Introduction
The growing emphasis on sustainable practices and animal welfare has driven the food industry to explore innovative ways of producing milk, meat, and other animal-derived products without relying on traditional livestock. While plant-based substitutes have been widely marketed, their inability to fully replicate the sensory, nutritional, and functional qualities of animal products has limited consumer acceptance (Appiani et al., 2023; Jang and Lee, 2024). Options like margarine, one of the earliest substitutes for milk, highlight the ongoing debate over the benefits and limitations of replacing animal-derived products with non-animal ingredients (Pointke et al., 2022; Short et al., 2021). Recent technological advances have shifted attention toward producing identical animal components via biotechnological methods rather than seeking plant-based approximations.
The technological foundation of these advancements encompasses precision fermentation, cell culture, and tissue engineering technologies, which hold the potential to replicate animal-based products while reducing environmental impact. Startups and research initiatives have received substantial funding to refine these approaches, with their focus primarily on producing artificial milk molecules that mimic those found in bovine or human milk (Lurie-Luke, 2024). Regulatory frameworks and terminology surrounding such products remain under discussion, reflecting the novelty and complexity of these innovations. For clarity, this review defines artificial milk as any product created through these advanced technologies that contains components structurally or functionally identical to milk from lactating mammals.
The current landscape of artificial milk technology has evolved rapidly since 2022, with over $800 million in venture capital funding committed to companies developing precision fermentation and cellular agriculture approaches. The primary production focus has concentrated on individual milk proteins, particularly β-lactoglobulin (β-LG) and caseins, with several companies achieving regulatory approval in select markets. Notable technological developments include advances in strain engineering that have improved protein expression efficiency by 300%–400%, development of continuous fermentation systems that reduce production costs by 40%–60%, and emerging approaches for producing complex structures like casein micelles (Change Foods, 2023; Perfect Day, 2024). While most efforts target bovine milk equivalents, significant research also addresses human milk components for infant nutrition applications. Investment in the artificial milk sector has expanded significantly since 2022, with precision fermentation dominating as the primary technology platform, focusing mainly on protein production. Most companies remain in pre-commercial development phases, with scaling challenges and production costs being primary barriers to wider market adoption. Strategic partnerships with established food companies represent the predominant commercialization pathway for emerging technologies.
Milk represents a uniquely complex biological fluid comprising hundreds of molecular components organized in sophisticated structural assemblies. Unlike many foods, milk constituents demonstrate both compositional and structural complexity. Its major components include proteins like caseins and whey that dominate nutritional relevance, while its unique sugars such as lactose and multifaceted fat structures contribute to functionality. Casein micelles provide the backbone for cheese-making (D’Alessandro et al., 2011), while the milk fat globule membrane (MFGM) plays a significant role in texture and flavor (Ozturk et al., 2022). The structural complexity of milk underscores the challenge of replicating its components accurately. Apparently, milk texture, nutritional value, and functionality arise from dynamic interactions between components rather than from ingredients in isolation.
Current research in artificial milk production integrates knowledge from food engineering, biotechnology, and dairy science to address scalability and cost challenges. The production trajectory follows two complementary approaches. Rather than tackling milk in its entirety, some efforts focus on producing high-purity components for specific applications. Isolating proteins or lipids for high-value dairy derivatives could yield sustainable solutions with immediate commercial potential. This targeted approach aligns with the dairy industry trend toward “milk refinery” techniques, which fractionate milk into specialized products. Simultaneously, the field faces intricate challenges regarding regulatory approval pathways, consumer acceptance barriers, and scale-up economics (Antuma et al., 2023; Nielsen et al., 2024).
As artificial milk technologies advance, they hold the potential to redefine the dairy sector by offering sustainable alternatives without sacrificing quality. Unlike previous reviews that address isolated technological aspects, this integrative analysis examines artificial milk production through a multidimensional framework encompassing compositional requirements, production methodologies, and implementation barriers. This review systematically analyzes the scientific and practical challenges involved in producing milk-like products without traditional mammals, offering a perspective on how emerging technologies might reshape dairy production. It covers the most common routes to produce artificial milk and components with special focus on proteins, fats, and carbohydrates without direct usage of animals via the bottom-up and top-down approaches to milk synthesis. While most progress focuses on bovine and human milk, these methods could extend to milk from other mammals. By establishing milk’s compositional complexity as the foundation for technological assessment, this review creates a uniquely comprehensive evaluation framework for discussing production routes and the technological and practical hurdles that remain.
Composition of Milk and Their Importance
Milk functions as a complex nutritional resource that has evolved over time, specifically designed to aid in the growth and survival of young in various environmental settings. Multiple factors influence milk composition, including the nutritional requirements of young animals characterized by rapid growth rates, as well as external conditions such as temperature and water availability. Thus, mammalian milk demonstrates remarkable compositional variability across different species, which presents both challenges and opportunities when replicating its essential characteristics in artificial systems. As shown in Table 1, human milk differs substantially from bovine milk in protein content (1.0% vs 3.4%), casein-to-whey ratio (40:60 vs 82:18), and oligosaccharide concentration (5–15 g/L vs 0.03–0.06 g/L), illustrating the species-specific optimization that artificial milk production must consider. This compositional diversity across species represents a critical parameter for artificial milk development. Human milk is significantly lower in protein but higher in lactose compared to ruminant milk, reflecting the different developmental needs of human infants. Similarly, the casein-to-whey ratio varies substantially across species, with human milk having the lowest proportion of casein, creating significant implications for protein digestibility and functional properties. The extraordinarily high concentration of oligosaccharides in human milk (5–15 g/L) compared to bovine milk (0.03–0.06 g/L) represents a particularly important distinction with significant implications for infant gut microbiome development.
Milk components segregate into three functional categories based on their origin and purpose. The first group includes components synthesized directly within lactating cells, such as proteins, sugars, lipids, and specific vitamins and minerals. These constituents serve intentional nutritional, immune-enhancing, and structural functions. The second group encompasses components actively transferred from other areas of the animal, like antibodies, specific fatty acids, and immunomodulatory molecules (He et al., 2023; Purba et al., 2020b). These elements enhance the immune-protective and functional properties of milk. The third group consists of contaminants or incidental inclusions, including mineral residues, pesticides, heavy metals, and pathogens (Nag, 2010). In artificial milk production, manufacturers typically exclude these undesirable elements to maintain safety and quality standards while attempting to replicate beneficial components.
Certain milk components challenge simple categorization due to their formation mechanisms and multifunctional roles. The MFGM, formed during fat secretion, exemplifies this complexity. Traditionally viewed as a byproduct of secretion, MFGM contains bioactive properties promoting brain development, enhancing infection resistance, and reducing inflammation, rendering it a significant element in specific formulations. Recent research has identified over 200 proteins in the MFGM, many with enzymatic activity and immune-modulating functions that contribute significantly to milk nutritional value beyond basic macronutrients (Lu et al., 2016). In infant formula, MFGM incorporation mimics breast milk benefits, supporting overall infant health and cognitive development. Similarly, components like urea, frequently overlooked as mere contaminants (Paengkoum et al., 2021), play crucial roles in enhancing milk heat stability and demonstrate important functional implications.
Functional replication of milk can sometimes achieve targeted properties through simplified component selection. Single components can achieve specific functions like emulsification and foaming through proteins such as β-LG or through mono- or diglycerides and phospholipids. Desired sweetness levels require only lactose. However, achieving a comprehensive match to milk properties typically requires combining multiple functional elements in precise arrangements. The challenge for artificial milk production lies in determining which components require exact replication versus those that can be substituted or simplified while maintaining desired nutritional and functional profiles (Antuma et al., 2023). It is important to recognize that composition varies considerably based on stage of lactation, nutrition, and genetic factors. Values typically represent mature milk composition, as colostrum composition differs significantly with higher protein and bioactive component concentrations. Ovine (sheep) milk has the highest content of total solids, fat, and protein among common mammalian species, while equine (horse) milk most closely resembles human milk in its whey-to-casein ratio and lactose content. These compositional differences; thus, have substantial implications for the nutritional profile and functional properties of milk from different species, requiring careful consideration when designing artificial milk systems targeted at specific applications.
Lipids contribute essential sensory, nutritional, and functional properties to milk through a diverse blend of fatty acids and complex lipid components derived from multiple biological pathways. Milk lipid sources encompass de novo synthesis occurring in mammary cells, adipose tissue release, and dietary intake (Wilmot et al., 2024). Additionally, studies suggest potential microbial lipid production within milk itself (Purba et al., 2020a; Stinson and George, 2023). This complex interaction of lipid sources creates distinctive profiles across mammalian species and dietary contexts, presenting significant challenges for artificial reproduction.
In mammary glands, specialized lactocytes produce fatty acids through de novo synthesis, assembling them from smaller precursors into triacylglycerols, milk primary lipid storage form. Animal breed, lactation stage, and nutritional status significantly influence fatty acid production efficiency and types. During energy deficits, animals mobilize stored fat from adipose tissue into the bloodstream, contributing particularly to milk saturated fatty acid content. Diet directly impacts milk lipid composition, with fatty acids from feed incorporated into milk or metabolized before mammary gland secretion.
