Introduction
Constipation is one of the most common gastrointestinal disorders and is associated with bowel obstruction, insufficient bowel movements, and the feeling of incomplete evacuation (Filipović et al., 2017). It is influenced by changes in diet and psychological and social factors, often leading to chronic gastrointestinal issues (Crowell et al., 2007). There is increasing interest in improving gastrointestinal disorders and alleviating fecal problems with probiotics (Jung et al., 2022; Yu et al., 2017; Zhao et al., 2015), such as Bacillus and Lactobacillus, as they have shown beneficial effects in improving intestinal health, including constipation, bowel movements, and intestinal barrier function (Rhayat et al., 2019; Zhang et al., 2024).
Probiotics are live microorganisms that confer health benefits on the host when administered in adequate amounts (Hill et al., 2014). Probiotics have been shown to have potential utility as a treatment for constipation with fewer side effects than chemical drugs. The existing research results indicate that probiotics can affect intestinal motility by altering the levels of neurotransmitters and short-chain fatty acids (SCFAs), and by regulating the gut microbiota and immunity (Dimidi et al., 2017). Numerous trials of probiotic supplementation have been conducted in animals and humans to test the efficacy of probiotics against constipation (Chen et al., 2020, Qiu et al., 2022).
BIOVITA 3 bacterial species (BIOVITA 3) is a freeze-dried probiotic blend powder that contains three spore-forming bacteria (Clostridium butyricum IDCC 1301, Weizmannia coagulans IDCC 1201, and Bacillus subtilis IDCC 1101). It was developed in 1959 and was the probiotic nutritional supplement in Korea used to improve the function of children’s intestines. BIOVITA 3 have different oxygen requirements, and consequently are known to proliferate throughout the gastrointestinal tract, from the small intestine to the large intestine, and exert positive effects on intestinal function (Kim, 2021). A recent in vivo study found that C. butyricum IDCC 1301 improved high fat diet-induced colonic inflammation (Choi et al., 2023). B. subtilis IDCC 1101 and W. coagulans IDCC 1201 have been evaluated as safe probiotics for human use (Bang et al., 2021; Kim et al., 2022). Although the gut health benefits of their microbial components are expected, no studies have reported on the constipation improvement of blend probiotics BIOVITA 3.
The purpose of this study therefore was to investigate the effects of BIOVITA 3 bacterial species on loperamide-induced constipation. Body weight, fecal parameters (fecal number and water content), gastrointestinal transit (GIT) ratio, and histopathological analyses were conducted, and changes in fecal SCFAs and microbial communities were observed.
Materials and Methods
B. subtills IDCC 1101, W. coagulans (formerly B. coagulans, commercially L. sporogenes) IDCC 1201, and C. butyricum IDCC 1301 were obtained from Ildong Bioscience (Pyeongtaek, Korea). BIOVITA 3 was manufactured by mixing the three probiotics powder listed above (B. subtills IDCC 1101, W. coagulans IDCC 1201, and C. butyricum IDCC 1301) while according to the in-house manual of Ildong Bioscience (Lot. No. ID-R0301).
Male Sprague-Dawley (SD) rats (6 weeks old) were purchased from Orientbio (Seongnam, Korea), and housed two per cage. Animals were kept at a temperature of 22±3°C and relative humidity of 30%–70% and were given free access to tap water and a regular rodent diet (Envigo, Indianapolis, IN, USA). Rats were acclimated for seven days prior to use and in vivo experiments were conducted in accordance with the Efficacy Evaluation Center at Dt&CRO (220112, 2022.04.28). Loperamide hydrochloride (L0154) was purchased from Tokyo Chemical Industry (Tokyo, Japan). Phosphate-buffered saline (PBS, 10010023) was purchased from Gibco (Grand Island, NY, USA). Dried starch was provided by Ildong Bioscience.
Rats were randomly divided into six groups (n=10 per group): G1 (non-constipated group; Normal), G2 (loperamide-induced constipation group; Constipation), G3 (B. subtilis IDCC 1101), G4 (W. coagulans IDCC 1201), G5 (C. butyricum IDCC 1301), and G6 (BIOVITA 3). During the administration period, constipation was induced by the oral administration of loperamide (4 mg/kg) for seven days in rats in all groups except for G1. Starch (probiotic powder excipient) or probiotic powder was orally administered to the rats in each group for seven days. Rats in the G1 and G2 groups were orally administered 0.2 g of starch. Rats in the test groups were orally administered probiotic powder at 1.0×109 CFU/day. All administered samples were dissolved in PBS before use. Body weights and food and water intake were recorded daily. All rats were euthanized at the end of the administration period, and fecal and colon tissue samples were collected for subsequent analysis.
