Introduction
Chicken eggs are one of the best natural food products that are consumed all over the world (Abeyrathne et al., 2013a), and play a significant role in the human nutrition. Eggs are a rich source of protein and most of the egg proteins are present in the egg white and yolk, which account for 50% and 44%, respectively, of total egg proteins (Wu, 2014). Among the egg white proteins ovalbumin (54%), ovotransferrin (11%), ovomucoid (12%), globulins (8%), lysozyme (3.0%), and ovomucin (3.5%) are the major proteins (Abeyrathne et al., 2013a).
Ovotransferrin is a single-chain glycopeptide consists of 686 amino acids with a molecular weight of 78 kDa (Wu and Acero-Lopez, 2012). The native ovotransferrin is present in two forms: metal-free (apo) and metal-bound (holo), and the chemical and physical characteristics of the two differ significantly (Abeyrathne et al., 2013a). Apo-ovotransferrin is highly susceptible to chemical and physical treatments, whereas holo-ovotransferrin is resistant to chemical and thermal denaturation (Ko and Ahn, 2008). Furthermore, being a member of the transferrin family, ovotransferrin has an antimicrobial activity due to its iron-chelating capability (Wu and Acero-Lopez, 2012). Apo-ovotransferrin shows a high affinity to iron ions, and the antimicrobial activity of egg white is through the removal of iron from being used by microorganisms. The precipitation technique in the presence of ammonium sulfate (Warner and Weber, 1951), DEAE affinity-gel blue chromatography (Chung et al., 1991), Duolite C-476 (Guerin and Brule, 1992), and counter-current chromatography (Shibusawa et al., 1998) were commonly used to separate ovotransferrin, but the purity was low. To improve the purity of the ovotransferrin, chromatographic techniques such as immobilized-metal affinity chromatography (Al-Mashikhi and Nakai, 1987) and cation exchange chromatography (Guérin-Dubiard et al., 2005) were developed. Although the chromatographic methods improved the purity of ovotransferrin (over 89%), those methods were not practical for scale-up production. Ko and Ahn (2008) introduced an economic and simple purification procedure for the large-scale production of ovotransferrin, and Abeyrathne et al. (2013b) introduced a new separation method without using ethanol. Recently, Ji et al. (2020) sequentially separated ovotransferrin along with 5 major egg white proteins with 90% purity and 77% yield.
The native ovotransferrin displays multiple bioactivities that include antimicrobial activity for a wide spectrum of bacteria, fungi, yeasts, and parasites (Cooper et al., 2019; Ibrahim et al., 1998; Ko et al., 2008a; Ko et al., 2008b; Moon et al., 2012; Valenti et al., 1983), antioxidant activity (Ibrahim et al., 2007; Moon et al., 2015), anticancer activity against colon and breast cancer (Ibrahim and Kiyono, 2009), and immunomodulatory activity (Chiurciu et al., 2017; Lee et al., 2018; Xie et al., 2002; Zhang et al., 2020; Zhu et al., 2019). However, Moon et al. (2017) reported that native ovotransferrin does not contain any inhibitory activity against angiotensin-converting enzyme (ACE). Furthermore, ovotransferrin was reported to have various food applications; use as a κ-carrageenan-based packing material once combined with ethylenediaminetetraacetic acid (EDTA) (Seol et al., 2009), surfactant-free food-grade Pickering emulsion (Wei and Huang, 2019) and medical and pharmaceutical applications; metal supplement (Abdallah and Chahine, 1999), bone health promoter (Shang and Wu, 2019), heteroprotein complexes (e.g., ovotransferrin-lysozyme) for deliver hydrophobic nutraceutical such as curcumin (Wei et al., 2019), and therapeutic agent for the reproductive health of cows (Talukder et al., 2019).
Bioactive peptides are specific protein fragments that are inactive within the sequence of the native protein but have positive impacts on body functions or conditions once released by proteolysis or fermentation (Noh and Suh, 2015). Several peptides from egg proteins have been studied for their biological activities. The bioactive peptides in the native ovotransferrin were produced using various methods, including acid hydrolysis (Ibrahim et al., 2000; Lee et al., 2010), autocleaving under reduced conditions (Ibrahim and Kiyono, 2009; Moon et al., 2015), and enzymatic hydrolysis (Huang et al., 2010; Ma et al., 2020; Moon et al., 2013; Shen et al., 2010; Wang et al., 2017). All the studies indicated that the functionality of ovotransferrin increased after the hydrolysis.