The MFGM represents one of the most structurally sophisticated elements requiring replication in artificial milk systems. This complex tri-layer membrane surrounding fat globules contains phospholipids, sphingolipids, cholesterol, and proteins. Beyond stabilizing fat globules, MFGM contains bioactive compounds contributing to health benefits, particularly in infant nutrition. Phospholipids like phosphatidylcholine and sphingomyelin serve essential roles in emulsification and nutrient delivery, supporting fat-soluble vitamin absorption. Sphingolipids, particularly sphingomyelin, aid neuronal development and immune system support, protecting neonates from infections (Bernal-Vega et al., 2023; Chuh et al., 2018). As illustrated in Fig. 1 (right panel), this trilayer architecture presents particular challenges for artificial production systems.
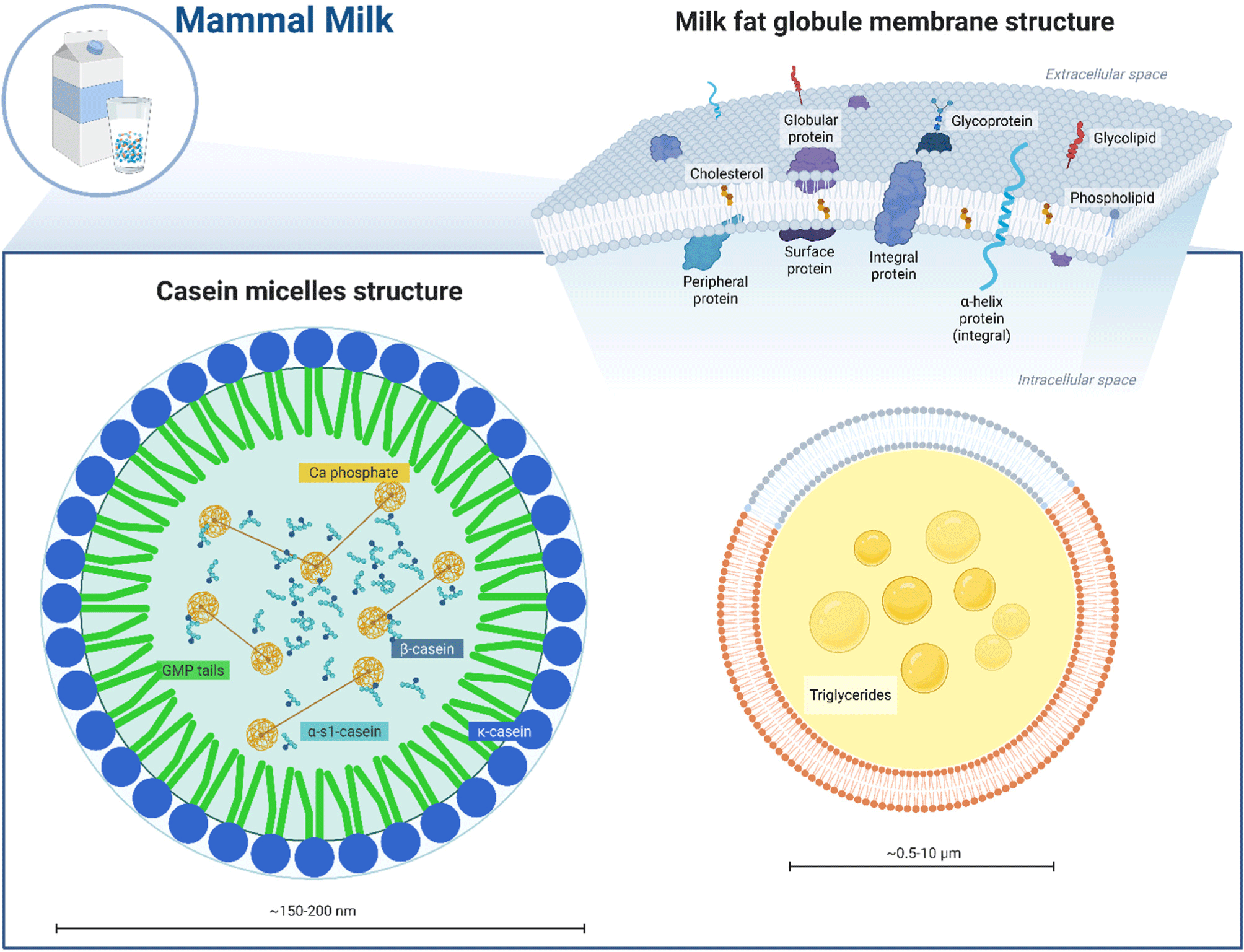
The MFGM protein fraction contains unique membrane-associated proteins involved in lipid transport, cell signaling, and enzymatic activity. However, processing can compromise MFGM structural integrity. Homogenization, common in artificial milk production, significantly damages MFGM by disrupting its tri-layer structure and reducing functional capabilities (Wang et al., 2024; Wilmot et al., 2024). During homogenization, milk fat globules break down into smaller droplets with increased surface area, exposing new surfaces often coated by milk proteins like caseins and whey, providing stabilization but sacrificing original MFGM structure integrity. In products like plain milk, evaporated milk, and certain yogurts, this disruption may not significantly affect stability (Obeid et al., 2019; Yao et al., 2024). However, maintaining MFGM structure integrity proves crucial for products where texture, emulsification, and bioactivity remain critical, such as infant formulas, whipping cream, or ice cream.
Despite processing challenges, incorporating MFGM components into artificial milk formulations offers potential nutritional and functional benefits. Bovine MFGM, while different from human MFGM due to processing, retains similar bioactive lipids and proteins supporting cognitive development and immune function. Research demonstrates that bovine MFGM supplementation in infant formulas improves cognitive development and reduces infection risk (Fontecha et al., 2020; Silva et al., 2021). Furthermore, MFGM lipid composition, particularly phospholipids and sphingolipids, offers valuable emulsifying properties enhancing fat-soluble nutrient delivery. Understanding MFGM interactions with proteins and lipids during processing, and developing methods for preservation or reconstruction in artificial milk, remains essential for developing milk alternatives mimicking natural milk nutritional and functional qualities.
Milk proteins function as the structural and nutritional foundation of milk, enabling essential mineral transport, supporting immune health, and creating the unique colloidal system underlying dairy product functionality. From their synthesis pathways to their complex hierarchical assemblies, these proteins present multidimensional challenges for artificial production systems. Understanding protein biosynthesis in lactating cells, particularly casein micelle formation and post-translational modifications (PTMs) such as glycosylation and phosphorylation, reveals precisely why replication outside biological systems has proven so challenging.
Though governed by the central dogma of molecular biology, the milk protein production pathway requires sophisticated post-translational processing to achieve functional maturity. First, protein synthesis begins with DNA encoding in the cell nucleus. This genetic information then is transcribed into messenger RNA (mRNA), which carries the protein blueprint to ribosomes in the rough endoplasmic reticulum (RER). Once arriving at this cellular workshop, the mRNA sequence directs amino acid assembly into polypeptide chains. What happens next transforms these simple chains into functional biomolecules, including nascent proteins receive critical PTMs within the RER environment (Pegolo et al., 2018). Among these essential modifications are disulfide bond formation between cysteine residues that stabilize tertiary structure; oligomerization of multiple protein subunits into larger complexes; and N-glycosylation attachment of sugar chains to specific asparagine residues.
The specific arrangement of sugar units in N-glycans creates diverse glycoforms influencing ultimate protein functionality. Signal sequences, short amino acid stretches directing protein to proper cellular location, go through cleavage within the endoplasmic reticulum (ER). A sophisticated quality control mechanism ensures correct protein folding before secretory pathway progression, involving chaperone proteins assisting proper folding and preventing misfolded protein aggregation (Bukau et al., 2006). As proteins move from ER to Golgi apparatus, initial casein interactions become observable. Within the Golgi apparatus, further refinement occurs through additional PTMs including O-glycosylation, sugar chain addition to serine or threonine residues particularly relevant for κ-casein, and phosphorylation, attachment of phosphate groups to specific amino acid residues primarily affecting other caseins. These modifications create extensive milk protein heterogeneity even within individual animals, presenting significant challenges for recombinant production in alternative host organisms. Since PTMs lack direct DNA encoding, they depend heavily on host cellular machinery, influencing the types and extent of modifications and impacting final protein structure and function (Kolb et al., 2011). Within secretory vesicles, larger casein structures and micelles begin assembling in preparation for mammary gland lumen secretion.
Further, what makes casein micelles so remarkable? These sophisticated supramolecular assemblies serve dual roles in calcium phosphate transport and colloidal stabilization. Structurally, they exist as complex spherical structures composed of casein proteins and calcium phosphate. Their primary biological role involves functioning as vehicles delivering essential minerals like calcium and phosphorus to offspring. Simultaneously and equally important, they prevent mineral precipitation within the mammary gland, ensuring bioavailability and preventing calcification (Manguy and Shields, 2019; Oftedal, 2012). Without this dual function of nutrient delivery and mineral stabilization, neonatal development would be compromised. Evolution has thus optimized these structures over millions of years, explaining their remarkable conservation across mammalian species. As depicted in Fig. 1 (left panel), the complex arrangement of calcium phosphate nanoclusters within the casein micelle structure presents significant challenges for artificial replication.