Fecal pellets in cages were collected on the sixth day of administration. The collected feces were counted and measured as the fecal pellet weight. After drying in the oven at 60°C for 24 h, the feces were weighed. The water content (%) in fecal samples was calculated as follows: (initial weight of feces – dried weight of feces) / initial weight of feces × 100. All feces were stored at –80°C for subsequent analysis.
The intestinal transit ratio was measured by a modification of the method of Xu et al. (2012). Rats were fasted for 18 h on the last day of treatment and administered loperamide and the test substances. Then, a charcoal meal (10% charcoal powder) and 5% arabic gum were immediately administered. All rats were fasted and denied water for 30 min, euthanized by CO2 inhalation, and their intestines were excised. Total intestinal length and distance traveled by the charcoal meal were measured. The intestinal transit ratio (%) was calculated as the transit distance of the charcoal meal / total length of the intestinal tract × 100.
Colon tissues were fixed in 10% formaldehyde, dehydrated, and embedded in paraffin. Then, 5 μm sections were prepared and stained with hematoxylin and eosin (H&E). Pathological changes were observed using an E600 (Nikon, Tokyo, Japan) research microscope.
SCFAs were quantitated with a high-performance liquid chromatography-ultraviolet (HPLC-UV) system according to the method of Leite et al. (2023). Feces (1 g) were extracted with 8 mL of distilled water by vortexing for 15 min. The extracted samples were centrifuged for 15 min, 8,640×g at 4°C. The supernatant was filtered through a 0.45 μm Millipore filter (Whatman, Kent, UK), and the filtrate was injected by HPLC (Alliance e2695; Waters, Milford, MA, USA) equipped with a UV detector (2998; Waters) and a COREGEL 87H3 C18 column (7.8 mm×300 mm×5 μm; Concise Separations, Seattle, WA, USA). The column temperature was set at 35°C, and the sample tray temperature was set at 4°C. The mobile phase was 5 nM H2SO4 in water and the flow rate was 0.6 mL/min. The injection volume was 10 μL and the detection wavelength was 210 nm. The concentrations of acetic, propionic, and butyric acid were determined by constructing calibration curves using the respective standard reagents.
16S rRNA gene sequencing was performed to analyze the microbiome in SD rats with loperamide-induced constipation. DNA was extracted using a PowerSoil® DNA Isolation Kit (Cat. No. 12888, MO BIO, Carlsbad, CA, USA) according to the manufacturer’s instructions. Each sample was prepared according to Illumina Sequencing Library protocols. Extracted DNA was amplified with V3–V4 region and sequenced using MiSeqTM (Illumina, San Diego, CA, USA) by Macrogen (Seoul, Korea). The microbiome was investigated using the 16S rRNA gene-based Microbial Taxonomic Profiling platform of EzBioCloud Apps (ChunLab, Seoul, Korea). ChunLab’s 16S rRNA database (DB ver. PKSSU4.0) was used for the taxonomic assignment of 16S rRNAamplicon reads (Yoon et al., 2017). Alpha diversity and beta diversity were subsequently analyzed. Alpha diversity was evaluated by ACE, Chao1, Shannon, and Simpson indices. Beta diversity was investigated by generalized UriFrac, using principal coordinates analysis (PCoA) to analyze the differences between samples. Linear discriminant analysis (LDA) effect size was analyzed using the linear discriminant analysis effect size (LEfSe) algorithm to identify the bacterial biomarkers differentially represented between the groups at different taxonomic levels.
The results were analyzed using the Statistical Package for Social Sciences version 27.0 (SPSS, Chicago, IL, USA) and are presented as the mean±standard deviation. An analysis of variance (ANOVA) test was used to determine differences among samples at a significance level of p<0.05. Microbial taxonomic abundance and gut microbiota diversity were compared using the Wilcoxon rank-sum test in the EzBioCloud Apps platform (ChunLab).
Results and Discussion
No significant differences in body weights (p>0.05) were seen among the groups (Fig. 1a). Body weights increased from 247.6–250.8 g on day 1 to 282.2–289.3 g on day 6 of the administration period. The body weight gain, food intake, and FER of group G1–G6 are shown in Table 1. There was no significant difference (p>0.05) in body weight gain among all groups (G1–G6). There was no significant difference (p>0.05) in food intake among all groups except G6. Following the induction of constipation, the food intake of the G6 group (21.3 g/day) was significantly lower (p<0.05) than that of the G1 group (24.6 g/day). However, the FER of the G6 was not significantly different from those of the other groups (G1–G5). The FER for all groups (G1–G6) ranged from 0.29 to 0.33, with no significant difference in FER among groups.