The application of natural bioactive compounds and peptides has received great attention as potential agents to improve human health in recent years (Wu, 2014). Extensive scientific evidence proved that the bioactive peptides derived from foods have beneficial effects in improving human health and preventing diseases (Möller et al., 2008). Ibrahim et al. (2000) reported that the peptide located within 109–200 sequences of the N-lobe of ovotransferrin showed a strong antimicrobial activity against Escherichia coli through a membrane damage mechanism. Furthermore, peptides of ovotransferrin possess antimicrobial activities against Gram-positive Staphylococcus aureus, Listeria monocytogenes, Bacillus subtilis, and Gram-negative E. coli, Pseudomonas aeruginosa (Ma et al., 2020), and Gram-negative Salmonella typhimurium (Zohreh et al., 2014). Also, many scientific findings proved that peptides derived from ovotransferrin showed antioxidant activity.
Ovotransferrin hydrolysates showed stronger superoxide anion scavenging activity, oxygen radical absorbance capacity (ORAC), and 2,2-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity than native ovotransferrin (Kim et al., 2012). Two tetrapeptides (Trp-Asn-Ile-Pro and Gly-Trp-Asn-Ile) derived from ovotransferrin showed significant antioxidant activities when sonicated and hydrolyzed with thermolysin (Shen et al., 2010). An ovotransferrin peptide with the sequence of Ile-Arg-Trp is reported to have a significant oxygen radical-scavenging effect (Huang et al., 2010). Also, ovotransferrin hydrolysates obtained from promod 278P, thermolysin, and their combination had strong DPPH radical scavenging activities (Lee et al., 2017). Two ovotransferrin peptides with the amino acid sequence of Asp-Gln-Lys-Asp-Glu-Tyr-Glu-Leu-Leu and Lys-Asp-Leu-Leu-Phe-Lys showed the antiviral activity against Marek’s disease infection (Giansanti et al., 2005). Especially, ovotransferrin hydrolyzed with a food-grade promod 278P enzyme had a strong ACE-inhibitory activity (Moon et al., 2017). Majumder et al. (2015) reported that a peptide (Ile-Arg-Trp) derived from ovotransferrin contributed to antihypertensive activity by increasing ACE2 and decreasing pro-inflammatory genes expression. Lee et al. (2006) found that one peptide from ovotransferrin with the peptide sequence of Lys-Val-Arg-Glu-Gly-Thr-Thr-Tyr has an ACE-inhibitory activity that can be used as a pro-drug to reduce blood pressure. Recently, Yu et al. (2019) exhibited potent ACE-inhibitory of ovotransferrin hydrolysates of 19 enzymes and one combination obtained from in silico hydrolysis. In addition, the peptides of ovotransferrin possess various immunomodulatory activities (Jiao et al., 2019; Liu et al., 2017), anti-inflammatory (Wang et al., 2017), and cytotoxic (Yi et al., 2017) activities. Ovotransferrin-derived peptides were also reported to have potentials for many medical and pharmaceutical applications: the use of Ile-Arg-Trp, Ile-Gln-Trp, and Lys-Val-Arg-Glu-Gly-Thr to treat cardiovascular diseases (Chen et al., 2017), and Ile-Arg-Trp to decrease insulin-stimulated glucose uptake (Son et al., 2018).
Hence, the functional peptides derived from ovotransferrin may have great potentials to be used as antimicrobial and antioxidative agents in the food industry (Giansanti et al., 2015). Thus, the production of bioactive peptides from egg proteins will increase the values and potential applications of eggs. Recently, functional peptides derived from ovalbumin, ovomucoid, and ovomucin using single and two enzymes combined hydrolysis with strong bioactivities such as antioxidants, metal chelating, and ACE inhibitory activities were reported (Abeyrathne et al., 2014; Abeyrathne et al., 2015; Abeyrathne et al., 2016). There are many previous studies on ovotransferrin hydrolysates and their activities, but little work on analyzing multi-functionality of ovotransferrin hydrolysates in a study is available. This study determined the multiple functions of ovotransferrin hydrolysates, including antimicrobial, antioxidant, and metal-chelating activities. Also, the functionalities of the hydrolysates produced depend on the amino acid composition of peptides in the hydrolysates and their physical natures such as solubility. However, enzyme hydrolysates will lead to either improving or destroying existing activities. In this study we showed that enzymatic hydrolysis can also destroy the activities of ovotransferrin under the investigated conditions. The objectives of this research were to produce functional peptides from ovotransferrin using the single-enzyme treatments and to analyze the functional properties of the hydrolysates produced from ovotransferrin.