Following synthesis, caseins sustain crucial PTMs, primarily phosphorylation and glycosylation, significantly influencing their functionality. Phosphorylation particularly impacts caseins interaction with calcium phosphate nanoclusters, forming casein micelle cores (Farrell et al., 2003) and preventing precipitation. Glycosylation influences micelle size and stability, particularly involving κ-casein (Aimutis, 2004). The specific phosphorylation and glycosylation patterns lack direct DNA encoding and vary depending on host organism, creating significant challenges for casein production in non-native systems for artificial milk.
Within the Golgi apparatus, modified casein proteins initiate interaction, forming larger structures developing into micelles within secretory vesicles. κ-casein plays a critical role in micelle structure and stability, positioned on the surface where its hydrophilic glycomacropeptide (GMP) portion provides steric hindrance (McClellan et al., 2008), preventing excessive aggregation and maintaining colloidal stability, essential for preventing sedimentation and ensuring efficient calcium and phosphorus transport. Consequently, casein micelle size and aggregation properties prove crucial for functionality in both natural and artificial milk.
Moreover, casein micelles contribute significantly to dairy product properties through their response to enzymatic and pH modifications. In cheese making, for instance, enzymatic cleavage of κ-casein GMP tail by rennet disrupts steric stabilization, promoting micelle aggregation and curd formation. In yogurt production, lowering pH causes isoelectric casein micelle precipitation, creating characteristic gel structure (Rankin et al., 2010). These gelation processes depend on casein micelle structure, hydration, and interactions, highlighting the importance of replicating these properties in artificial milk formulations. High casein micelle hydration contributes to dairy gel water-holding capacity (Gai et al., 2021), affecting viscosity and potential micelle concentration in artificial milk production.
Whey proteins constitute a significant portion of milk protein, playing crucial roles in neonatal nutrition and contributing to milk unique properties. The three main whey proteins including β-LG, α-lactalbumin (α-LA), and whey acidic protein have evolved with specialized functions, although their expression varies across species, with some mammals lacking one or more proteins (McClellan et al., 2008). α-LA plays a central role in lactose synthesis within the mammary gland, functioning as a regulatory subunit of the lactose synthase enzyme complex responsible for producing lactose, the primary carbohydrate in milk. Fundamentally, lactose synthesis provides energy to neonates and regulates milk volume by controlling osmotic pressure (Layman et al., 2018).
Of particular note, α-LA abundance varies significantly between human milk (approximately 22% of total protein) and bovine milk (around 3.5%), reflecting varying nutritional requirements across mammalian offspring. Additionally, α-LA serves as an essential amino acid source, including tryptophan, lysine, branched-chain amino acids, and sulfur-containing amino acids, vital for infant development. β-LG represents the most abundant whey protein in bovine milk, although its function remains incompletely understood. While potentially transporting retinol, its primary function appears to provide essential amino acids, particularly cysteine and methionine, less prevalent in caseins (McClellan et al., 2008). This proves especially important for neonates, as these amino acids support protein synthesis and metabolic processes. β-LG properties, including water solubility and heat stability, make it a valuable food ingredient.
Furthermore, it is worth noting that the distinct properties and functionalities of whey proteins have significant implications for artificial milk formulation. Replicating the natural balance and composition of whey proteins proves crucial for achieving nutritional equivalence to human milk. The varying proportions of α-LA and β-LG in different mammalian milks underscore the need for species-specific formulations. The absence of specific whey proteins in certain mammals; hence, highlights the challenges in creating universal artificial milk formulations.
Understanding individual whey protein roles, including contributions to lactose synthesis, amino acid delivery, and immune protection, remains essential for optimizing artificial milk composition and ensuring optimal infant nutrition. The complexities of whey protein functionality and their interactions with other milk components, such as caseins and MFGM, present ongoing challenges for artificial milk development. Research focusing on these interactions and the impact of processing on whey protein structure and bioactivity remains crucial for refining artificial milk formulations and improving their nutritional value and digestibility.
Milk proteins originate from a sophisticated interplay between mammary and extra-mammary sources, adding another layer of complexity to artificial replication efforts. While the mammary gland serves as the primary milk production site, not all milk proteins originate within its epithelial cells. Serum albumin, a major blood protein synthesized in the liver, reaches the mammary gland via bloodstream transport (Audic et al., 2003). Immunoglobulins, crucial components of the adaptive immune system produced by B and T cells, transport to the mammary gland, providing passive immunity to neonates (Puppel et al., 2019; Purba et al., 2025). This transfer holds particular importance during the first days after birth when colostrum, rich in protective proteins, undertake production. Substantially, correct immunoglobulin glycosylation requires involvement of various B cells.
In addition, milk contains diverse enzymes catalyzing biochemical reactions with various origins. Their sources vary widely. Some ongoing synthesis within the mammary gland, while others derive from blood plasma, somatic cells, or contaminating microorganisms (Dallas et al., 2015). Not all enzymes serve the same purpose in milk’s complex system. Some milk enzymes, such as lactoperoxidase, offer commercial value and contribute to natural milk preservation. With its well-documented antimicrobial properties, lactoperoxidase serves in various food preservation applications beyond dairy products (Khan et al., 2019). Other enzymatic actors play more complicated roles. Plasmin, while fulfilling important biological functions, can contribute to quality and shelf-life issues in dairy products through its proteolytic activity. This is massive phenomenon. Such interactions subsequently trigger gradually breaking down proteins and leading to undesirable changes in milk texture and flavor (Dallas et al., 2015). Perhaps most interesting from a food safety perspective is alkaline phosphatase. This alkaline phosphatase serves as a vital signal for appropriate pasteurization due to its heat sensitivity, functioning as a natural indicator that milk has been properly heat-treated (Rankin et al., 2010).
It appears that understanding milk protein origins and modifications, proves crucial for developing artificial milk formulations. Replicating the complex composition and functionality of natural milk presents significant challenges. The host-dependent nature of PTMs, such as glycosylation and phosphorylation, poses a major hurdle in producing caseins in non-native systems. Artificial milk production must consider these modifications to mimic the properties of natural casein micelles, essential for mineral transport, bioavailability, and gelation processes. The inclusion or exclusion of specific enzymes, like lactoperoxidase and plasmin, respectively, represent important considerations for artificial milk quality and preservation. Furthermore, mimicking the diversity of immunoglobulins found in natural milk, with their correct glycosylation patterns, presents a significant challenge.
Beyond well-known components like lactose, fats, and proteins, human milk contains a diverse class of carbohydrates called human milk oligosaccharides (HMOs) representing the third most abundant solid component after lactose and lipids. These complex sugars play crucial roles in infant health and development by shaping gut microbiome composition, influencing immune function, and potentially contributing to cognitive development (Urashima and Saito, 2005).
In most instances, HMOs are synthesised in the mammary gland by specific glycosyltransferases that sequentially add monosaccharide units to a lactose core. The process involves adding monosaccharides including N-acetylglucosamine (GlcNAc), galactose (Gal), fucose (Fuc), and sialic acid (N-acetylneuraminic acid, NeuAc) to the lactose [Gal(β1-4)Glc] starting point. Glycosyltransferase expression, particularly fucosyltransferases (FucTs) and sialyltransferases, varies among individuals based on genetic factors like blood group and secretor status (Urashima et al., 2023). For example, α1,2-fucosylated galactose units, characteristic of the secretor type, depend on FucT II activity (Rudloff and Kunz, 2012). FucT III expression in Lewis (a+b–) individuals leads to α1,4 linkage formation to subterminal GlcNAc residues. This genetic variation contributes to the remarkable structural diversity observed among HMOs, with over 100 distinct structures identified, ranging from three to more than twenty monosaccharide units (Fischöder et al., 2019). This structural diversity has important implications for biological functions, as specific HMO structures interact with distinct receptors and microorganisms in the infant gut.
HMO metabolic fate research has intensified, though this complex area remains incompletely understood. A significant ingested HMO portion, estimated at several grams daily, reaches the lower intestine intact, escaping human enzyme digestion (Rudloff and Kunz, 2012). In the colon, HMOs serve as prebiotics, selectively promoting beneficial bacteria growth such as Bifidobacterium. These bacteria possess specialized enzymes degrading HMOs and releasing short-chain fatty acids like butyrate, which demonstrate anti-inflammatory and immunomodulatory effects (Ehrlich et al., 2018). A smaller HMO fraction enters the bloodstream and continues urinary excretion, suggesting potential systemic effects beyond the gut, possibly influencing immune development and other physiological processes. Maternal serum HMO presence has been observed associated with maternal glucose homeostasis. Evidence suggests serum 3-sialyllactose concentration changes in response to glucose load, indicating a relationship between maternal glucose metabolism and HMO synthesis (Weiser-Fuchs et al., 2023).
