Introduction
Increasing awareness on healthy foods have led to increasing interests on natural food products and nutraceuticals such as probiotics. Probiotics have been defined as ‘living microorganisms which when administered in adequate amount confer a health benefits on the host’ (FAO/WHO 2001). Probiotic microorganisms have shown much health beneficial effects via in-vivo trials, accompanied by much promising new potentials as developed by in-vitro experiments (Ewe et al., 2010; Liong and Shah, 2005). In general, probiotics have been demonstrated to improve intestinal microbial balance, provide protection against gut pathogens and modulate immune system.
Trend for probiotics products was first observed to gain momentum in Japan in late 1980 and soon spreaded into areas such as Asia Pacific, European Union and United States (Arora et al., 2013). In present, given the greater understanding of the linkage between diet, nutrition and health, market for functional foods especially the probiotics, are rapidly expanding. The public has also been increasingly accepting alternative therapies which include probiotics, in replacing synthetic drugs.
The current probiotic market has reached $33.19 billion (2015) and expected to reach $46.55 billion by 2020, indicating a compound annual growth rate (CAGR) of 7.0%. The probiotic market was dominated by Asia Pacific, followed by Europe in 2014, attributed to the increasing demand for dietary supplements. The future probiotics market of Asia-Pacific is projected to be dominated by China, Japan and India (Markets and Markets, 2015).
Emergence of new probiotic products and needs has spurred increasing number of research groups exploring new probiotic strains and potential novel health function of probiotics (Fung et al., 2011). While consumption of probiotics is previously known to restore and stabilize the gut microbial population, ongoing researches have suggested that probiotics have potentials in preventing cancer and ageing processes (Xu et al., 2003), combating hypercholesterolemia (Choi et al., 2014; Ooi et al., 2010), and reducing risks and severity of myocardial infarction (Lam et al., 2012) via various mechanisms of decreased in-vivo toxicity and normalized gut microbiota.
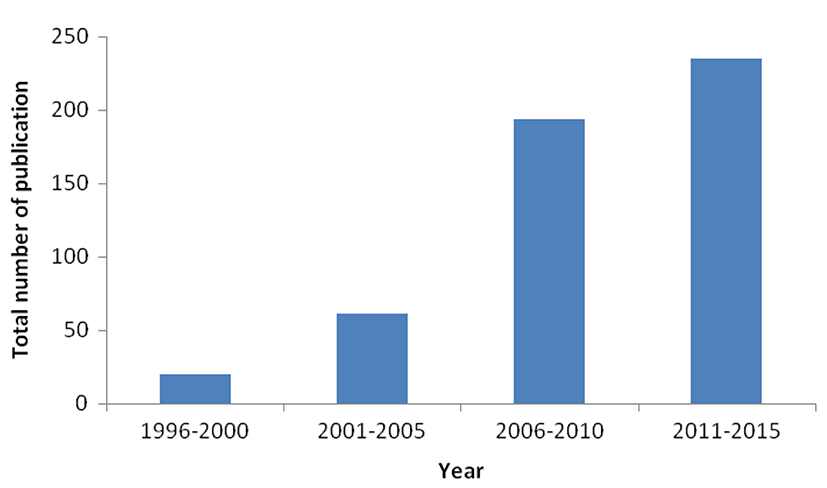
In addition to human consumption, probiotics are also increasingly applied to animals especially in poultry industries (Fig. 1), with the probiotic market for animals currently growing at a CAGR of 7.7% (Probiotics Ingredients Market, 2015). Probiotics are supplemented into animal feed such as that for ducks, broilers and chickens, cattles and in aquaculture for fishes and prawns. Genera of probiotic microorganisms commonly used for animals include Bifidobacterium, Lactococcus, Lactobacillus, Bacillus, Streptococcus and yeast such as Candida. Feeding of probiotics have been reported to have beneficial impacts on the commercial animals by enhancing weight gain, increasing feed conversion efficiency, increasing egg/milk production, lowering the incidence of disease as well as lowering mortality rates (Crittenden et al., 2005).