1) G1, normal group; G2, loperamide-induced constipation group; G3, Bacillussubtilis IDCC 1101 and loperamide-induced constipation group; G4, Weizmannia coagulans IDCC 1201 and loperamide-induced constipation group; G5, Clostridium butyricum IDCC 1301 and loperamide-induced constipation group; G6, BIOVITA 3 and loperamide-induced constipation group<
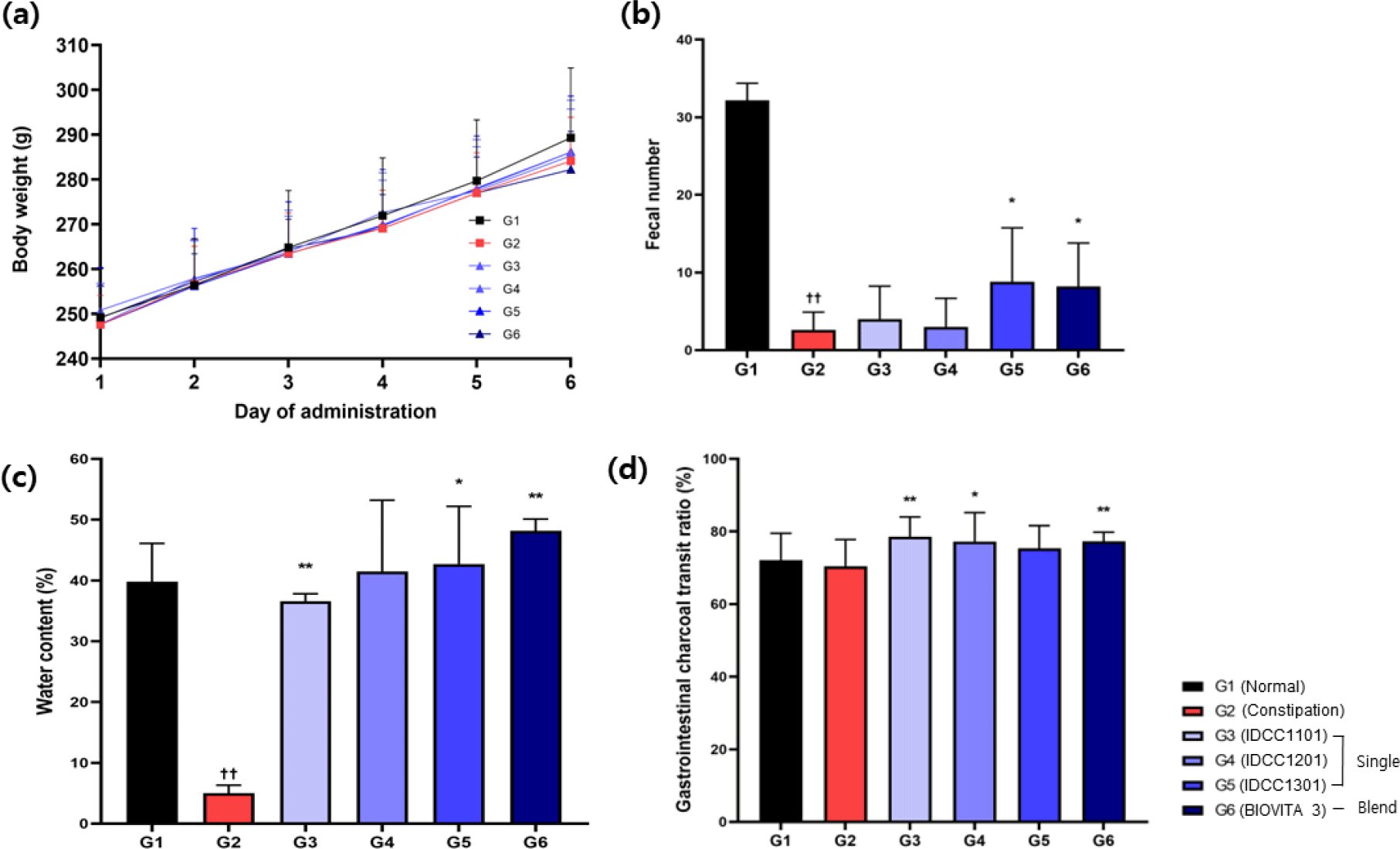
The fecal number and water content of feces were measured to investigate the fecal parameters of rats with loperamide-induced constipation treated with the three IDCC strains and BIOVITA 3. The average numbers of fecal pellets per cage were 32.3, 2.6, 4.0, 3.0, 8.8, and 8.2 in G1–G6 groups, respectively (Fig. 1b). A statistically significant decrease (p<0.01) in fecal number was seen in the G2 with constipation compared to G1. Statistically significant increases (p<0.01) in the fecal number were seen in the G5 and G6 groups compared to G2. The water content in each group (G1–G6) was 39.8%, 29.7%, 36.6%, 41.5%, 42.7%, and 48.2%, respectively (Fig. 1c). A significant reduction was seen in the G2 group (p<0.01) compared to the G1 group. However, there were statistically significant increases in the G3 (p<0.05), G5 (p<0.05), and G6 (p<0.01) groups compared to G2. Therefore, the results of the G2 group confirmed that loperamide-induced constipation in SD rats, and that the IDCC strains and BIOVITA 3 had significant effects on fecal number and water content in constipated groups.