The limited natural HMO availability has driven biotechnological method development for large-scale production. In response to these limitations, biotechnology advances, particularly in metabolic engineering and enzyme catalysis, have revolutionized HMO production. One approach employs metabolically engineered bacteria, such as Escherichia coli, designed to produce specific HMOs (Priem et al., 2002). By manipulating glycosyltransferase expression and other metabolic pathways, researchers have successfully produced several HMOs, including lacto-N-neotetraose and sialyllactose, at high yields. Complementing this cellular approach, another strategy uses enzymatic cascade synthesis, utilizing an enzyme series to build complex HMO structures in vitro (Fischöder et al., 2019). This approach allows production of well-defined HMOs serving as reference standards for analytical techniques like capillary gel electrophoresis with laser-induced fluorescence detection (xCGE-LIF). As a result of these methodological advances, these biotechnological methods have enabled large-scale production of high-purity individual HMOs, advancing research and potential applications in infant formula and other therapeutic areas.
Beyond complex carbohydrates, it is also important to consider such small molecules in milk. Those extends include lactose, citric acid, and urea. These small molecules influence milk heat stability and contribute to its overall functionality. Lactose serves as an energy source and contributes to milk osmotic balance. Similarly challenging but equally important, replicating milk flavor profile presents a significant challenge, as milk flavor arises from complex interplay between volatile and non-volatile compounds, many originating from the mammal feed. Interactions between aroma-active components and proteins and other constituents can modulate flavor perception (Urashima and Saito, 2005).
The interplay of proteins, lipids, and small molecules, along with their structural arrangements, defines unique milk properties and presents multifaceted challenges for artificial replication. Establishing a single milk type as a “gold standard” for artificial milk development proves misleading given the extensive variation in milk composition across species and the impact of processing methods (Urashima et al., 2023). Apparently, the focus should address understanding individual milk component functional roles and their interactions, considering the wide variation consumers accept in existing dairy products. While replicating natural milk full complexity remains challenging, producing artificial milk products meeting consumer expectations represents an achievable goal. This requires understanding individual milk components and their combined effects on digestion, flavor, stability, and other properties. Additional research focusing on detailed characterization and synthesis of complex milk components like HMOs, and development of efficient and scalable production methods, remains crucial for advancing artificial milk production.
Routes to Artificial Milk Via Bottom-Up and Top-Down Strategies
Having established the compositional complexity that artificial milk technologies must replicate, we now examine the production methodologies that have emerged in response to these challenges. The manufacturing approaches developed thus far reflect strategic decisions about which milk components to prioritize and which structural elements are essential for functional equivalence. These technological pathways represent different philosophical approaches to the fundamental question of how closely artificial systems must mimic biological processes to achieve desired outcomes.
The quest for artificial milk production has yielded two conceptually distinct yet increasingly complementary technological trajectories (Fig. 2) The bottom-up strategy (Fig. 2A) disassembles milk into its molecular constituents, synthesizing each component individually before recombination. This reductionist approach enables unprecedented control over composition but faces significant challenges in replicating complex structural assemblies. Conversely, the top-down approach (Fig. 2C) leverages cellular machinery to recapitulate natural lactation processes, preserving structural complexity at the expense of compositional flexibility.
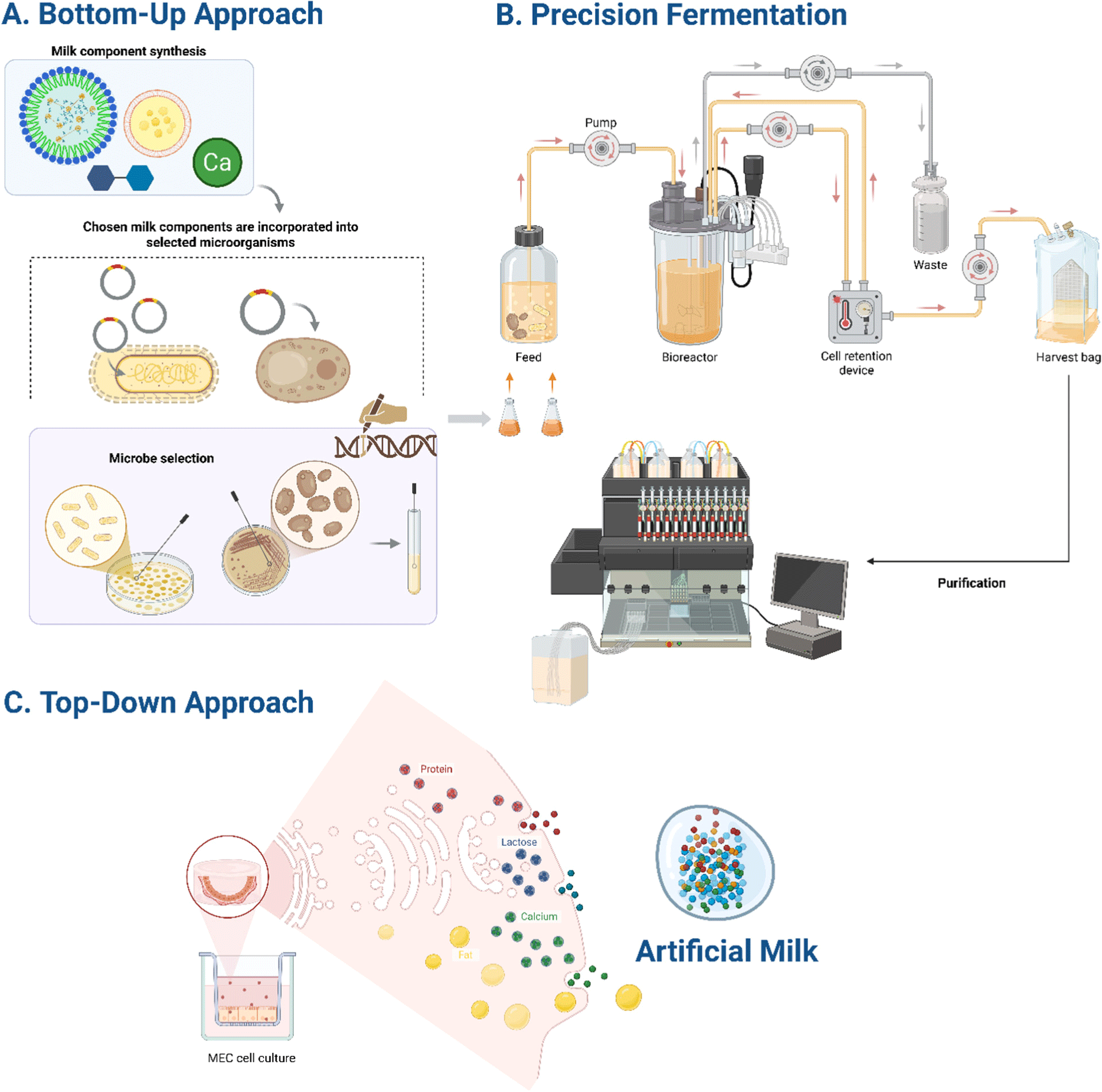
Precision fermentation, positioned at the intersection of these approaches (Fig. 2B), represents a technological bridge combining advantages from both methodologies. By employing genetically modified microorganisms as biofactories, precision fermentation enables production of structurally authentic milk proteins while maintaining component-level control characteristic of bottom-up approaches. This hybrid positioning explains its dominance in the commercial landscape, with approximately 76% of artificial milk ventures utilizing this technology (Table 2).
The bottom-up strategy excels in producing simpler components like specific fatty acids, vitamins, and mineral complexes. Organic acids found in milk can be synthesized through established chemical or biochemical pathways, while many bioactive compounds like carotenoids and flavonoids can be extracted directly from plant sources. However, as component complexity increases particularly for proteins with specific PTMs and higher-order assemblies the bottom-up approach encounters significant technological barriers that precision fermentation helps overcome.
Top-down approaches through cellular agriculture most closely mimic natural lactation processes by culturing mammary epithelial cells (MECs). This methodology potentially yields the most complete milk-like fluid with naturally assembled structures. The preservation of native assembly processes offers particular advantages for replicating complex structures like the MFGM, whose trilayer architecture and intricate protein-lipid composition present formidable challenges for alternative production methods.