Antibiotics as Growth Promoters for Poultry
The use of antibiotics in broiler production was first introduced in the 1940s to enhance growth and feed efficiency as well as reduce mortality (Castanon et al., 2007). Consequently, addition of antibiotics as growth promoters in poultry feed became common practices without rigorous testing. The mechanisms of action in promoting growth induced by antibiotics is closely related to the reduction of pathogenic bacteria in the intestines (Dibner et al., 2005) However, concentration of antibiotics used for growth promotion is often lower than the concentration used for therapy and prophylaxis, and the former concentration is commonly referred as “sub-therapeutic concentration”.
Antibiotics that are provided to healthy animals as growth promoters are usually at concentrations lower than 200 g per ton of feed for more than 14 d (Graham et al., 2007)These sub-therapeutic doses of antibiotics often create a conducive condition to selection for resistance in bacteria. Association of the use of antibiotic growth promoters (AGPs) and the occurrence of resistant bacteria including Campylobacter, Salmonella, Enterococcus and Escherichia coli was well documented in previous studies. This close link has been most thoroughly studied on the connection between avoparcin and glycopeptideresistant enterococci (Van Immerseel et al., 2004).
It has been suggested that animal feed could serve as a reservoir for antibiotic-resistant bacteria that may rapidly spread across the food chain and eventually to the human. Propagation of antibiotic-resistant bacteria could spread to food products during slaughtering and processing. Past studies have shown that the presence of enterococci resistant to antibiotic in food products derived from animal fed with AGPs (McDonald et al., 2001). Furthermore, the contaminations of antibiotic-resistant bacteria and active antibiotics have also been extensively documented to spread into the environment. An eight month period investigation on soil bacteria revealed that treatment of farmland with pig manure slurry elevated the level of resistant of the bacteria to tetracycline (Sengeløv et al., 2003).
While the transmission of the antibiotic resistant bacteria to human could be attributed to the consumption of the food animals and products, a direct transmission of the bacteria could happen during the handling of the farm animals by the farm workers. High prevalence of vancomycin-resistant enterococci was reportedly to be detected in the fecal samples of healthy poultry workers at antibiotic-exposed farm (van den Bogaard et al., 1997). Antibiotic resistant properties of the bacteria could also be acquired by other distantly related microorganisms via horizontal transfer of gene material, a process in which substantial amounts of DNA are inserted into and deleted from the chromosomes (Ochman et al., 2000). As a result of the alarming risk of elevated resistance level of the bacteria towards antibiotics, the European Union (EU) has decided to ban the use of AGPs in poultry industry. This removal of antibiotics as growth promoters is soon implemented by countries such as the USA due to consumer pressure (Huyghebaert et al., 2011).
Following the phasing out of use of AGPs, impact of the termination was assessed on the aspect of animal health and productivity. The discontinuation of AGPs has been reported to led to animal performance issues and increased incidence of animal diseases such as necrotic enteritis (Wierup, 2001). Enteric disease is one of the prime concern in poultry industry after the exclusion of AGPs due to reduction in productivity, increased mortality as well as associated food products contamination (Patterson and Burkholder, 2009). Other apparent syndromes reported as consequences of termination of AGPs use are bacterial overgrowth in small intestine, increased water content in faeces, malabsorption and poor condition of the gut (Huyghebaert et al., 2011). Thus, with the ban on sub-therapeutic antibiotic usage, search for potential alternative to AGPs has gained momentum.
Application of Probiotics in Poultry Industry
Probiotic supplementation in farm industry dated back to the 1960s, although the details of selection for the poultry probiotics were often rarely provided (Santini et al., 2010). According to Kabir (2009), in order to fit the criteria as functional probiotic for poultry production, the bacteria must possess the following desirable traits: the bacteria must be a gut inhabitant, it must be able to adhere to the intestinal epithelium and withstand harsh condition such as high acidity environment in stomach and tolerance to bile salts in the intestines, and competes against other gut microorganisms for colonization in the gastrointestinal (GI) tract, as well as able to exert beneficial effects in host and maintain high viability under normal storage condition and after industrial processes such as lyophilisation. Advancement in the research technology has currently enabled more promising selection of functional probiotics as many in-vitro assays have been made available for evaluation of competitiveness of the probiotics. Further selection of the most potential probiotic for poultry industry can be enhanced by monitoring the efficacy of administration via in-vivo study using live broilers.