Intestinal transit times reflect intestinal motility, which can be assessed by the transit speed of the digestive tract (Inatomi and Honma, 2021; Kim et al., 2017). The intestinal charcoal transit ratio is important in the diagnosis of constipation and for the G1–G6 groups were 72.1%, 70.4%, 78.6%, 77.2%, 75.4%, and 77.3%, respectively, as shown in Fig. 1d. The transit ratio in G2 decreased by 1.7% compared to G1, but the differences between the two groups were not significant (p>0.05). There was a statistically significant increase in probiotic-treated groups G3 (p<0.05), G5 (p<0.01), and G6 (p<0.01) compared to G2.
To confirm that probiotics could alleviate the histopathological alterations in constipated SD rats, H&E staining was performed to observe the morphology of the colon. As shown in Fig. 2b, the mucosal layer length of G2 (272.0 μm) was significantly lower than that of G1 (322.4 μm), and all groups treated with probiotic powders showed increased lengths, compared to G2. In particular, there was a significant difference in the G6 (p<0.05) compared to the G2. In terms of the muscle layer, the G2 (156.4 μm) was significantly shorter than that of the G1 (207.2 μm; Fig. 2c). G3 (183.2 μm), G4 (207.4 μm), G5 (184.6 μm), and G6 (162.6 μm) showed increased lengths compared to G2. Among them, G4 was significantly different (p<0.01) compared to G2. The results of this study were consistent with previous studies in which the thickness of the mucosal layer of colonic tissue decreased in the constipation-induced group compared to the normal group, while it increased in the probiotic-treated group (Kim et al., 2015; Kim et al., 2017). The mucosal layer and muscle layer of colon tissue play important roles in both maintaining intestinal functions (intestinal motility, absorption and secretion, immune function, etc.) and creating a healthy gut environment. These results showed that G4 and G6 significantly improved the muscle layer and mucosal layer, respectively. Moreover, when leukocyte infiltration was observed in colon tissue, the number of animals observed was reduced in both G4 (n=2) and G6 (n=1) compared to G2 (n=4), while the frequency of inflammation was reduced in BIOVITA 3 (G6), thus indicating an improvement over G4. Consequently, most of the constipation improvement scores showed significant improvements with the G6.
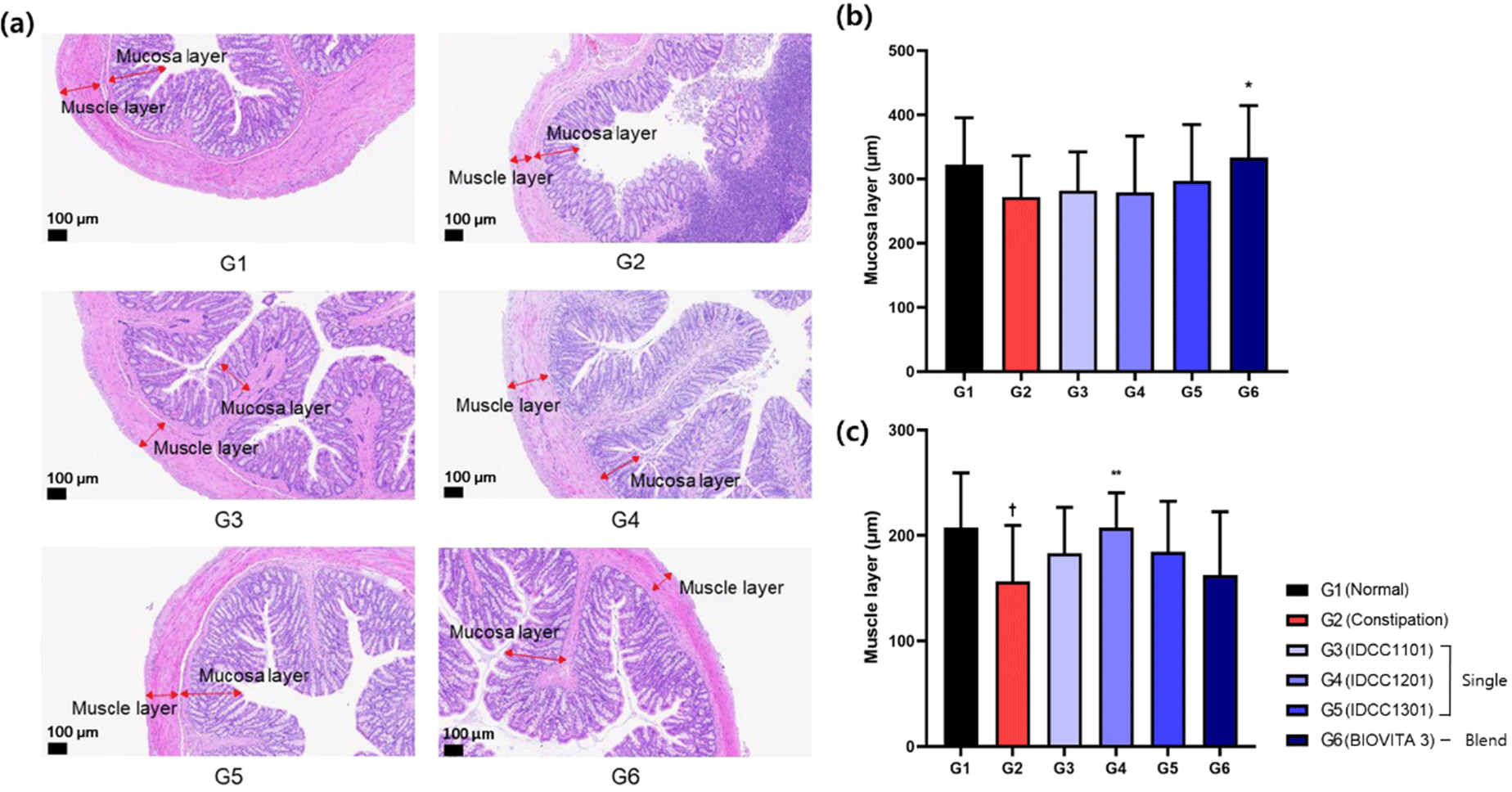