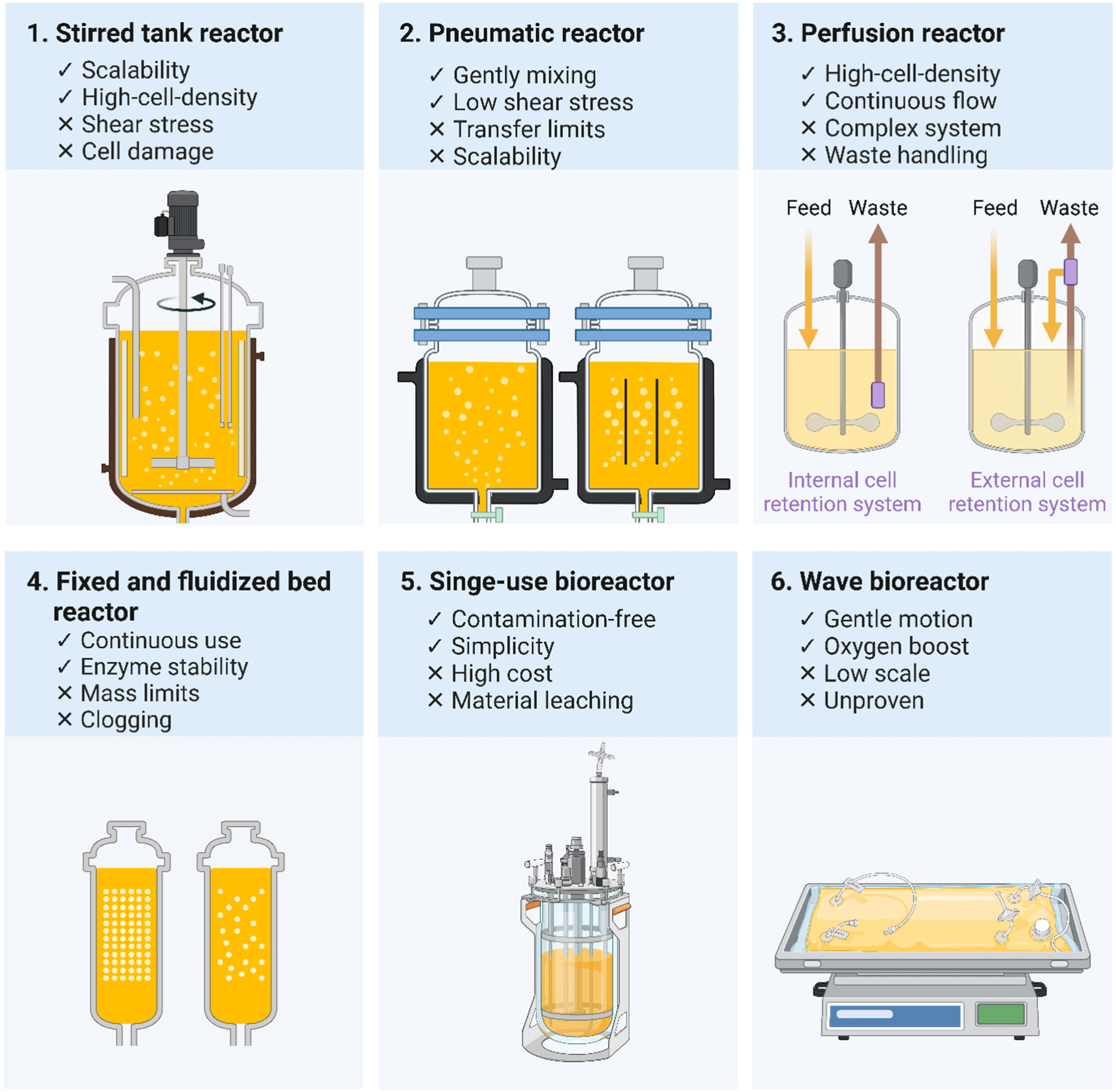
The integration challenges between these approaches center on three critical dimensions such as economic viability, structural fidelity, and regulatory pathway complexity. Economic analyses reveal substantial cost differentials between conventional dairy and precision fermentation ($15–25/kg versus $210–310/kg for proteins), highlighting the need for technological advances in strain engineering and bioprocess intensification (Lurie-Luke, 2024). Furthermore, as illustrated in Table 3, bioreactor selection significantly impacts production economics, with stirred tank reactors offering superior scalability but potentially compromising structural integrity of complex proteins. It can be reasonably inferred that media costs typically represent 60%–75% of total production costs across all bioreactor types, while energy requirements increase significantly with scale, particularly for stirred tank reactors. Downstream processing complexity varies by product, with proteins typically requiring more sophisticated purification than simple metabolites. Single-use bioreactors are increasingly preferred for flexibility and rapid changeover between products, though commercial production of artificial milk components currently favors stirred tank reactors due to their scalability and established protocols.
| Bioreactor type | Protein/fat produced | Microorganism | Volume (L) | Yield | Temperature (°C) | pH | Aeration rate (vvm) | Reference1) |
|---|---|---|---|---|---|---|---|---|
| Stirred tank reactor | Beta-lactoglobulin (protein) | Pichia pastoris | 5–1,000 | 5–10 g/L | 20–28 | 5.0–6.5 | 0.5–1.5 | Ostergaard et al. (2000); Reihani and Khosravi-Darani (2019) |
| Stirred tank reactor | Triglycerides (fat) | Yarrowia lipolytica | 50–500 | 30%–60% lipid of dry cell weight | 25–30 | 5.5–7.0 | 0.5–1.0 | Abghari and Chen (2014); Ledesma-Amaro and Nicaud (2016) |
| Perfusion reactor | Lactoferrin (protein) | Saccharomyces cerevisiae | 10–200 | 2–4 g/L | 28–32 | 4.5–6.0 | 0.2–1.0 | Janakiraman et al. (2015); Ostergaard et al. (2000) |
| Fixed bed reactor | Casein micelles (protein) | Escherichia coli | 10–50 | 1–2 g/L | 30–37 | 6.8–7.2 | 0.1–0.5 | Fang et al. (2022); Ostergaard et al. (2000) |
| Single-use bioreactor | Whey proteins (protein) | Kluyveromyces lactis | 1–500 | 8–12 g/L | 25–30 | 4.5–6.0 | 0.2–1.0 | Oda and Nakamura (2009); Ostergaard et al. (2000) |
| Wave bioreactor | Lipid globules (fat) | Mortierella alpina | 1–20 | 15%–20% lipid content | 22–28 | 5.5–6.5 | 0.5–1.0 | Jones et al. (2017); Ostergaard et al. (2000) |
Milk proteins embody complex biological molecules requiring sophisticated systems for accurate reproduction outside their native context. Attempts to produce them through alternative pathways often result in amino acid chain variations. These variations, sometimes necessary for protein transport or stability within the cell, might not put up with enzymatic cleavage, resulting in non-identical protein sequences that affect secondary and tertiary structures, component interactions, and ultimately, functionality (Li et al., 2024).
Sequence discrepancies pose considerable regulatory approval hurdles despite natural milk proteins exhibiting significant genetic variability across species and even within species. Any deviation from naturally occurring sequences may impact regulatory approval pathways. Proteolysis, particularly concerning caseins, presents another significant challenge. In cow milk, plasmin-mediated casein proteolysis operates under control of a complex activator and inhibitor system (Timlin et al., 2024). Alternative production organisms may exhibit higher proteolytic activity, potentially reducing purity and yield. Engineering the host organism to minimize proteolytic activity represents an important mitigation strategy currently employed by several companies in the field.
PTMs significantly influence protein stability, with some modifications proving desirable (phosphorylation and glycosylation), while others, such as fusion with carrier proteins, generally appear undesirable as they alter target proteins in ways not found in natural milk. Faithfully replicating a protein requires matching not only amino acid sequence and PTMs but also ensuring correct folding and aggregation behavior, essentially achieving identical primary through quaternary structure. This has sparked ongoing debates among regulators regarding acceptable similarity levels between artificial and natural milk proteins.
Amyloid fibril formation, common in various caseins, can limit protein concentration during production and cause downstream processing complications. In lactating cells, chaperones such as β-casein and αS1-casein often inhibit fibril formation. Therefore, efficient production necessitates careful consideration of protein production rate, potential degradation, and avoidance of virus or toxin formation in the production organism. Advanced expression system development has revealed that higher organisms often offer advantages over bacteria or yeast in facilitating correct PTMs and preventing problematic aggregation.
Milk lipids primarily consist of triglycerides (TGs) along with phospholipids, cholesterol, and various minor components, collectively contributing to milk energy content and enhancing its nutritional and functional attributes. The synthesis occurring within MECs involves complex processes requiring precise regulation for effective lactation. Similar challenges apply when attempting to replicate these processes beyond the mammary gland.
This perspective is further supported by TG synthesis involves complex enzyme and metabolic pathway interplay within the mammary gland. Fatty acids primarily obtained from the bloodstream, take forward incorporation into TGs via glycerol esterification. The exact fatty acid composition varies depending on animal diet and genetics (Stock and Wells, 2023), influencing milk nutritional value and physical properties. Replicating this intricate process artificially, particularly achieving a specific fatty acid profile, presents a substantial challenge requiring precise control over fatty acid type and proportion incorporated into TGs.
The MFGM functions beyond a passive TG container, playing critical roles in milk lipid stability, digestion, and bioavailability (Ozturk et al., 2022). It comprises a unique protein and lipid collection, many exhibiting biological activity such as immune modulation and gut health benefits. Indeed, replicating the MFGM complex composition and structure presents significant challenges for both bottom-up and top-down approaches to artificial milk production, with structural accuracy representing a particularly formidable barrier.
Current artificial milk production advancements focus on two main technological routes precision fermentation and cellular agriculture. Precision fermentation uses genetically engineered microorganisms to produce individual milk components, including fatty acids and other lipid precursors (Nielsen et al., 2024). While promising for producing specific fatty acids, precision fermentation struggles to replicate the complex TG assembly into MFGs and MFGM formation.
Similar patterns emerge when addressing cellular agriculture. Cellular agriculture involves culturing MECs to produce milk lipids in a controlled environment (Jedrzejczak and Szatkowska, 2014). This approach offers potential to recreate the natural TG assembly into MFGs and MFGM formation, thereby producing a more comprehensive and possibly more nutritionally similar milk product. However, maintaining MEC culture viability and productivity over extended periods remains challenging, and animal-derived media components in culture systems raise ethical concerns and complicate truly animal-free milk creation (Maga et al., 2013).
