol and extracted for 1 h by ultrasonication, and stored 24 h at room temperature. After extraction, they were filtered by 0.2 μm filter and analyzed using Dionex Ultimate 3000 HPLC system (Dionex, USA). Used column for analysis was VDSpher 100 C18-E (4.6 mm × 150 mm, 3.5 um/VDS optilab, Germany), and mobile phase A was 40 mM sodium phosphate dibasic (pH 7), and mobile phase B was acetonitile:MeOH:distilled water=45:45:10(v/v/v). Amino acid standard (Agilent tech., USA), borate buffer (Agilent tech., USA), and OPA (ortho-phthaladehyde) reagent (Agilent tech., USA) were used as reagent for analysis.
This experiment was designed as two treatments (NP and SP) and six storage periods (0, 1, 7, 14, 21 and 30 d). The data was expressed as mean and standard error of means (SEM). ANOVA (one way analysis of variance) was performed to determine significant difference in pH and water activity during the storage periods and an independent t test of the results from proximate composition, TBARS, pH, water activity, color (CIE L*, CIE a* and CIE b*), total plate count, lactic acid bacteria, fatty acid, free amino acid compositions, texture analysis and electronic tongue was used to determine the significant difference between NP and SP after ripening process at 30 d. p<0.05 was considered significant, and a trend was noted at p<1.0.
Results and Discussion
Proximate compositions of the fermented sausages are shown in Table 2. The moisture and fat contents of SP and NP were not significantly different (p>0.05) in this study. The protein and ash contents of SP were higher than those of NP (p<0.05). Similar result for ash contents was reported by Kim et al. (2014). Researchers reported that meat products made with sulfur-fed pork or cattle had higher moisture contents and lower fat contents than meat products made with normal pork or cattle (Kim et al., 2015; Lee et al., 2009). However, there were no significant differences in moisture and fat contents in this study (p>0.05).
| Composition | NP | SP | SEM | p-value |
|---|---|---|---|---|
| Moisture (%) | 25.54 | 23.16 | 0.21 | 0.17 |
| Protein (%) | 22.59 | 23.53 | 2.34 | 0.04 |
| Fat (%) | 36.75 | 38.66 | 1.28 | 0.46 |
| Ash (%) | 4.23 | 5.20 | 0.20 | 0.01 |
| TBARS 2) | 1.94 | 0.96 | 0.05 | 0.01 |
All values are the means of three replicates.
1)NP, group, processed fermented sausage with non-sulfur fed pork; SP, group, processed fermented sausage with processed sulfur-fed pork. 2)TBARS (Thiobarbituric acid reactive substance), malondialdehyde mg/kg. 3)SEM, standard error of the means.
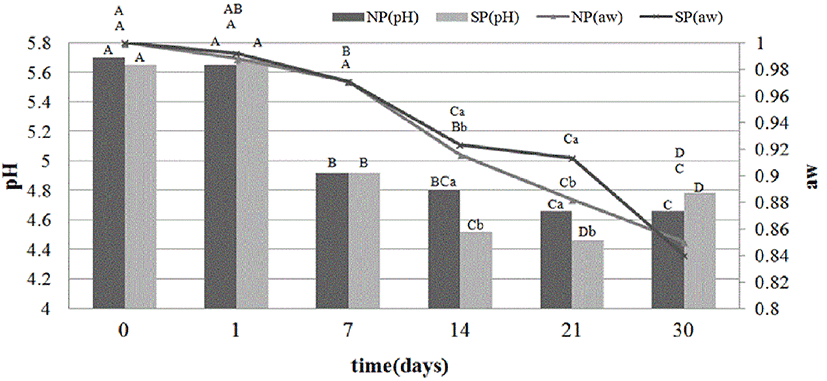
Fig. 1 shows the changes in water activity (aw) and pH during the manufacturing process. The aw in both samples decreased significantly during the dry-curing process (p<0.05). The aw of the samples showed no significant difference (p>0.05) between 0 and 7 d. However, the aw of SP was significantly higher than the aw of NP on 14 and 21 d (p<0.05). At the end of the process (30 d), there was no significant difference between NP and SP. The results on 14 and 21 d may be explained by the higher water holding capacity of processed sulfur fed pigs (Lee et al., 2009).