Materials and Methods
Lyophilized apo-ovotransferrin (over 85% purity and over 83% yield), which was prepared according to the method described by Abeyrathne et al. (2013b), was obtained from Iowa State University, Ames, USA. Standard enzymes; protease (from Bacillus licheniformis; Alcalase® 2.4L; ≥2.4 U/g solution; EC 3.4.21.64), papain (from papaya latex; ≥10 U/mg protein; EC 3.4.22.2), elastase (from the porcine pancreas; ≥4.0 U/mg protein, EC 3.4.21.70), trypsin (from the bovine pancreas; ≥7,500 BAEE U/mg solid; T9201) and α-chymotrypsin (from the bovine pancreas; ≥40 U/mg protein; EC 3.4.21.1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were purchased either from Sigma-Aldrich, Daejung Chemical and Materials (Siheung, Korea), HiMedia Laboratories (Mumbai, India), or Research Lab Fine Chem Industries (Mumbai, India).
The enzymatic hydrolysis of ovotransferrin was performed according to the method described by Abeyrathne et al. (2014) with some modifications. Ovotransferrin (20 mg/mL) solution was prepared by dissolving lyophilized ovotransferrin in distilled water and then the pH of the ovotransferrin solution was adjusted for the optimal condition for each enzyme (protease pH 6.5, papain pH 6.5, elastase pH 8.0, trypsin pH 7.8, α-chymotrypsin pH 7.6) using 1 N HCl, 1 N NaOH, 0.1 N HCl and 0.1 N NaOH under room temperature. Standard enzymes were separately added to the ovotransferrin solutions with the enzyme: substrate ratio of 1:100 (w/w) and then incubated at the optimal temperature of each enzyme (protease 55°C, papain 37°C, elastase 25°C, trypsin 37°C, α-chymotrypsin 37°C) for 0 (immediately after addition of enzymes), 3, 6, 9, 12, and 24 h. After incubation, the samples were heated at 100°C for 15 min to inactive the added enzymes and the resulting solutions were freeze-dried and considered as the enzyme hydrolysates of ovotransferrin.
The degree of hydrolysis was analyzed using 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) described by Green and Sambrook (2014) under reduced conditions using Mini-Protean® Tetra System (Bio-Rad; Bio-Rad Laboratories, Hercules, CA, USA) and stained with coomassie brilliant blue R-250 (Ameresco, Solon, OH, USA).