Artificially produced milk lipid composition will inevitably differ from animal-derived counterparts to some degree, raising concerns regarding regulatory approval and consumer acceptance. Demonstrating artificial milk lipid safety and nutritional equivalence to animal-derived lipids remains crucial for gaining regulatory approval and ensuring consumer trust.
Precision fermentation represents a hybrid approach connecting bottom-up and top-down methodologies for artificial milk production. This technology utilizes genetically engineered microorganisms to produce specific milk components with high purity and customizability, while leveraging biological systems that mimic natural biosynthetic pathways. By serving as this technological bridge, precision fermentation offers unique advantages in integrated production systems for artificial milk.
Precision fermentation typically employs bacteria or yeasts grown in bioreactors, representing a well-established technology for producing individual ingredients. While heterotrophic or phototrophic microalgae can serve similar purposes, they typically fall outside precision fermentation categorization and require different bioreactor systems. The application of precision fermentation in food production has substantial precedent; citric acid production in Aspergillus niger has operated as an established industrial process since 1917 (Moeller et al., 2012). Other examples include insulin, vitamin C, lactic acid, microbial rennet, and riboflavin production.
A recent analysis by Eisner (2024) highlights the potential livestock industry disruption by precision fermentation, noting that for some products (insulin, microbial rennet), the transition was driven by factors beyond cost or animal welfare. For instance, microbial rennet offers greater specificity and higher yield in cheese production than calf rennet. Several HMOs have achieved commercially viable production using precision fermentation (Zhou et al., 2021), demonstrating the technology applicability to complex carbohydrate structures.
The current commercial focus in precision fermentation centers on single proteins, particularly β-LG and human or bovine lactoferrin. β-LG has gained regulatory approval in several countries, while lactoferrin approval remains limited to the US, with potential new approvals anticipated elsewhere in the near future. The production of animal-identical TGs currently represents an active research area, though it would oversimplify to assume precision fermentation could easily provide viable methods for producing specific milk lipids given the complex genetic modifications required to generate individual fatty acids or more complex lipids.
A prevalent protein production method involves locating the gene encoding the desired protein within the animal genome and subsequently transferring it to a host organism for production. The host organism undergoes cultivation in fermenters, with protein production initiated by specific growth medium triggers. Careful induction media selection proves essential to prevent potential consumer acceptance challenges. Downstream processing, typically including filtration and chromatography, separates and purifies the protein from the fermentation broth, representing a significant portion of overall production costs.
Despite precision fermentation reaching technological maturity, cost continues to pose a substantial obstacle to traditional dairy ingredient replacement. The significant expense associated with bovine protein production through precision fermentation, estimated at $210–310/kg compared to conventional methods priced at $15–25/kg, underscores the necessity for additional cost reduction efforts. Within the main parameter of this review, the existing bioreactors used for precision fermentation (Table 3), along with the benefits and drawbacks of different bioreactor models applied in precision fermentation (Fig. 3), are currently outlined. It is important to note that Fig. 3 includes Pneumatic reactor for illustrative purposes; however, their application in industrial-scale recombinant protein and milk fat production is limited due to scalability and mass transfer constraints. Precision fermentation primary constraint lies in its output, which generally remains limited to one or a small number of components rather than complete milk replication. For different fermentation products, bioreactor selection must balance numerous factors including shear stress, oxygen transfer, scalability, and product recovery. This multifactorial optimization process becomes increasingly complex given that yields and operating parameters vary significantly based on specific strains, media compositions, and process optimizations, which subsequently introduces additional technical challenges as scale-up considerations often necessitate modifications to operating parameters at industrial scales. These technical constraints manifest in protein-specific production strategies, wherein complex milk proteins requiring correct folding and PTMs generally necessitate lower-shear systems or mammalian expression systems rather than conventional stirred tanks. From an operational efficiency perspective, continuous fermentation systems demonstrate energy efficiency improvements of 30%–45% compared to batch processing by eliminating repeated heating, cooling, and cleaning cycles.
Higher organisms including plants, insects, and transgenic animals offer alternative routes for producing milk components with distinct advantages and limitations compared to microbial systems. Plants, particularly, offer environmental and economic advantages due to their relatively simple growth requirements. While tobacco plants have historically served as expression platforms, recent research explores common agricultural plants like soybeans, rice, potatoes, and edible insect (Lu et al., 2024), potentially enhancing consumer acceptance through familiarity with food crops. Additional insights can be derived from both intracellular ER accumulation and extracellular secretion pathways remain feasible, depending on the chosen expression strategy. Plants possess pathways for phosphorylation and glycosylation, enabling correctly folded protein production with PTMs, although glycosylation patterns may differ from mammalian patterns. Both stable transgenic lines and transient expression through Agrobacterium tumefaciens infection present viable options with different production timelines and scaling characteristics (Laible et al., 2017).
Protein degradation in plants varies significantly depending on specific location within plant tissue, highlighting the importance of carefully selecting the production site within the plant. Seed-based expression often provides better protein stability than leaf-based systems. Although insect or transgenic animal production systems could theoretically produce milk components with appropriate PTMs, they fail to resolve the ethical issues linked to animal agriculture that drive alternative production development.
Non-mammalian organism application for milk component production builds upon established methods in areas like antibody production (Campos et al., 2025). However, this approach does not align with animal-free milk production objectives. Transgenic mammals can produce proteins not naturally found in their milk, but this approach similarly fails to address the fundamental goal of producing milk without animal involvement.
Cellular agriculture, specifically referring to animal cell cultivation to produce animal products, offers a promising avenue for artificial milk production by mimicking natural biological processes. MEC cultures have demonstrated capacity to produce milk-like fluids when supplemented with lactogenic hormones like prolactin and other essential medium components (Jedrzejczak and Szatkowska, 2014). Given these outcomes, one must consider, a key challenge involves separating culture medium from produced milk, effectively mimicking the blood-milk barrier in mammals. Researchers explore approaches using intact cell layers or submerged cells with downstream processing for separation. While several proteins, lactose, and TGs have been produced in cell culture, achieving complete milk composition comparable to animal milk remains an ongoing challenge. The potential need to mimic developmental changes in mammary glands and MECs throughout lactation represents an unresolved question requiring further research.
Many current MEC culture systems rely on animal-derived media components such as fetal bovine serum, which conflicts with non-animal milk production goals. In turn, efforts to replace these media with plant-based alternatives continue to advance but face challenges in providing all necessary growth factors. The resulting artificial milk composition will likely differ from animal milk to some degree, raising important questions about consumer acceptance and regulatory approval pathways.
Moreover, it is worth noting that MEC cultures offer two key advantages over precision fermentation including facilitation of correct PTMs and potential for direct higher-order structure production like milk fat globules with original MFGM or extracellular vesicles. These capabilities address fundamental limitations in microbial production systems, particularly regarding structural complexity. Additionally, cultivating other milk-derived cells, such as leukocytes or macrophages, could enable extracellular vesicle production and enzymes like plasminogen activator with potential antiviral properties.
In most instances, precisely replicating milk taste and aroma presents significant challenges in cellular agriculture. These sensory properties depend on animal diet, environment, enzymatic changes, and microbiota influences. However, media design incorporating necessary precursors offers opportunities to modulate these aspects, potentially allowing customization beyond what conventional production permits. These technological approaches, precision fermentation and cellular agriculture, establish the scientific foundation for artificial milk production. However, the transition from technological possibility to commercial reality depends on navigating complex regulatory landscapes and addressing consumer acceptance barriers. The interplay between production technology selection and regulatory classification creates a critical feedback loop that shapes commercialization strategies and investment priorities.
Regulatory Frameworks and Consumer Acceptance of Artificial Milk
How do regulators approach artificial milk production? Significant jurisdictional variation exists, reflecting both the novelty of these technologies and different philosophical approaches to food innovation policy. Consider the United States such as the Food and Drug Administration (FDA) has established a Generally Recognized As Safe (GRAS) notification process; apparently, a pathway several companies have successfully navigated for recombinant milk proteins. At its core, the FDA approach primarily assesses substantial equivalence to conventional counterparts. Rather than focusing on production methods, compositional analyses and safety evaluations take precedence. The result? Relatively rapid commercialization of certain components, particularly whey proteins.
In striking contrast stands the European Union’s approach through its Novel Foods Regulation [Regulation (EU) 2015/2283]. Before any market approval can occur, comprehensive safety assessments must be completed. Under this more cautious framework, most artificial milk components are categorized as novel foods, a classification requiring thorough evaluation by the European Food Safety Authority (EFSA) prior to market authorization. Not only composition but also production processes experience greater scrutiny in the EU approach. This procedural thoroughness comes at a cost such as longer approval timelines. Despite several years of development, only limited approvals had been granted for recombinant milk proteins in the European market as of early 2025.
Of particular note that product classification poses particular challenges for regulatory review, as artificial milk components may receive different categorization depending on production method, composition, and intended use. Some jurisdictions distinguish between proteins produced through precision fermentation and those derived from cellular agriculture, applying different regulatory standards. Additionally, certain components may face classification as food additives rather than ingredients, triggering different review pathways. These classification distinctions have significant implications for labeling requirements, safety testing protocols, and market authorization processes.