Several studies have reported improvements in constipation by the oral administration of probiotics to loperamide-induced SD rats. In this model, the fecal number, water content, and GIT ratio decreased in the constipation-induced group compared to the normal group, while these constipation indicators increased in the probiotic-treated group, thus alleviating constipation symptoms (Inatomi and Honma, 2021; Jung et al., 2022; Kim et al., 2015). Furthermore, several studies have compared and analyzed the improvement in constipation by treatment with single and mixed probiotic strains, and these also demonstrated that mixed strains were more effective in improving constipation (Cheng et al., 2023; Kim et al., 2017). These previous research findings are consistent with our results, in which we found that BIOVITA 3 (mixed strains) had a therapeutic effect in alleviating constipation.
SCFAs are primary metabolites that are produced by gut microbiota or probiotics, which are mainly composed of acetic acid, propionic acid, and butyric acid (Shi et al., 2016). The concentration of SCFAs in the fecal samples were analyzed by HPLC-UV. Acetic, propionic, butyric acid, and total SCFAs content in the G2 were reduced compared to the G1, confirming the induction of constipation (Table 2). The total SCFAs content in G2 (1,638.18 ppm) was significantly lower than that in G1 (2,690.05 ppm). The three IDCC groups (G3–G5) and the BIOVITA 3 group (G6) all showed increased acetic acid, propionic acid, and total SCFAs compared to the G2 group. The total SCFAs content of G4 (3,673.67 ppm) and G6 (3,893.55 ppm) was significantly increased compared to G2 (p<0.05) and was highest in G6. G5 showed an increase in butyric acid compared to G2, which is considered to be the effect of C. butyricum IDCC 1301, which has the ability to produce butyric acid. These results are consistent with previous studies that reported increased SCFAs when probiotics were administered in loperamide-induced constipation (Cheng et al., 2023; Kim et al., 2015; Kim et al., 2017).
1) G1, normal group; G2, loperamide-induced constipation group; G3, Bacillus subtilis IDCC 1101 and loperamide-induced constipation group; G4, Weizmannia coagulans IDCC 1201 and loperamide-induced constipation group; G5, Clostridium butyricum IDCC 1301 and loperamide-induced constipation group; G6, BIOVITA 3 and loperamide-induced constipation group.