The antimicrobial activity of the hydrolyzed ovotransferrin was determined according to the agar well diffusion method described by Moon et al. (2012) with some modifications. Initially, all the bacteria were suspended in 0.1% w/v sterile peptone water and enriched for 2–3 h before culture. In the antimicrobial test, 20–25 mL of plate count agar (HiMedia, Maharashtra, India) for total plate bacteria was poured into sterilized Petri plates. After solidification, the agar surface was streaked with a sterilized cotton swab containing bacteria. After 30 min, 6 mm-diameter wells were aseptically punched on the agar surface using a sterilized cork-borer, and 100 μL aliquots (20,000 ppm, 10,000 ppm, 5,000 ppm, 2,500 ppm, 1,250 ppm, and 625 ppm) of hydrolysate was pipetted into it. Finally, the Petri dishes were kept for 30 min to complete diffusion and incubated at 37°C for 48 h. The bacterial inhibition zones of the hydrolysates were observed against the positive control containing Augmentin® (SmithKline Beecham, West Sussex, UK) and the negative control containing sterilized distilled water. The antimicrobial activity of the hydrolysates was calculated as the antimicrobial index using the following formula (Patra et al., 2009):
The 2-thiobarbituric acid reactive substance (TBARS) values of the hydrolysates were measured according to the TBARS assay described by Abeyrathne et al. (2014) with some modifications. One gram of olive oil (Aceites Agro Sevilla, Agrosevilla, Sevilla, Spain), 100 μL of Tween-20 (Research Lab Fine Chem, India), and 100 mL of distilled water were homogenized for 2 min in an ice bath using a homogenizer (D-500, Scilogex, Rocky Hill, CT, USA) at the maximum speed to prepare an oil-in-water emulsion. Samples for the TBARS assay were prepared by mixing 8 mL of an oil-in-water emulsion, 1 mL of distilled water, and 1 mL of ovotransferrin hydrolysates (20 mg/mL) followed by incubating at 37°C for 16 h. After incubation, 1 mL of sample was transferred to a 15 mL Falcon tube followed by adding 2 mL of thiobarbituric acid (TBA)/ trichloroacetic acid (TCA) solution (20 mM TBA/ 15% TCA w/v) and 50 μL of 10% w/v butylated hydroxyanisole (BHA) (Research Lab Fine Chem, Maharashtra, India) in 90% v/v ethanol. Then the solution was vortex-mixed and incubated at 90°C for 15 min to develop color. At the end of the incubation, the sample was cooled in ice water for 10 min and centrifuged at 3,000×g for 15 min at 5°C. Finally, the absorbance of resulting supernatant was measured at 532 nm against a blank (1 mL of distilled water and 2 mL of TBA/TCA solution). The TBARS value of the hydrolysates was expressed as milligrams of malondialdehyde (MDA) per liter of emulsion.
The Fe3+-chelating activity of the ovotransferrin hydrolysates was analyzed using the Ferrozine method (Carter, 1971) with slight modifications. In the experiment, 100 μL of ovotransferrin hydrolysates (20 mg/mL), 900 μL of deionized water, and 1 mL of 10 mg/kg FeCl3 (Research Lab Fine Chem, India) were vortex-mixed in a 15 mL Falcon tube followed by incubating at room temperature for 5 min. After proteins and peptides in the sample were precipitated by adding 900 μL of 11.3% w/v trichloroacetic acid (Sigma-Aldrich), the samples were centrifuged at 2,500×g for 10 min at 5°C. Then, 1 mL of supernatant from the sample was transferred to a culture tube followed by adding 1 mL of distilled water, 800 μL of 10% w/v ammonium acetate (HiMedia), and 200 μL of ferroin color indicator (75 mg of ferrozine, 75 mg of neocuprion, and 1 drop of 6 N HCl in 25 mL of distilled water) and vortex-mixed. Finally, the samples were incubated at room temperature for 5 min and the absorbance was measured at 562 nm. The Fe3+-chelating activity was calculated using the following formula:
All the tests were performed in triplicate and all the data were analyzed using Minitab® 17.1.0 statistical software (Minitab, State College, PA, USA). One-way ANOVA in a completely randomized design was used and Tukey’s test was performed for the significant differences (p<0.05) among mean values.
Results and Discussion
Proteolytic enzymes hydrolyze proteins into amino acid monomers and peptides at the target-specific peptide bonds under optimal temperature and pH (Tapal and Tiku, 2019). Over the decades, various studies have been carried out to produce functional peptides from egg-derived proteins. Ko and Ahn (2008) reported that apo-ovotransferrin can be easily hydrolyzed using proteolytic enzymes. In the previous studies, ovotransferrin was hydrolyzed using thermolysin, pepsin (Shen et al., 2010), promod 278P (Moon et al., 2017), chymotrypsin (Kim et al., 2012), protamex, alcalase, trypsin, neutrase, flavorzyme, maxazyme, collupulin and Protex (Kim et al., 2012).
In this study, ovotransferrin was hydrolyzed using protease, papain, elastase, trypsin, and α-chymotrypsin under optimal temperature and pH for 0, 3, 6, 9, 12, and 24 h. According to the visual observations, the hydrolysates with protease showed the lowest precipitation and turbidity compared with other enzymes. Among the treatments, the papain and elastase hydrolysates had the highest turbidity and precipitation. Although visual observations showed significant differences among the 5 treatments, it was very difficult to differentiate the best incubation time because they were very similar at all combination times. Therefore, visual observations were not effective in selecting the best hydrolysates with the best incubation time.