Labeling requirements remain contentious across markets, with ongoing debates about terminology like “milk,” “dairy,” and “animal-free.” The FDA has maintained that terms like “milk” should refer exclusively to lacteal secretions from animals, while other jurisdictions have shown more flexibility when products demonstrate functional equivalence. Consequently, industry stakeholders and regulatory bodies continue working toward standardized nomenclature that balances accurate consumer information with fair market access for innovative products.
Consumer acceptance of artificial milk products depends on multiple interacting factors that vary across demographic segments and markets. Technological familiarity plays a significant role, with consumers showing greater acceptance for precision fermentation (which resembles traditional fermentation processes) compared to cellular agriculture. A 2024 market survey revealed that 62% of consumers expressed willingness to try precision-fermented dairy products after receiving basic information about the technology, compared to 43% for cell-cultured alternatives (Banovic and Grunert, 2023; Engel et al., 2024).
This phenomenon can be attributed to environmental concerns function as adoption drivers among environmentally conscious consumers, particularly when clear sustainability advantages receive demonstration. Studies indicating that precision-fermented dairy proteins could reduce greenhouse gas emissions by 91%–97% and land use by 78%–90% compared to conventional production resonate strongly with sustainability-motivated consumers (Nielsen et al., 2024; Purba and Sangsawad, 2025). These environmental benefits represent powerful marketing messages when substantiated through credible life-cycle assessments.
Price sensitivity remains a significant barrier, as current production costs for artificial milk components substantially exceed conventional dairy. Early adopters may accept premium pricing, but broader market penetration requires cost parity or near-parity with conventional products. Market research suggests most consumers will not pay more than a 15%–20% premium for environmental benefits alone, underscoring the importance of achieving cost competitiveness through technological improvements and scaling effects.
Sensory expectations strongly influence acceptance, with consumers demanding taste and texture equivalent to traditional dairy (Mahendra et al., 2023). Early products facing taste or functionality compromises have encountered limited market success despite environmental or ethical advantages. This highlights the critical importance of sensory optimization alongside nutritional equivalence, particularly for products targeting mainstream rather than niche markets.
Health perceptions vary widely, with some consumers viewing artificial milk as potentially safer (free from hormones, antibiotics, or pathogens) while others express concerns about “unnaturalness” or unknown long-term effects. This perception dichotomy necessitates targeted educational approaches addressing specific consumer segments with different primary concerns. Transparency regarding production methods, compositional analysis, and safety testing plays a crucial role in building consumer trust across demographic groups.
Cultural and demographic factors also demonstrate important roles in acceptance patterns, with younger, urban consumers typically showing greater openness to novel food technologies. Studies indicate that Generation Z and Millennial consumers express approximately twice the willingness to try artificial dairy compared to Baby Boomers (Coderoni et al., 2025; Fasanelli et al., 2025). Geographic variations also appear significant, with highest acceptance in Asia-Pacific markets (particularly Singapore and Japan), followed by North America and Northern Europe (National Frozen & Refrigerated Foods Association [NFRA], 2024).
As artificial milk technologies evolve, regulatory frameworks will likely adapt in several important dimensions. Standardized product definitions and categories will likely emerge to provide clarity for producers and consumers navigating this new category. Several industry coalitions have proposed unified terminology frameworks that distinguish production methods while emphasizing nutritional and functional equivalence to conventional products. Furthermore, international harmonization efforts may develop to facilitate global trade in these products while ensuring consumer safety. Organizations including the Codex Alimentarius Commission, for instance, have initiated discussions on appropriate standards for recombinant food proteins and cellular agriculture products. These harmonization efforts fundamentally aim to prevent unnecessary trade barriers while maintaining appropriate safety oversight across jurisdictions.
Regulators will need to consider appropriate environmental impact assessment methodologies that account for artificial milk production system unique aspects. Traditional agricultural impact frameworks may not adequately capture the distinct environmental footprint of biomanufacturing approaches. Development of specialized assessment tools for biotechnology-derived food products represents an active area of regulatory science development.
The novel protein database and safety assessment protocols will continue expanding as more recombinant proteins enter the market. This growing knowledge base will likely facilitate more streamlined safety evaluations for structurally related proteins based on established precedents. Notably, regulatory agencies have increasingly signaled willingness to consider categorical approaches that reduce redundant testing for similar proteins.
Life-cycle assessment standards specific to artificial milk production will likely develop to enable accurate sustainability comparisons. These standards will need to address unique aspects of biotechnology manufacturing, including media inputs, energy consumption, and waste stream handling. Several non-governmental organizations have initiated work on standardized methodologies to prevent greenwashing while enabling valid environmental benefit claims.
Intellectual property protections for novel production methods and engineered organisms will significantly shape the competitive landscape and potentially impact regulatory approaches. Patent portfolios covering key production technologies, microbial strains, and product formulations have grown rapidly in recent years. These intellectual property considerations intersect with regulatory frameworks through issues including data protection periods, approval transfer mechanisms, and biosimilar product pathways.
The extensive research and development efforts in artificial milk production are evidenced by the growing patent landscape (Tables 4 and 5). Recent patents in recombinant proteins demonstrate particular attention to addressing the structural challenges described in earlier sections, including modifications to enhance functionality while reducing allergenicity. The parallel development of patents for recombinant fat production highlights industry recognition of the importance of both protein and lipid components in creating comprehensive milk alternatives. Importantly, these intellectual property developments reflect the methodological sophistication underlying precision fermentation approaches and illustrate how theoretical understanding of milk composition directly informs technological innovation.
| Description of selected patents | Reference |
|---|---|
| The recombinant milk protein pointed out herein can be a recombinant β-lactoglobulin that includes an amino acid sequence featuring amino acid residue N152 of Bos taurus β-lactoglobulin, along with non-native N-glycosylation at that amino acid residue. | Geistlinger et al.(2019) |
| A micelle composition comprising both alpha casein and kappa casein, where at least one of the proteins is a recombinant or modified version of the original protein. | Radman et al.(2021) |
| Preparations of reconstituted or recombined milk, along with milk powder and its derivatives, which do not include any non-milk fats or non-milk proteins, along with the relevant methods involved. | Geistlinger et al.(2017) |
| Recombinant milk protein that has significantly reduced or nearly eliminated allergenicity, while maintaining one or more functional properties of the natural protein. | Bhatt et al.(2021) |
| A liquid colloid featuring a micellar form, which includes a recombinant α casein protein, a recombinant κ casein protein, and at least one salt, while explicitly excluding β casein protein from the micellar form. | Gibson et al.(2020) |
| The recombinant milk protein is an innovative form of recombinant β-lactoglobulin, characterized by an amino acid sequence that incorporates one or more residues selected from T4, T6, T18, S21, S27, S30, S36, T49, T76, T97, S110, S116, T125, S150, N152, and T154 of Bos taurus β-lactoglobulin. This protein features non-native glycosylation found on one or more specific amino acid residues. | Geistlinger et al.(2020b) |
| The egg replacer contains recombinant β-lactoglobulin, which shows a similarity of at least 80% to bovine β-lactoglobulin. | Geistlinger et al.(2020a) |
| A method for generating a purified milk product that contains secretory IgA (sIgA) derived from cultured mammary cells and plasma cells. | Strickland(2021) |
| A collection of recombinant proteins, including β-lactoglobulin, κ-casein, α-lactalbumin, β-casein, α-S2-casein, α-S1-casein, and serum albumin, where at least one of these proteins features a sequence exhibiting at least 70% identity to the amino acid sequence of bovine proteins, and is synthesized within a fungal cell. | Pandya et al.(2015) |
| Description of selected patents | Reference |
|---|---|
| The invention applies to microbial cells that contain triacylglycerol (TAG) with short chain fatty acids (SCFA), along with methods for utilizing these cells to generate lipids that include TAG with SCFAs. | El Tahchy et al.(2021) |
| A methodology for the production of docosahexaenoic acid in microbial cell culture involves the introduction of a vector that encodes a polypeptide from a polyketide synthesizing system into the microbial cells. | Facciotti et al.(2007) |
| The invention describes techniques for producing renewable compounds by the modification of novel triglyceride oils, utilizing C8, C10, C12, or C14 fatty acid chain lengths as substrates. | Franklin et al.(2012) |
| Genetically engineered Yarrowia lipolytica strains produce oil with over 50% EPA and a certain EPA% TFA ratio, resulting from overexpression of enzymes and deletion of peroxisome biogenesis factor protein. | Hong et al.(2010) |
| The invention emphasizes on producing oils, fuels, and oleochemicals from microorganisms, particularly oil-bearing microalgae, and involves genetically modifying them to enhance efficiency and oil composition. | Franklin et al.(2014) |
| Polyketide synthase systems for polyunsaturated fatty acids, sourced from non-bacterial organisms, are utilized in the synthesis of bioactive compounds and the discovery of novel microbes. | Metz et al.(2002) |
| Methods to generate ω-3 and/or ω-6 fatty acids in an oleaginous yeast host through the expression of the enzymes involved in the ω-3/ω-6 fatty acid biosynthesis pathway. | Picataggio et al.(2004) |
| A method that entails the heterologous establishment of an oxygen-dependent pathway in Saccharomyces cerevisiae grown on a non-fatty acid substrate to generate polyunsaturated fatty acids containing four or more double bonds. | Förster et al.(2005) |
Economic Challenges and Production Costs
The economic viability of artificial milk production faces significant challenges compared to conventional dairy, with production economics representing a primary barrier to widespread adoption. Current production costs for milk proteins through precision fermentation range from $210–310/kg, substantially higher than conventional methods ($15–25/kg; Wood and Tavan, 2021). This cost differential places artificial milk components in premium product categories rather than mainstream alternatives, limiting market penetration despite technological readiness. In addition, media costs constitute the largest expense in precision fermentation, typically representing 60%–75% of total production costs (Augustin et al., 2024). The specialized nutrients required for optimal protein expression and purification significantly contribute to this expense. Traditional media formulations containing complex ingredients like yeast extract and peptones drive costs but offer efficiency advantages compared to chemically defined alternatives. Recent innovations in waste valorization have demonstrated potential to reduce media costs by utilizing agricultural side-streams as nutrient sources.