Poultry animals are constantly subjected to various environmental stresses. Stressful experiences happen in broilers during their adaptation to post hatching period, transportation, processing at the hatchery and high stocking densities (Burkholder et al., 2008) Also, before slaughtering, feed withdrawal are implemented on the birds in order to reduce intestinal volume, for the purpose of minimizing risk of carcass contamination following the rupture of intestinal tract during processing (May and Deaton, 1989). Fluctuation in temperature in seasonal environments has also been documented as additional stressors for poultry animals (Traub-Dargatz et al., 2006). Exposure to stressors could eventually threaten the animals’ health via weakening immune functions and predisposing the animals to enteric diseases induced by pathogenic microorganisms. Evidence of stress study on poultry animals has showed that fecal shedding of pathogens was markedly increased in the event of stress, which is one of the main factors that contribute to carcass contamination. This could subsequently pose threat to food safety and detrimental to human health. Thus, probiotic could prove to be a reliable strategy to control bacterial shedding and improve poultry animal welfare.
Benefits of Probiotics Application in Poultry Industry
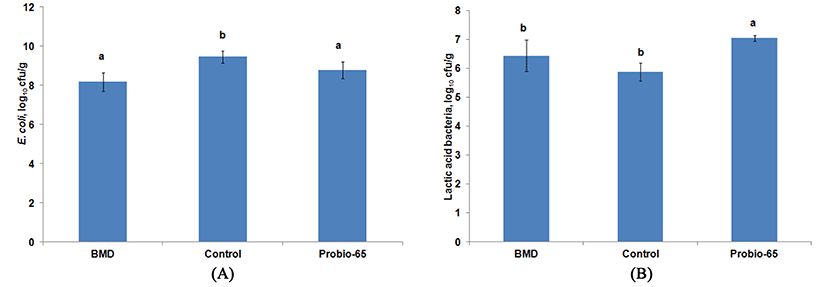
Successful probiotics colonization is essential for immunoregulatory function and inhibition of pathogenic bacteria in the gastrointestinal tract. The inhibition of pathogen by probiotics is suggested to occur via competition for adherence site on the intestinal wall and nutrient as well as production of antimicrobial compounds (Patterson and Burkholder, 2003). Adherence of probiotics consists of Lactobacillus acidophilus and Streptococcus faecium to intestinal mucosa and subsequent colonization in the gut are able to reduce colonization and shedding of Campylobacter jejuni in the intestinal tract of chicken (Morishita et al., 1997). Colonization of probiotic Lactobacillus strains have been demonstrated to have a preventive function against Salmonella enterica serovar Enteritidis infection in chicken (Van Coillie et al., 2007). Approach using probiotics against pathogenic bacteria has been shown to be effective to reduce food-borne illnesses such as campylobacteriosis and salmonellosis in consumer in view of the absence of AGPs. Besides suppressing the colonization of E. coli as efficient as antibiotic bacitracin methylene disalicylate (BMD), application of probiotic using L. sakei Probio-65 was also observed to increase the population of lactic acid bacteria (Fig. 2). Lactic acid bacteria have been widely acknowledged for its importance in exerting inhibitory and antagonistic effects against pathogenic bacteria.
Numerous studies have been documented that the probiotics can exert antimicrobial effect against pathogenic bacteria via production of metabolites such as short chain fatty acids (SCFAs) and bacteriocins. Increased concentration of butyric acid has been demonstrated to reduce Salmonella infection in poultry animals whereas elevated concentration of SCFAs as a result of probiotic Bacillus subtilis effectively reduced coliform counts while increased population of Lactobacillus in broiler chickens (Milián et al., 2013; Van Immerseel et al., 2004]. On the other hand, bacteriocins which are ribosomally synthesized peptides or proteins with antimicrobial properties have been reported to show promising growth inhibition potential against intestinal pathogenic bacteria. Bacteriocins derived from Lactobacillus salivarius exhibit strong antagonistic activity against Campylobacter jejuni and Garmpositive bacteria (Pilasombut et al., 2006). Similarly, bacteriocins produced by probiotic Escherichia coli strain have been shown to greatly reduce Salmonella contamination in poultry industry (Stern et al., 2006).