The pH value showed a significant decrease until 21 d in all samples (p<0.05). However, between 21 and 30 d, the pH values of the samples were slightly increased or remained stable. This pH evolution is similar to the change in other fermented sausages (Fernández-López et al., 2007). These results are because of the microbial activity of lactic acid bacteria, which metabolize sugar in meat to produce lactic acid (Fernández-López et al., 2007). The pH value of the two samples had no significant differences on 0, 1 and 7 d (p>0.05), whereas the pH value of SP was significantly lower than that of NP on 14 and 21 d (p<0.05). Generally, a high pH value in meat and meat product is due to the hydrolysis of urea and accumulation of ammonia, sulphides, indole and amines (Hernández-Jover et al., 1996). On the other hand, a low pH value in meat product can inhibit pathogenic microorganisms and decrease biogenic amines (Suzzi et al., 2003).
The TBA values of the final products are shown in Table 2. The TBA value of NP was significantly higher than that of SP (p<0.05). Komarnisky et al. (2003) reported that sulfur-containing compounds such as cysteine, glutathione and lipoic acid protect against oxidative stress through the scavenging and reduction of various oxidants in biological systems. Some researchers found that glutathione, which is an important antioxidant that prevents damage caused by free radicals, peroxides and lipid peroxides (Pompella et al., 2003), was formed from the transsulfuration pathway during the consumption of sulfur-containing feeds (Kim et al., 2015; Song et al., 2013). Song et al (2013) demonstrated that sulfur-containing antioxidants such as glutathione enzyme, methionine and taurine were increased as fed high sulfur-containing diet to pigs. Similar results showed an antioxidant effect from sulfur-fed pork applied to meat products. (Cho et al., 2015; Kim et al., 2015). Also, the higher value of TBA meat products have, rancid odor increased, the sensory quality of meat product might be negatively affected (Younathan and Watts, 1959).
Table 3 shows the color of the final products. The lightness value (CIE L*) of NP was significantly higher than that of SP (p<0.05), whereas the redness value (CIE a*) was significantly lower than that of SP at the end of the processing period (p<0.05). The yellowness value of the NP was significantly higher than that of SP (p<0.05). Lactic acid bacteria metabolize carbohydrates, generating a red color in fermented sausages (Van Schalkwyk et al., 2011). The higher redness value in SP might be explained by higher lactic acid bacteria activity at the lower pH of SP. According to Rosenstein et al. (2009), cysteine, which is a representative sulfur-containing amino acid, is an essential amino acid necessary for the growth of Staphylococcus carnosus. S. carnosus is a color fixing strain responsible for the typical red color of fermented sausages (Neubauer et al., 1999). The yellowness increased when lipid oxidation was increased during processing (Kim et al., 2015). Therefore, the lower yellowness value of SP could be related to lower lipid oxidation in fermented sausages.
All values are the means of three replicates.
1)NP, group, processed fermented sausage with manufactured with non sulfur-fed pork; SP, group, processed fermented sausage with processed sulfur-fed pork. 2)SEM, standard error of the means.
The microbial properties of fermented sausages are shown in Table 3. The total plate counts (TPC) of SP were significantly lower than those of NP (p<0.05). The lactic acid bacteria (LAB) counts of SP and NP showed no significant differences in the finished products (p>0.05). Microorganisms damage lipids and this process is usually called lipid oxidation (Kohen et al., 2002). Sahoo and Anjaneyulu (1997) proved the positive correlation between microbiology population and TBARS. Similar results of lower TPC with sulfur-fed pork were reported by Kim et al. (2014). In general, TPC can be used as a parameter for evaluating the deterioration of meat products (Ciuciu et al., 2011). Hence, these results indicate that fermented sausages processed with sulfur-fed pork can prevent spoilage by microorganisms.
The fatty acid composition of the fermented sausages is shown in Table 5. The SFA (saturated fatty acids) content of SP was lower than that of NP, while the MUFA (monounsaturated fatty acids), especially oleic acid, in SP was significantly higher than that in NP (p<0.05). However, the PUFA/SFA ratio was not significantly different between SP and NP. Yang et al (2015) demonstrated that basal diet supplementation with 0.3% processed sulfur increased MUFA in pigs. Also, children who eat foods containing lots of MUFA have healthier serum lipid profiles (Sanchez-Bayle et al., 2009). According to Lunt and Smith (1991), meat products that have more oleic acid were evaluated to have better taste and flavor in a sensory test. Cameron and Enser (1991) also reported better taste in meat when MUFA was higher and PUFA was lower. Therefore, dry-cured sausage made from 0.3% processed sulfur pigs might enhance the nutritional balance needed for health.
All values are the means of three replicates.
1)NP, group, processed fermented sausage with non sulfur-fed pork; SP, group, processed fermented sausage with processed sulfur-fed pork. 2)SEM, standard error of the means. *p<0.05
Table 4 shows the texture profile analysis (TPA) results for the fermented sausages. There were no significant differences in TPA (p>0.05) between SP and NP. Texture profile analysis (TPA) is the most commonly used technique for measuring the textual properties of foods (Herrero et al., 2008). Hardness, cohesiveness, gumminess and chewiness are related to water activity and moisture contents (Lorenzo et al., 2014). When the water activity and moisture contents of sausages decrease, the hardness, cohesiveness, gumminess and chewiness of the sausages increase.