Energy consumption in bioreactors, particularly for aeration and temperature control, forms another substantial cost category, accounting for 10%–15% of total production expenses (Drewnowski et al., 2019). Continuous fermentation systems have demonstrated energy efficiency improvements of 30%–45% compared to batch processing by eliminating repeated heating, cooling, and cleaning cycles. The renewable energy transition offers significant opportunities to reduce both costs and environmental impact, with several companies implementing on-site renewable generation or procuring renewable energy credits.
Downstream processing, including filtration, chromatography, and other purification steps, represents 15%–25% of costs and presents particular challenges for scaling. Current purification technologies designed for pharmaceutical applications often prove prohibitively expensive for food ingredients. Novel separation technologies including membrane-based systems and continuous chromatography have demonstrated cost reduction potential of 40%–60% compared to traditional batch methods. It is also important to consider that labor and overhead costs. While significant in absolute terms, typically constitute a smaller percentage of total costs and decrease proportionally with scale. Automation and process monitoring technologies have reduced labor requirements in recent production facilities. However, skilled labor availability remains a constraint for rapidly scaling operations, with specialized training programs developing to address workforce gaps.
Several approaches offer promise for improving artificial milk production economic competitiveness. Media optimization through defined media formulations, recycling and reuse systems, and alternative nutrient sources from agricultural side-streams could reduce this major cost component by 30%–50% (Lee et al., 2024). Companies have reported success with approaches including cell culture supernatant recycling, targeted supplementation of depleted components, and development of minimal media formulations specifically engineered for industrial production strains.
Strain engineering to improve yield, productivity, and robustness offers potential for 2–4 fold increases in production efficiency. Advanced metabolic engineering approaches have targeted flux optimization, reduced byproduct formation, and enhanced protein secretion capabilities. Recent developments in synthetic biology tools including CRISPR-based genome editing have accelerated strain improvement timelines from years to months, enabling rapid iteration toward optimized production platforms.
Process intensification approaches, including continuous fermentation, improved bioreactor designs, and integrated downstream processing, could yield 40%–60% cost reductions. Continuous processing eliminates unproductive downtime between batches and allows higher average cell densities and volumetric productivity. Several manufacturers have reported successful implementation of perfusion-based production systems achieving titers exceeding 15 g/L, dramatically improving space-time yields compared to batch processes (Yongky et al., 2019).
Scaling effects will progressively reduce costs as production volumes increase, with cost reductions of 50%–70% possible at commercial scale compared to pilot scale operations. These improvements derive from economies of scale in equipment, labor efficiencies, and improved capacity utilization. The investment required to capture these scaling benefits represents a significant barrier, creating a “valley of death” between proof-of-concept and commercial viability that several companies have struggled to cross.
Since 2018, over $500 million in venture capital has flowed into the artificial milk sector. Why such enthusiasm? Investment has concentrated primarily in precision fermentation platforms, reflecting their nearer-term commercialization potential compared to cellular agriculture approaches. While the sector’s early days saw investors focused on technology development, a notable shift has occurred, specifically more recent funding rounds have emphasized scaling and commercialization capabilities.
Adding momentum to this investment trajectory, corporate funding from established food and ingredient companies has accelerated. This is not merely about capital, but rather about access. Strategic partnerships between startups and established players have facilitated product launches through existing distribution channels and manufacturing capabilities. Through licensing agreements, supply contracts, and co-development arrangements, these collaborations reduce commercialization barriers for innovative technologies. What might take a startup years to build independently can now be accessed through strategic alignment with established market players.
Furthermore, public funding supporting research in both precision fermentation and cellular agriculture has increased in North America, Europe, and Asia, particularly targeting sustainability improvements. Government grants, tax incentives, and public-private partnerships have addressed fundamental research challenges while simultaneously reducing financial risk for early-stage companies. For example, Singapore has emerged as a particularly supportive ecosystem through its 30×30 initiative, which aims to produce 30% of nutritional needs domestically by 2030 and includes substantial investment in alternative protein technologies.
Complementing these various funding sources, impact investors focused on environmental sustainability have shown particular interest in artificial milk’s potential to reduce greenhouse gas emissions and land use compared to conventional dairy. The environmental benefits of biomanufacturing compared to traditional animal agriculture have attracted capital seeking both financial returns and positive impact. As a result, several specialized investment funds now focus exclusively on sustainable protein technologies, thereby bringing both capital and domain expertise to portfolio companies. Together with corporate partnerships and governmental initiatives, these investments create a robust financial ecosystem supporting artificial milk development from laboratory research through commercial scale-up.
Artificial milk technologies present significant opportunities for customizing milk composition to address specific nutritional requirements. Applications include infant formula with higher whey protein content and β-casein enrichment, and cheese-making milk with casein dominance. Additionally, technologies enable eliminating components causing technological challenges like the plasmin-plasminogen system or allergens, while modifying nutritional value through sugar or fatty acid composition alteration.
The production of proteins currently unavailable commercially, such as human lactoferrin, represents another significant opportunity. Cell cultures could enhance accessibility to milk from rare species or colostrum, providing research tools and specialized nutritional products. The similarity extent needed between artificial and animal milk for both consumer and regulatory approval remains unresolved, especially regarding higher-order structures such as casein micelles or MFGM. It is also important to consider that artificial milk components with varying molecular structures provide valuable research tools for understanding structure-function relationships. The environmental impact of artificial milk production compared to conventional methods requires further investigation, partly due to limited extensive production data and considerable variability among conventional dairy farming systems globally. Artificial milk production offers several potential advantages including stable product output regardless of seasonal changes, enhanced climate change resilience, and animal farming elimination as a zoonoses and antibiotic resistance contributor.
Maintaining current genetic diversity of milk proteins across different species and breeds may require additional conservation efforts as production technologies advance. Sterile production systems would eliminate negative impacts associated with milk from mastitis-affected animals. The dairy industry has engaged in non-animal milk production transition discussions for over twenty years, with recent technological advancements and growing commercial interest significantly accelerating this transition. Although the potential economic and social changes appear significant, the global implications for approximately 140 million dairy farms sustaining around 1,000 million people require careful consideration. The elevated costs associated with artificial milk and required production facility investments suggest that widespread adoption in developing countries where substantial milk production greenhouse gas emissions originate will require considerable time. The pathway toward an integrated dairy system incorporating both traditional and biotechnological production methods represents the most likely near-term scenario, with gradual shifts driven by technological progress, consumer preferences, and environmental considerations.
Conclusion
The biotechnological synthesis of milk-identical components has transcended theoretical possibility to achieve technical validation, though substantial impediments persist in establishing economic parity and structural verisimilitude with conventional dairy systems. Precision fermentation occupies a privileged methodological position at the biosynthetic nexus, simultaneously preserving component-level customizability while leveraging biological pathways that approach, yet do not fully recapitulate, the sophisticated quaternary architectures characteristic of natural milk constituents. The economic differential between precision fermentation and traditional production methodologies (approximately tenfold) establishes a deterministic commercialization trajectory wherein initial market applications will necessarily prioritize high-value, functionally distinct components with simplified structural requirements. Indeed, this economic reality engenders a bifurcated implementation pathway, wherein specialized applications with compelling value propositions will precede broader market integration, while technological advancements in strain engineering and process intensification progressively narrow the cost disparity. Heterogeneous regulatory frameworks across jurisdictions create variable commercialization timelines, introducing additional complexity to market entry strategies beyond purely technological considerations. It is also important to note that consumer adoption dynamics reveal a paradoxical tension wherein environmental sustainability benefits serve as powerful motivating factors primarily when sensory equivalence has been established, a hierarchy of preferences that constrains marketing strategies but clarifies product development priorities. The inevitable progression toward an integrated dairy production paradigm suggests not a revolutionary displacement but rather an evolutionary complementarity, wherein biotechnological approaches establish specialized market segments differentiated by functional attributes and price sensitivity. Collectively, the evidence presented herein supports reconfiguration of production systems to address sustainability imperatives while preserving the nutritional and organoleptic qualities that define consumer expectations.