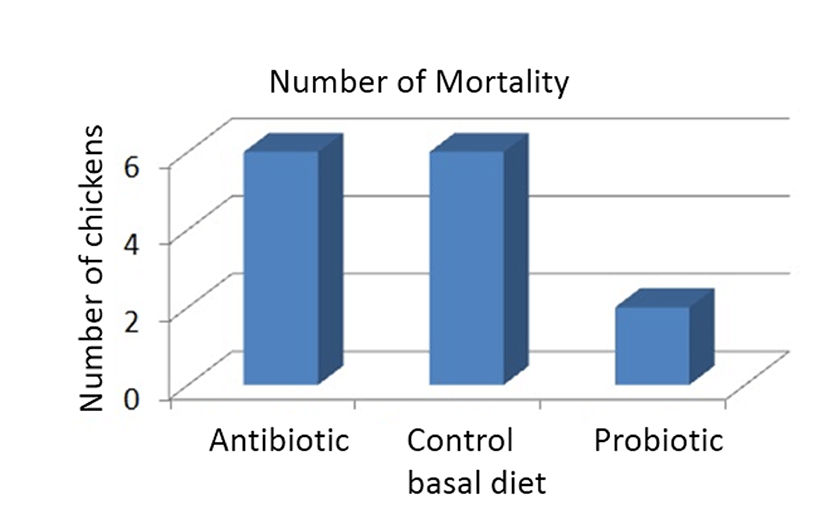
Role of probiotics in stimulation of protective immune response have been proposed to be one of the main element that help suppress growth of potential gut pathogens in poultry animals (Panda et al., 2007). Stable establishment of enteric microbiota by administration of probiotics is often associated with immunologically balanced intestinal inflammatory activity. It has been shown that failure in maintaining the balance of gut microbiota could initiate activation of the mucosal immune system and lead to chronic inflammatory response in the intestine (Haller et al., 2000). Exaggerated synthesis of pro-inflammatory cytokines such as interleukin (IL)-12 and interferon (IFN)-γ in inflamed intestine has been demonstrated to have dev astating effect contributing to intestinal tissue damage (Pallone and Monteleone, 2001). Intestinal tissue damage accompanied by challenges imposed by opportunistic pathogens often lead to aberrant changes in gut microbiota. Gut inflammation and subsequent increased abundance of pathogenic bacteria decrease the animal performance and would eventually increase mortality rate of the broilers. However, this unfavorable condition could be reversed via probiotic supplementation in broiler diet. The administration of L. sakei Probio-65 reduced mortality of broilers as compared to chickens fed with antibiotic and chickens that were fed with feed void of antibiotics or probiotics (Fig. 3).
The dynamics of probiotics related to immune responses evaluated by Kabir et al. (2009) demonstrated that antibody production was elevated in broilers fed with probiotics Lactobacillus compared to control chickens. The modulation of immune responses by probiotics is also apparently observed in broilers exposed to stress conditions. Lactobacillus-based probiotics administration was observed to ameliorate heat-stress related problems in broilers which are accompanied with improved antibody production as compared to controls (Zulkifli et al., 2000). In addition, supplementation of probiotic Lactobacillus in broilers diet revealed that probiotic could enhance intestinal immunity against coccidiosis by altering population of intestinal intraepithelial lymphocyte (IEL) expressing surface markers CD3,CD4, CD8, and αβTCR (Dalloul et al., 2003). Probiotics has also been suggested to augment Toll-like receptor (TLR) signaling in which TLR play a crucial role for activation of T-cells in the intestinal immune system. Recent study showed that probiotic product consists of Lactobacillus fermentum and Saccharomyces cerevisiae increased the level of mRNA expression of TLR-2 and 4 in the foregut of the chickens compared to those administered with control diet and antibiotic (Bai et al., 2013). Furthermore, basal diet supplemented with probiotics mixture containing Lactobacillus acidophilus, Lactobacillus casei, Enterotococcus faecium and Bifidobacterium thermophilus elevated the concentration of IgG and IgM levels in turkeys and the enhancement of the immunoglobulins level have been proposed to contribute to more positive growth performance, production and resistance of the animals towards diseases (Cetin et al., 2015).