The three SCFAs play an important role in human health with physiological activities ranging from immune system regulation to metabolic pathway modulation (Xiong et al., 2022). Increased SCFAs in the colon have been correlated with a reduced risk of certain conditions including constipation and cancer (Hooper et al., 2002), and the content of fecal SCFAs was reported to be significantly lower in constipated patients than in healthy individuals (Shi et al., 2016). SCFAs are anions in the colon that promote the absorption of sodium and water, promote the proliferation of colonic epithelial cells and mucosal growth, and act as important nutrients (Fukunaga et al., 2003; Ruppin et al., 1980). Acetic acid and butyric acid released in the colon have been shown to increase water content and GIT rate, and to alleviate the symptoms of constipation in a microbial-dependent manner (Wang et al., 2020). Therefore, an increase in total SCFAs is associated with constipation relief. We consider it to be the case that BIOVITA 3 and its component strain administered in this study increased total SCFAs in loperamide-induced constipated rats, thereby improving fecal water content and GIT, and ultimately relieving constipation.
However, the profile of fecal SCFAs in this study exhibited some differences from previous studies, with the content of propionate being higher than acetate. Acetate was predominant on the second day of administration (data not shown), but propionate was predominant on the sixth day. According to Annison et al. (2003) “SCFAs can be sensitive to changes in substrate supply,” and the ratio of acetate, propionate, and butyrate was shown to vary depending on the type of starch. We conclude that the proportion of SCFAs changed because of the effect of the test substances during the administration period.
At the phylum level, Firmicutes and Bacteroides were the major gut microbiota in SD rats (Fig. 3a). At the family level, Porphyromonadacea was only found in rats in the G6 group (Fig. 3b). We primarily found increases in the genus Parabacteroides. These bacteria were recently reported to enhance the physiological properties of carbohydrate metabolism and the secretion of SCFAs (Cui et al., 2022; Wang et al., 2019). Enterobacteriaceae appear similar in G1 and G6 but not in G2.
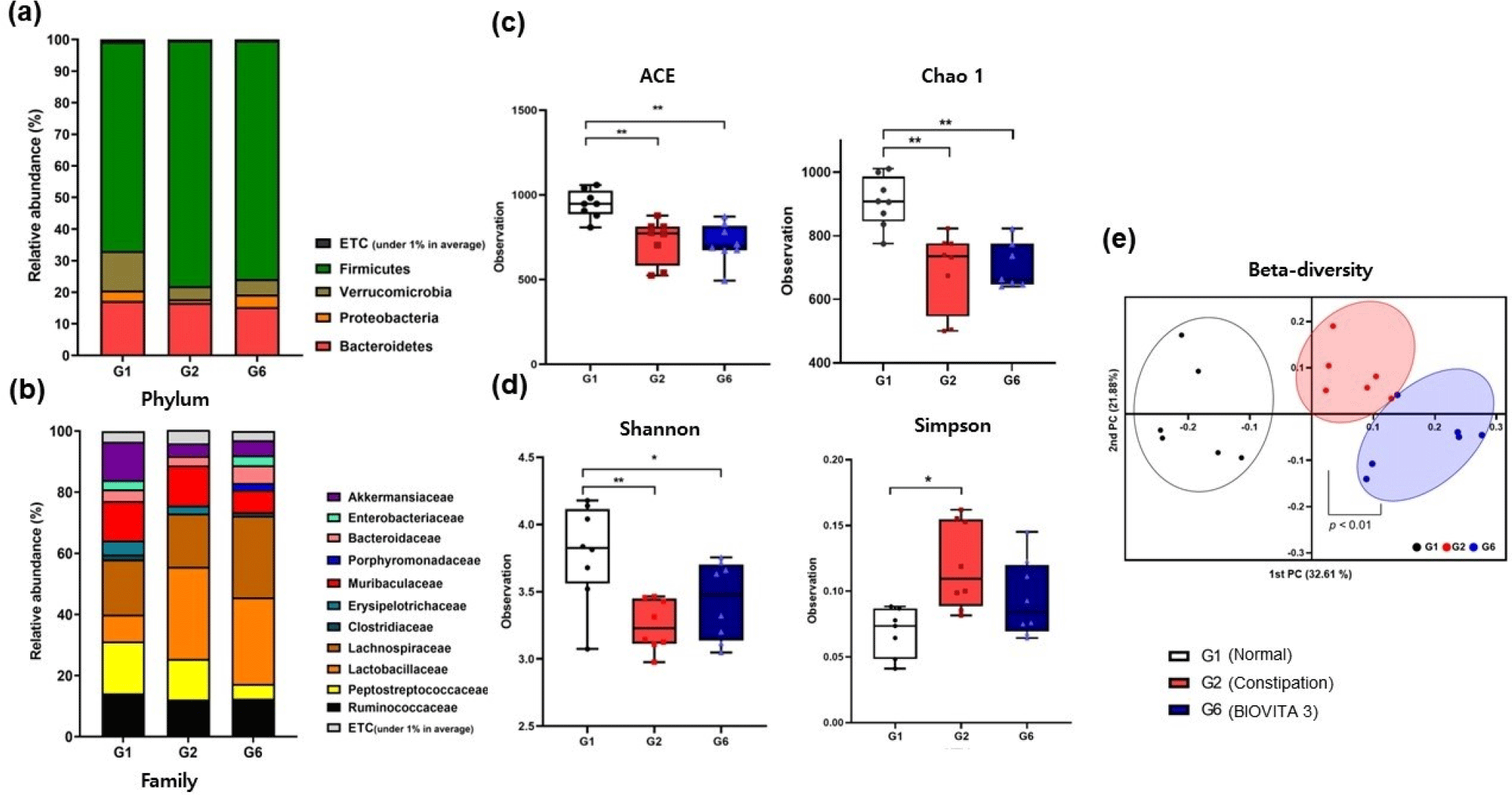