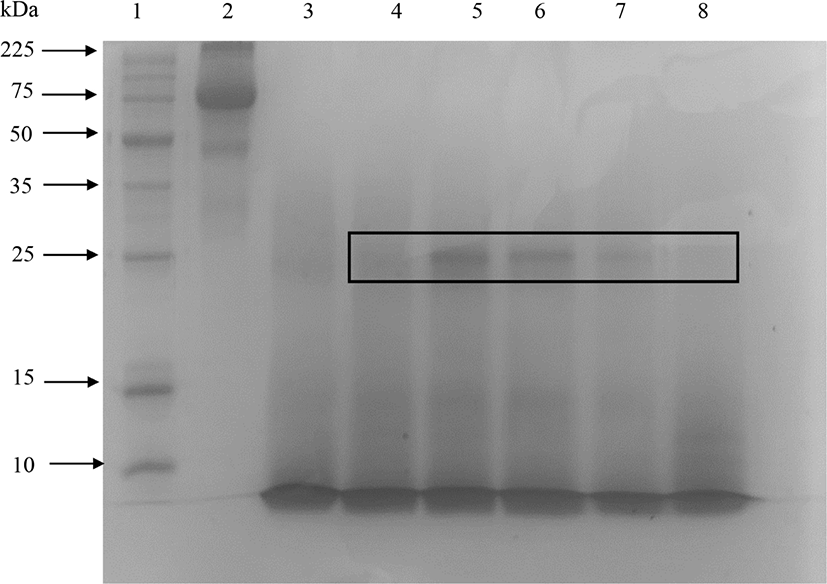
The peptides with very small molecular weight were not retained in the 15% SDS-PAGE gel (Abeyrathne et al., 2014). Fig. 1 showed that protease almost completely hydrolyzed ovotransferrin after 3 h of incubation. However, elastase produced partially hydrolyzed ovotransferrin products even after 24 h of incubation. Treating ovotransferrin with papain produced peptides with MW<10 kDa, but another clear band appears around 25 kDa and smear showed between 25–10 kDa area (shown by the box in Fig. 2). Therefore, it cannot be considered as a completely hydrolyzed product. Trypsin also hydrolyzed the ovotransferrin, but it was not as effective as α-chymotrypsin (data not shown).
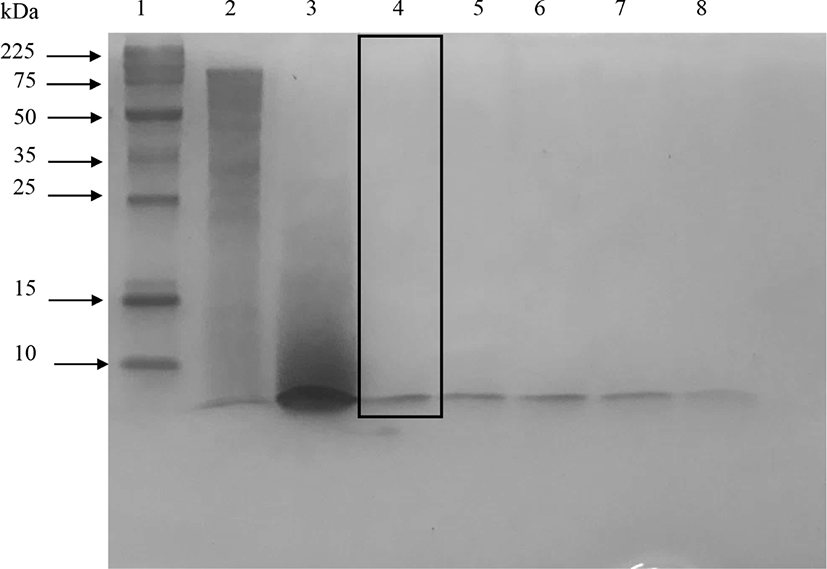
Elastase did not hydrolyze ovotransferrin well even after 24 h of incubation (shown by the box in Fig. 3) and a significant number of bands were found in 15% SDS-PAGE (Fig. 3). Therefore, elastase was not effective to produce ovotransferrin hydrolysates with low molecular weights. Kim et al. (2012) also discussed that acids, alcalase, and maxazyme treatments were more effective than neutrase and flavozyme in producing low-molecular-weight peptides from ovotransferrin.