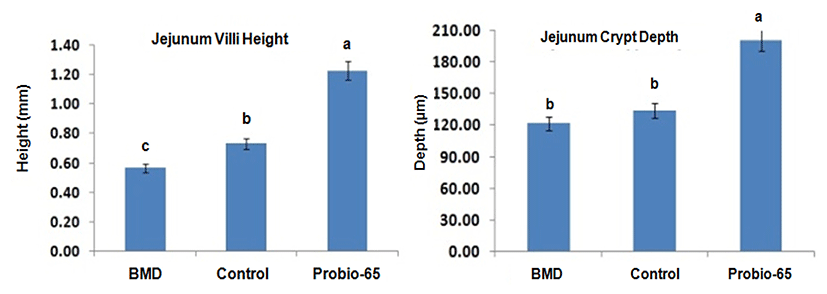
Numerous studies have been carried out to investigate the effects of probiotic administration on the histomorphology of the intestine. Dietary treatment with probiotic Lactobacillus sp. was reported to influence the villi height and crypt depth in the small intestine of broilers (Bai et al., 2013). The administration of Lactobacillus sakei Probio-65 increased villi height and crypt depth in jejunum of broilers as compared to chickens fed with antibiotic and chickens that were fed with feed void of antibiotics or probiotics (Fig. 4). Probiotics are proposed to increase the length of villi by activating cell mitosis and induce gut epithelial-cell proliferation (Samanya and Yamauchi, 2002). Increased of villi height by the probiotics is beneficial to the broilers as the increased surface area of the villi enhanced the absorption of nutrient (Caspary, 1992). On the other hand, deeper crypt depth promoted in the presence of probiotics allow higher turnover rate of villi tissue and replenish villi which may lost due to sloughing or inflammation in response to pathogen infection (Wegener, 2003). It has been suggested that alteration in villi length and crypt depth may lead to poor nutrient absorption, digestive enzymes secretion in the GI tract and eventually lower growth broilers performance (Xu et al., 2003).
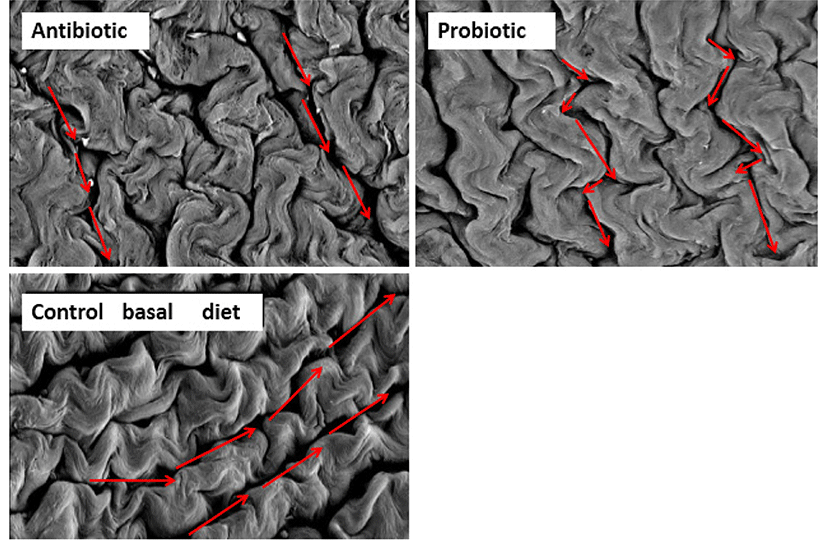