The alpha diversity index was analyzed by species richness (ACE and Chao 1) and community diversity indices (Shannon and Simpson). There was a significant difference in the ACE and Chao1 indices between the G1 group and loperamide-induced constipated groups (G2 and G6), but there were no significant differences between the G2 and G6 groups (Fig. 3c). The Shannon index, which is an indicator of microbiota diversity, was significantly different between the loperamide-induced constipated groups (G2 and G6) and the G1 group. An increased Shannon index was seen in G6 compared to G2, but the difference was not significant (Fig. 3d). The Simpson index showed a contrasting trend with the Shannon index, indicating increased microbial diversity. The PCoA of beta diversity at the genus level showed that the three groups (n=6) were divided into PC1 (32.61%) and PC2 (21.88%; Fig. 3e). There was a significant difference between G2 and G6 (p<0.01).
This study utilized the LEfSe algorithm to identify core gut microbiota for constipation responses to probiotic treatment. Fecal samples from the G1, G2, and G6 groups were analyzed at the genus and species levels (Fig. 4). The results showed that the loperamide-induced constipation samples (G2) had a higher abundance of Lactobacillus and a lower abundance of Romboutsia, Turicibacter, and Pseudoflavonifractor than the normal samples in the G1 group. Additionally, Ruminococcus and Fusicatenibacter were identified as core gut microbiota for treatment responses in the G6 group, whereas Lactobacillus was identified as core gut microbiota in the G2 group. Furthermore, EU622720_s, Lactobacillus reuteri group, Blautia faecis, and Fusicatenibacter saccharivorans were identified as core gut microbiota in the G6 group, whereas FJ880321_s and Lactobacillus intestinalis were identified as core gut microbiota in the G2 group.
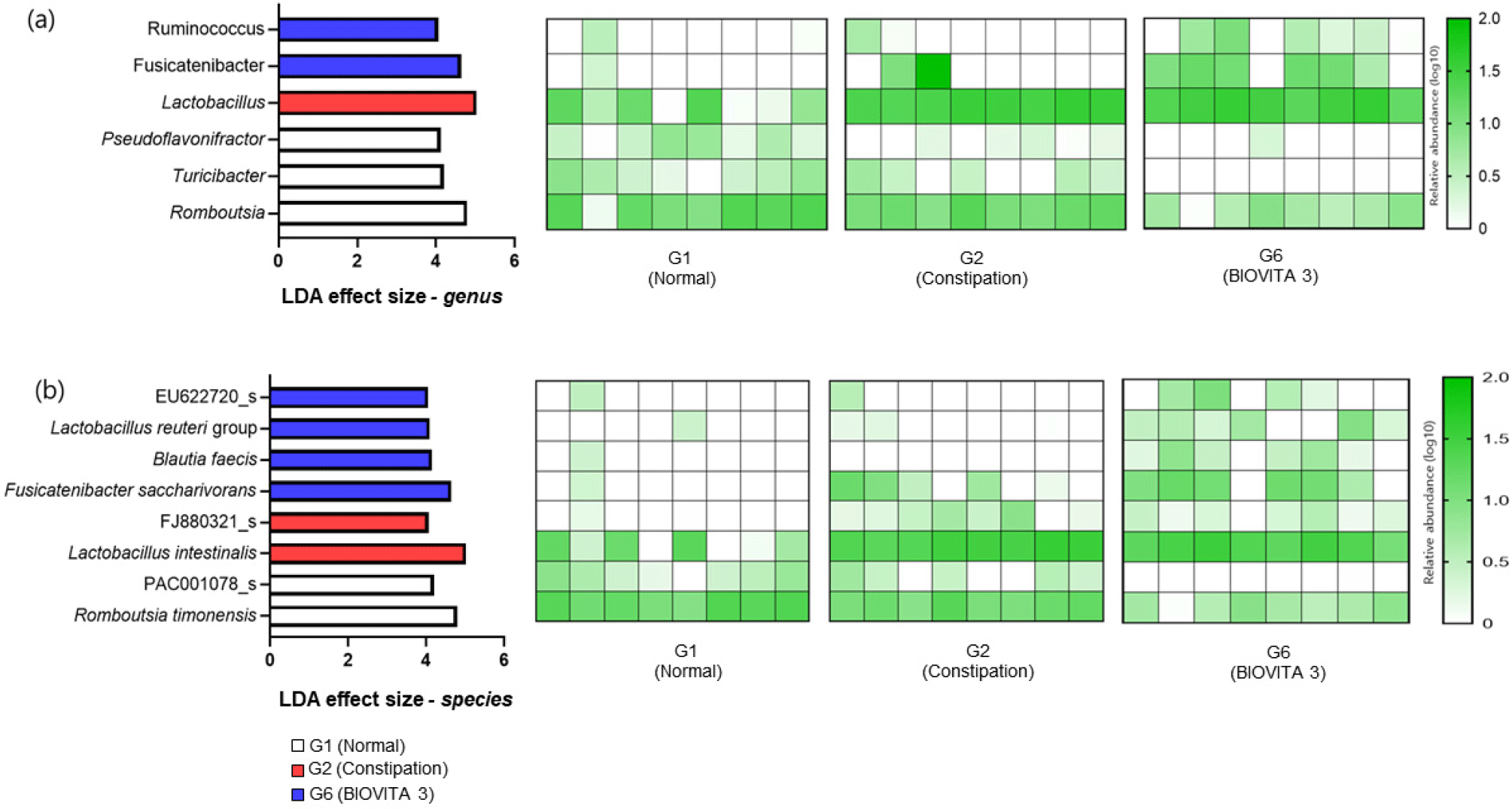
Ruminococcus and Fusicatenibacter are fiber-degrading bacteria that produce SCFAs as a byproduct of fiber fermentation (Belenguer et al., 2007). The baterial-derived SCFAs are known to play a role in promoting healthy bowel movements by stimulating the growth of beneficial bacteria, reducing inflammation, and improving gut motility (den Besten et al., 2013). Limosilactobacillus reuteri is a bacterium that produces luterin, an antibacterial substance that has antibacterial activity against pathogenic microorganisms (Kim et al., 2015). Recently, Blautia facis has been found to be a acetic acid-producing bacterium belonging to the genus Blautia that has attracted substantial attention as a gut microbiota that brings about potential health benefits such as the