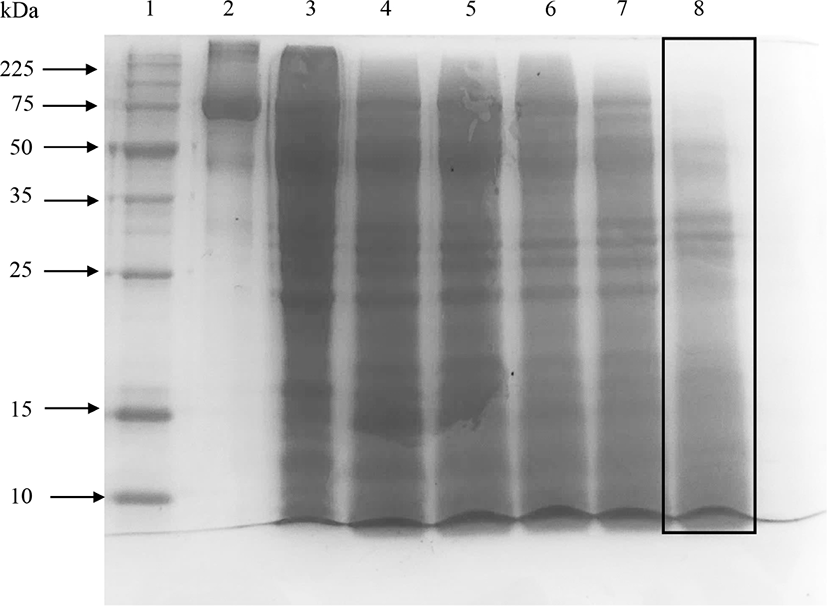
All the single enzyme treatments with the best hydrolysates from ovotransferrin [protease 3 h at 55°C (OTPro), papain 3 h at 37°C (OTPap), elastase 24 h at 25°C (OTEla), trypsin 3 h at 37°C (OTTrp) and α-chymotrypsin 3 h at 37°C (OTChy)] were selected and used for the analysis of the functional properties.
Antimicrobial peptides are abundant in many tissues and cells of plants and animals. The amino acid composition, amphiphilicity, cationic charge, and the size of peptides allow them to attach and penetrate membrane bilayers (Brogden, 2005). The antimicrobial peptides, usually with the molecular weight below 10 kDa, inhibit cell growth and kill several microorganisms (Kim and Wijesekara, 2010). Ibrahim et al. (2000) reported that the antimicrobial peptides from ovotransferrin (Leu109-Asp200) prevented E. coli through a membrane damage mechanism. They also stated that Zn2+-saturated ovotransferrin exhibited stronger antimicrobial activity than apo-ovotransferrin and other metal complexes. Compared with the antimicrobial activity of other egg white-derived peptides, little information on the antimicrobial activity of ovotransferrin-derived peptides is available.
According to present findings, 20 mg/mL ovotransferrin hydrolyzed products did not yield any inhibition zone in total plate counts compared to that of the Augmentin® (26.33±1.52 mm) under the investigated conditions. Similarly, Kim et al. (2012) noted that there was no clear zone at 20 mg/mL ovotransferrin against E. coli 0157:H7 and had a slight inhibition at 80 mg/mL concertation. Hence, the absence of antimicrobial activity of the ovotransferrin hydrolysates may be due to the destruction of antimicrobial and metal-binding sites by the enzyme hydrolysis. Also, the resistance of the bacteria and the ineffective concentration of the hydrolysate may have caused the loss of antimicrobial property under the investigated conditions. Furthermore, in order to express antimicrobial activity, protein materials should be completely diffused in the media. However, as per the visual observation, there were undiffused ovotransferrin hydrolysates even after incubation period, which can be a possible reason for ineffectiveness of antimicrobial activity. However, Zohreh et al. (2014) reported that the hydrolysis of ovotransferrin with trypsin and ficin showed antimicrobial activity against S. aureus (G+) and S. Typhimurium. Also, the peptides from lysozyme (Ibrahim et al., 2001; Pellegrini et al., 1997; You et al., 2010), ovalbumin (Pellegrini et al., 2004), and ovomucin (Kobayashi et al., 2004) exhibited strong antimicrobial activities. Further experiments are needed to analyze the antimicrobial activity of the hydrolysates with different enzyme treatments.