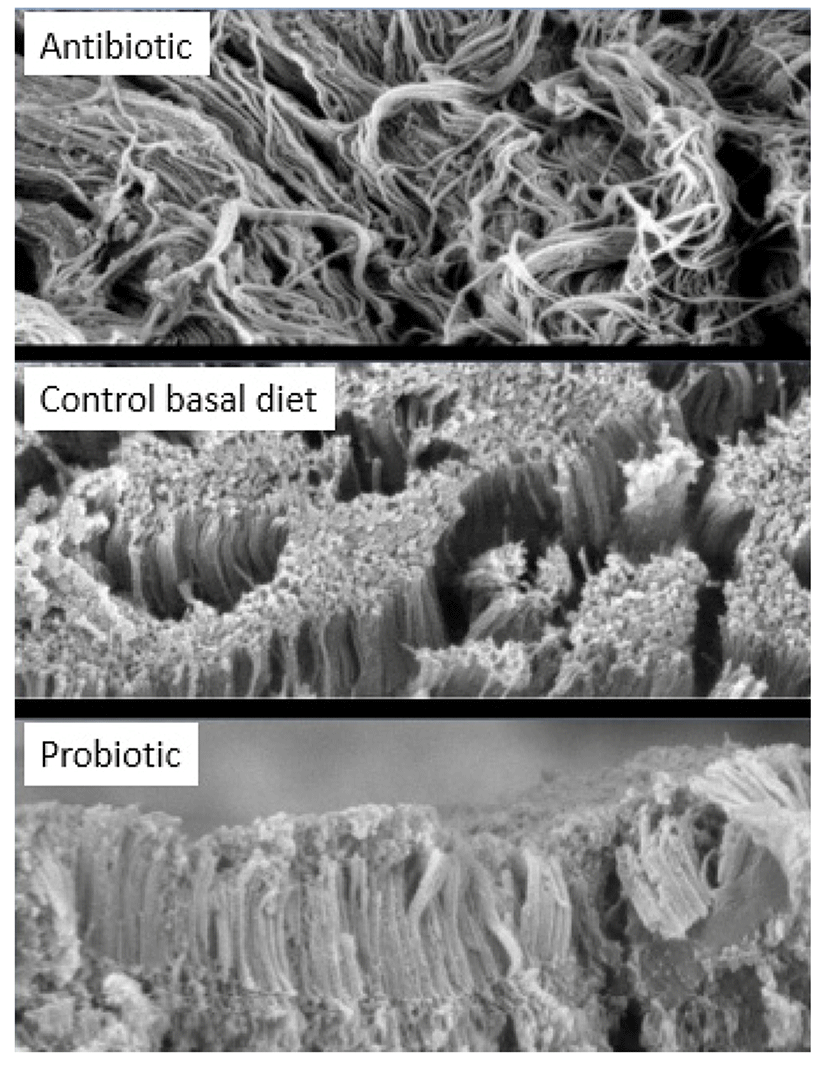
Pelicano et al. (2005) has described that villi in jejunum occur in zig-zag form, resembling wave pattern. It was suggested that the formation of villi in wave pattern enable better nutrient absorption than villi arranged in parallel or randomly positioned. Zigzag flux in the small intestine permits food to take a longer passage through the alimentary canal compared to the straight flux, and improve the contact between the nutrients and the absorption surface of the intestinal epithelium. Probiotic such as Lactobacillus sakei Probio-65 promoted waved-like arrangement of jejunum villi in broilers (Fig. 5), while this wave-like pattern was absent in the jejunum of broiler fed with antibiotic or were fed with feed void of antibiotics or probiotics. In addition, gut health of broilers was well-preserved as indicated by intact and densely packed microvilli upon administration with L. sakei Probio-65. On the other hand, the application of antibiotic led to less packed and scattered microvilli arrangement while chickens fed with feed void of antibiotics or probiotics showed normal arrangements of microvilli (Fig. 6). It has been suggested that reduced density of microvilli could compromise intestinal enterocyte integrity, leading to a reduced protective effect of the intestinal epithelium barrier (Merrifield et al., 2009).