prevention of respiratory infections and anti-inflammatory effects (Liu et al., 2021; Verstraeten et al., 2022). Therefore, the increase in these beneficial microbes (Ruminococcus, Fusicatenibacter, L. reuteri, B. facis) in the BIOVITA 3 group might have indirectly contributed to an improved gut environment, in addition to increased gut motility due to SCFA production. This also indicates that an increase in these genera may be a response to increased dietary fiber intake, which in turn, promotes healthy bowel movements. Further research will be needed to confirm these hypotheses.
Conclusion
The effect of BIOVITA 3 on loperamide-induced constipation was investigated in SD rats by analyzing the fecal characteristics (fecal number, water content), intestinal transit time, colonic morphology, SCFAs production, and gut microbiota.
The primary results of this study were as follows: First, this study confirmed the loperamide-induced constipation model. Second, single or blend probiotics were administered orally, and the blend probiotic BIOVITA 3 effectively improved constipation symptoms, significantly increasing fecal numbers, the fecal water content, GIT ratio, and the mucosal layer in colon. Third, BIOVITA 3 had a beneficial effect on alleviating constipation by altering the gut microbiome and increasing total SCFAs, which play an important role in promoting bowel regularity, maintaining intestinal barrier integrity, and regulating immune function.
In this study, BIOVITA 3 showed better effects in relieving constipation than using probiotics alone. We believe that the synergistic effects of the three probiotic-specific strains in BIOVITA 3 led to effects on improving constipation, and one of the key mechanisms is thought to be the production of SCFAs in the gut. The BIOVITA 3 increased the production of SCFAs in the gut, which led to improved GIT by promoting intestinal motility, increased fecal water content through enhanced water absorption, increased bowel movement frequency, and, ultimately, relief of constipation symptoms. We also suggest that BIOVITA 3 increases useful microorganisms and changes intestinal microbial diversity, and that it has the potential to maintain, and improve host intestinal health. The results strongly suggest that BIOVITA 3 supplementation has the potential to be developed as a therapeutic and preventive strategy for constipation. Further research is needed to understand the mechanisms of action by which these probiotics improve constipation.