Manso et al. (2008) explained that some substances termed as antioxidants should retard or at least attenuate the organic impairment by excessive oxidative stress at very low concentrations. Since there are potential health risks associated with synthetic antioxidants such as butylated hydroxytoluene (BHT) and BHA, natural antioxidants became an attractive choice for the food and pharmaceutical industries (You et al., 2010). Ovotransferrin is reported to have a strong antioxidant property when supplemented along with diets. Oxidative stress is related to the etiopathogenesis of several chronic diseases. Lipids are the most heavily involved class of biomolecules to oxidative stress and the oxidation of the lipids generates several secondary products. MDA is the principal product of polyunsaturated fatty acid peroxidation (Rio et al., 2005). TBARS is a commonly used method of measuring lipid peroxidation. MDA forms an adduct with 2-TBA molecules, which gives a pink color (Dasgupta and Klein, 2014). In the present study, oil emulsion prepared with olive oil had whitish pink color compared to that of other emulsions containing ovotransferrin hydrolysates. Overall results in Fig. 4 indicated that the oil emulsion with native ovotransferrin and its protease hydrolysate had lower TBARS values than the control; 0.029 mg MDA/L and 0.036±0.003 mg MDA/L, respectively, indicating that both ovotransferrin and its protease hydrolysates have some antioxidant activity. However, other hydrolysates had weak antioxidant properties. According to the visual observations, there were significant amount of undigested protein materials with low solubility. Hence, the solubility of the protein materials is an important factor to express functional properties in vitro.
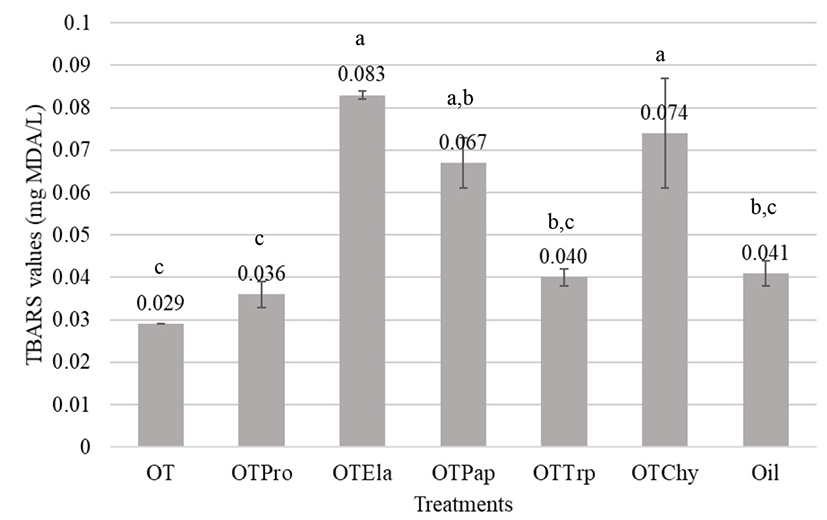
The antioxidant activity of protein hydrolysates depends on the amino acid composition (Alemán et al., 2011), which are affected by enzyme activity and the conditions of the hydrolysis process such as pH, temperature, enzyme-substrate ration, and incubation time (Shahidi and Zhong, 2008). Kim et al. (2012) obtained approximately 3.2 to 13.5 superoxide-anion-scavenging activity and oxygen-radical-scavenging activity for the ovotransferrin hydrolysates of protamex, alcalase, trypsin, neutrase, flavorzyme, maxazyme, collupulin, Protex, Promod 278P, and α-chymotrypsin. Shen et al. (2010) reported that two tetrapeptides (Trp-Asn-Ile-Pro and Gly-Trp-Asn-Ile) from Thermolysin hydrolysates of ovotransferrin had significantly higher ORAC, which increased with sonication. The hydrolysates obtained from ovalbumin with alcalase, pepsin, papain, and α-chymotrypsin (Abeyrathne et al., 2014), from ovomucoid with alcalase, trypsin and papain (Abeyrathne et al., 2016), and from ovomucin with alcalase, pepsin, trypsin, and papain (Abeyrathne et al., 2015) had lower TBARS values than that of the controls. However, the hydrolysates obtained from ovotransferrin under the investigated conditions were not much effective in preventing the formation of MDA in foods which leads to lipid oxidation as well as the occurrence of oxidative stress in animal cells.