Probiotic administration could alleviate histological changes in the intestine induced by feed contaminant such as deoxynivalenol. Deoxynivalenol is one of the richothecene mycotoxin commonly found in feedstuffs which involve in inhibition of protein synthesis that affects rapidly dividing cells such as those in GI tract (Leeson et al., 1995). Study has evidenced that probiotic feed additive is able reverse the morphology of short and thin villi which are negatively impacted by the feed contaminant (Awad et al., 2006).
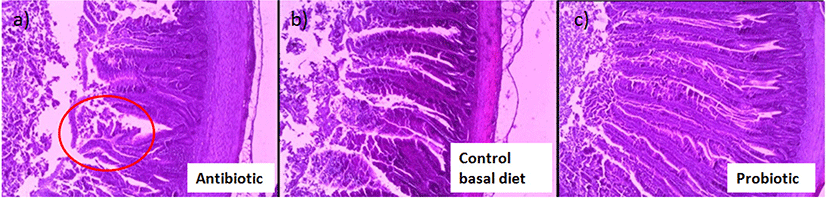
Furthermore, promotion of gut health by probiotic bacteria further strengthen the potential of probiotics as emerging alternatives to antibiotics as growth promoters in poultry industry. Gut condition was well preserved in the presence of probiotic such as Lactobacillus sakei Probio-65, accompanied by healthy development of the intestines of as compared to control broilers that were not fed with probiotics. Unlike probiotics, antibiotic damaged jejunal villi tip with prevalent shedding at the end of the villi tips (red circle; Fig. 7). Injuries of the intestinal walls have been much reported upon the administration of antibiotics, and are very often accompanied with thinning of the intestinal mucus layer and increased depletion of goblet cells (Wlodarska et al., 2011).
Effects of dietary supplementation of probiotics on growth performance of poultry animals have been extensively investigated. Most studies indicated that probiotics displayed great efficacy in promoting animal growth. Dietary inclusion of probiotics Lactobacillus has been reported to increase body weights and feed to gain ratio when compared to control broilers (Jin et al., 1998). Lactobacillus inclusion in broilers nutrition also resulted in higher broiler productivity index which is measured based on daily weight gain, feed efficiency, and mortality (Timmerman et al., 2006). While growth rates of the broilers are improved, the Lactobacillus administration reduced the mortality of the broilers which usually arised from pathogen infections. Moreover, probiotics supplementation to diet improved feed intake, feed efficiency, and carcass yield of broilers (Denli et al., 2003).
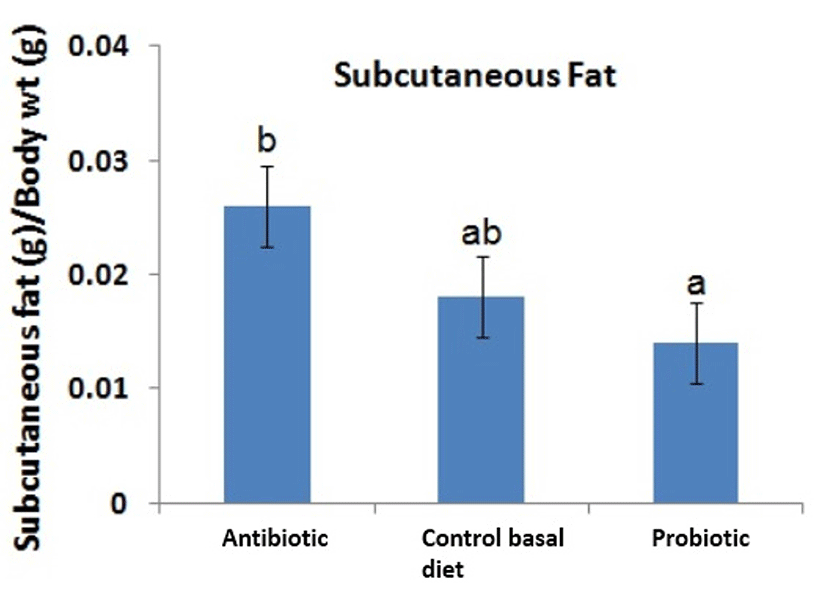
Recent evaluation of effects of probiotic supplementation on digestive enzymes activity in broiler chickens revealed that the probiotic Bacillus coagulans NJ0516 promotes higher activity of protease and amylase compared with controls. This finding suggests that the higher activity of the enzymes may lead to better digestibility of protein and starch, which in turn explains better growth in broilers fed with probiotics rather than control basal diet (Wang and Gu, 2010). On the other hand, dietary supplementation of probiotic Lactobacillus sporogenes lowered serum level of total cholesterol, low-density lipoprotein (LDL) cholesterol, very low-density lipoprotein (VLDL) cholesterol and triglycerides (Panda et al., 2006). Hypolipidaemic effect of probiotics on broilers was similarly reported by Kalavathy et al. (2003) where abdominal fat deposition was reduced by mixture of 12 probiotic Lactobacillus strains compared to control diet. The amount of subcutaneous fat beneath the skin of broiler chickens was lower in chickens fed with diet added with probiotic Lactobacillus sakei Probio-65 and higher in chickens fed with antibiotic BMD (Fig. 8). Such marked differences have been reported to be attributed to the roles of antibiotics that promote adiposity via disruption on gut microbiota and energy balance (Liou and Turnbaugh, 2012).