All values are the means of three replicates.
1)NP, group, processed fermented sausage with non sulfur-fed pork; SP, group, processed fermented sausage with processed sulfur-fed pork. 2)SEM, standard error of the means.
The free amino acid composition of fermented sausages is shown in Table 6. The Glutamic acid, Asparagines, Histidine, Glycine, Arginine, Alanine, Taurine, Tyrosine, Valine, Tryptophan, Isoleucine and Leucine contents of SP were significantly higher than those of NP (p<0.05). However, the Aspartic acid, Serine, Glutamine, Threonine, GABA, Methionine, Phenylalanine and Lysine contents showed no significant differences between SP and NP. The processed sulfur supplementation positively affected on protein degradation of dry cured loin during the manufacture and free amino acid composition was considered as an indicator of sensory quality (Kim et al., 2015). The free amino acids in fermented meat have a significant role of generating flavor and taste (Zhao et al., 2016). During the ripening process for fermented sausages, proteins are degraded into large peptides, and the large peptides are degraded into oligopeptides, which are finally degraded into free amino acids (Domínguez et al., 2016). Among these amino acids, glutamic acid has a synergy effect with salt in increasing Umami taste (Nishimura and Kato, 1988).
All values are the means of three replicates.
1)1)NP, group, processed fermented sausage with non sulfur-fed pork; SP, group, processed fermented sausage with processed sulfur-fed pork. 2) SEM, standard error of the means.
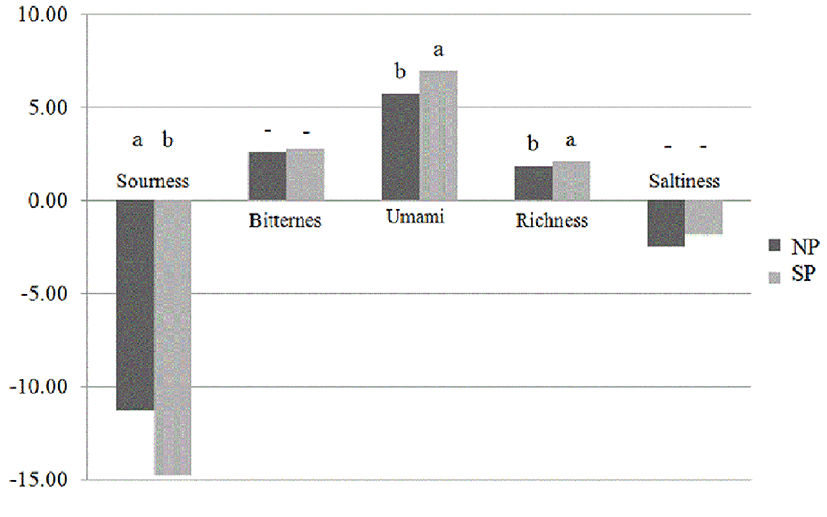
Fig. 2 shows the differences in electronic tongue values between SP and NP. Bitterness and Saltiness in the two samples had no significant differences (p>0.05) but NP had a significantly higher sourness value than SP. However, the Umami and Richness values of the SP were significantly higher than those of NP (p<0.05). Recently, instead of human sensory evolution, which is depend on daily mental and physical conditions, analytic sensory system (electronic tongue) is often used for evaluating foods taste because of objectivity (Nodake et al., 2013). Umami has a ‘savory’ or ‘delicious’ meaning derived from Japanese and important taste of fermented meat (Sentandreu et al., 2003; Zhao et al., 2016). Researchers concluded that Umami taste is related to glutamate-like taste from high amounts of glutamic acid and hydrophilic amino acid residues (Aristory and Toldra, 1995; Dang et al., 2015). Also, Kobayashi et al. (2010) reported that richness is Umami after-taste. Therefore, we could suppose the sensory values of SP were better than that of NP because of the amount of free amino acids in SP.
Conclusion
This study reports that the dry-cured sausages made from 0.3% processed sulfur-fed pork demonstrated better storage stability and meat quality than those made from normal pork. According to the result of electronic tongue analysis, umami and richness of the SP group were better than those of the NP group because the SP group had more free amino acids contents. The dry cured sausage form the 0.3% processed sulfur supplementation showed higher redness compared to the basal diet supplementation. In conclusion, the supplementation of pig feed with 0.3% processed sulfur improves oxidative stability, the nutritional quality and sensory properties of fermented sausages.