In this study, enzymatic hydrolysis did not significantly increase the ferric iron-binding ability of peptides in all treatments as shown in Fig. 5. However, the ovotransferrin hydrolysates obtained from elastase and α-chymotrypsin slightly increased the ferric iron-binding property (1.06±0.88%, 1.25±0.24%) compared to that of the native ovotransferrin (0.46±0.60%), which could be caused by the release of new iron-binding sites by the enzyme activity (Fig. 5). Rajapakse et al. (2005) stated that acidic (Asp and Glu) and basic (Arg and Lys) amino acid residues play important roles in the metal-chelating activity of the protein hydrolysates. Since elastase and α-chymotrypsin hydrolysates of ovotransferrin maintained a lower degree of hydrolysis, the hydrolysates maintained their iron-chelating residues even after hydrolysis. However, Keung et al. (1981) reported that the hydrolysis of holo-ovotransferrin with subtilin did not produce significant changes in the iron-binding capacity or the conformation of the iron-binding domains.
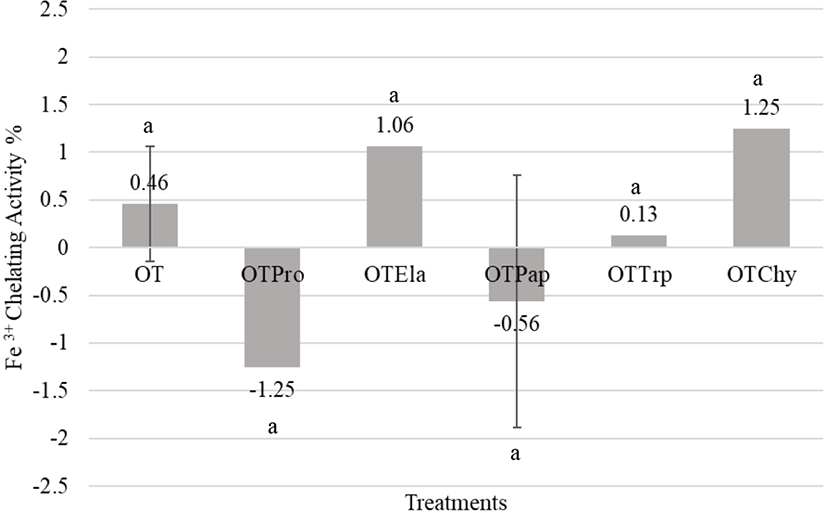
The native ovotransferrin is a member of the transferrin family and well-known for its iron (Fe3+)-binding capability (Ibrahim et al., 2000; Ko and Ahn, 2008; Lin et al., 1994). As previously described, the metal-binding peptides derived from egg white can retard lipid oxidation (Guérin-Dubaiard et al., 2007) as well as microbial growth (Ko et al., 2008b). Abeyrathne et al. (2013a) suggested that egg white peptides with metal-binding properties have great potentials as an iron carrier. However, the ovotransferrin hydrolysates produced from the current study were not effective in reducing oxidation nor metal-binding and it can be due to the destruction of metal-binding sites due to enzyme activity. Also, the physical structure of ovotransferrin hydrolysates may obstruct the metal-binding sites to prevent binding.
Conclusion
The ovotransferrin from chicken egg could be completely or partially hydrolyzed using protease, elastase, papain, trypsin, and α-chymotrypsin under the investigated conditions. However, all the hydrolysates of ovotransferrin had poor antimicrobial, antioxidant, and Fe3+-chelating activities at 20 mg/mL concentration. Hence, those hydrolysates cannot be utilized as antimicrobials, antioxidants, and iron carriers in the food and pharmaceutical industries. We also found that enzymatic hydrolysis destroyed some functionality of the native ovotransferrin under investigated conditions. Further research is needed to produce functional peptides from ovotransferrin using different enzymes or enzyme combinations or finding the functionalities other than tested here.