There is widespread agreement that probiotics supplementation could improve meat quality of broilers. Intramuscular lipid content is involved in determining meat quality particularly nutrition, tenderness, odor, tastes and flavor characteristics. Endo and Nakano (1999) reported a greater tendency of higher ratio of unsaturated fatty acids to saturated fatty acids in pectoral and thigh meat of broilers fed with probiotics-supplemented diet containing Bacillus, Lactobacillus, Streptococcus, Clostridium, Saccharomyces and Candida. The results suggested that the fat in meat was converted into favorable fat in the presence of probiotics, which in turn contributed to smoother meat texture. Improved tenderness which was indicated by decreased shear force was reported by Yang et al. (2010) when probiotic C. butyricum was added in diet of broiler. The decreased in shear force observed in the study was positively correlated with increased muscular fat content. The same study also demonstrated that probiotic inclusion in broiler diet modulated fatty acid composition in breast meat of broiler by increasing omega-3 fatty acids concentration especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) while contents of omega-3 fatty acids in meat of control broilers remain relatively low.
Sensory assessment on chicken meatballs conducted by Mahajan et al. (2000) revealed that overall organoleptic scores in terms of appearance, texture, juiciness and overall acceptability were higher in probiotic Lactobacillus fed broilers than counterparts fed with traditional basal diet. Evaluation of effects of probiotic on skin color of broiler elucidated that probiotic Lactobacillus salivarius could increased xanthophyll accumulation in tissue, thus improving the visual appearance of meat products (Zhu et al., 2009). Meat in broilers fed with probiotics Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Aspergillus oryzae, Streptococcus faecium and Torulopsis sp displayed higher content of moisture, protein and ash compared to the control (Khaksefidi and Rahimi, 2005). The results indicated that chicken fed with probiotics has better retention of minerals especially phosphorus, calcium and nitrogen as well as protein efficiency ratio. Higher protein efficiency ratio may subsequently help promote meat yield as observed by Hossain et al. (2012) where addition of probiotics increased breast meat absolute and relative weight. Besides, carcass quality of broilers was also reported to be improved by probiotics with lesser occurrence of Salmonella contamination.
Conclusions
Biotechnology plays an essential role in the development of effective poultry feed. Feed composition is crucial to promote growth and maintain health of broilers. A great amount of research has provided strong emphases that maximizing feed utilization could determine the functionality of feed. A well-balanced diet sufficient in nutrient and energy is also of significant importance to maintain gut in healthy state. In view of this, the concept of probiotics as feed additives has garnered much attention and support. Significant work and studies have increasingly demonstrated that probiotics provide means to a balanced gut microbiota in poultry, maintaining health status in broilers, preserving gut condition and improving immune system as well as enhancing nutrient absorption, which are all crucial and needed to promote growth of broilers.













