Introduction
Milk is the lactic secretion of mammals and contains valuable nutrients and immune components for optimum growth of neonates. The composition of milk varies depending on the lactation stage, energy requirements, and the growth rate of the neonate (Singh and Gallier, 2017). Fat is a major energy source in milk and provides essential fatty acids, phospholipids (PL), and cholesterol required for brain development (He et al., 2020). Fats are present as globules in milk and stabilized by a delicate membrane architecture known as the milk fat globule membrane (MFGM). The physical structure of the milk fat globules (MFG) plays an important role in digestion and postprandial metabolism (Baumgartner et al., 2017; Gallier et al., 2013a; Gallier et al., 2013b).
The composition and integrity of the MFGM are significantly altered in the production of infant formula (IF), and the MFGM is reorganized mainly with caseins and whey proteins during emulsification with vegetable oils. The changes in MFGM critically affect energy acquisition (Turgeon and Brisson, 2020), metabolic consequence (Bourlieu and Michalski, 2015), and gut maturation in infants (Li et al., 2018a).
Milk-based IF is commonly used for infants who cannot access human milk (HM). IF-fed infants showed greater body fats and body weight gain compared to HM-fed infants (Lönnerdal, 2014). The increased serum levels of amino acids, insulin, and urea observed in IF-fed infants are due to the increased utilization of protein rather than fat (He et al., 2019). Yuan et al. (2020) reported that fat is preferentially utilized in HM, and HM displays a faster fat digestion rate compared to IF. This suggests that the bioavailability of nutrients can be changed during the production of IF, and both quantitative and qualitative standardization should take into account for humanization of IF.
Whey and cream are two major sources for the production of MFGM and the composition of the MFGM varies depending on sources. Anhydrous milk fat contains only a trace amount of sphingomyelin (SM), whereas buttermilk (12–22 g/100 g PL) and butter serum (24–29 g/100 g PL) have a high level of SM (Rombaut et al., 2007). In addition, the PL content in dairy ingredients varies significantly depending on the analytical methods, season, and lactation period (Anto et al., 2020). In the present review, we would like to share up-to-date information about the role of the MFGM on fat digestion and infant nutrition.
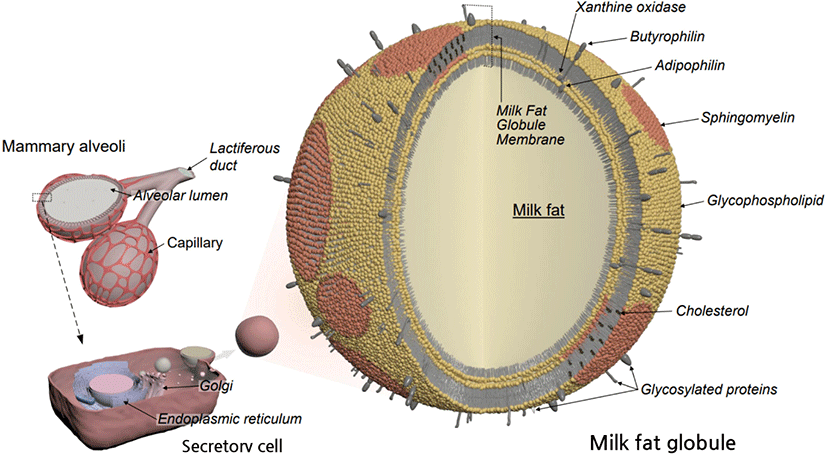
Structure of Milk Fat Globule Membrane (MFGM)
The MFGM is a triple-layered membrane (2%–6% of the globule mass) with 10–50 nm thickness. The MFGM is mainly composed of polar lipids (PL, glycolipids) and membrane-specific proteins (El-Salam and El-Shibiny, 2020). As shown in Fig. 1, the inner triacylglycerol (TAG) core is surrounded by glycerophospholipids and membrane proteins derived from the endoplasmic reticulum and external bilayers derived from the apical membrane and containing SM and ganglioside (GA) (Singh and Gallier, 2017). These MFGM components have lateral and asymmetrical organization. There are two distinct lipid domains in the lipid portion of MFGM. SM is closely associated with cholesterol and forms a densely packed liquid-ordered phase. The SM-rich domain is surrounded by a loosely packed glycerophospholipid matrix containing a high proportion of unsaturated PL (Simons and Vaz, 2004). SM and glycerophospholipids have different structural characteristics in terms of head group structure, hydrocarbon tail length, and degree of unsaturation. The predominant fatty acids of SM are long-chain saturated fatty acids and are esterified via an amide bond to the amino group of sphingosine, whereas two unsaturated fatty acids are bound to a glycerol backbone in glycerophospholipid such as phosphatidylcholine (PC; Li et al., 2015). The polarity of SM is significantly different from PC because of its asymmetric molecular structure and high hydrogen-bonding potential (Slotte, 2016). This segregated PL distribution influences the stabilization and digestion of MFG. The ordered domain contains low-digestible PL, namely SM and glycosphingolipid, whereas the highly unsaturated glycerophospholipid such as PC, phosphatidylethanolamine (PE), phosphatidylinositol, and phosphatidylserine, are readily hydrolyzed in the gut by the phospholipases (Lopez et al., 2019). Moreover, self-assembled SM-cholesterol clusters called lipid rafts act as cell signaling molecules (Rajendran and Simons, 2005). There are strong associations between the protein and lipid components in MFGM. Some fatty acids, including palmitic, stearic, and oleic acids, are strongly bound to the protein components of the membrane and possibly affects the membrane shape and plasticity (Keenan et al., 1982).
Composition of Milk Fat Globule Membrane (MFGM)
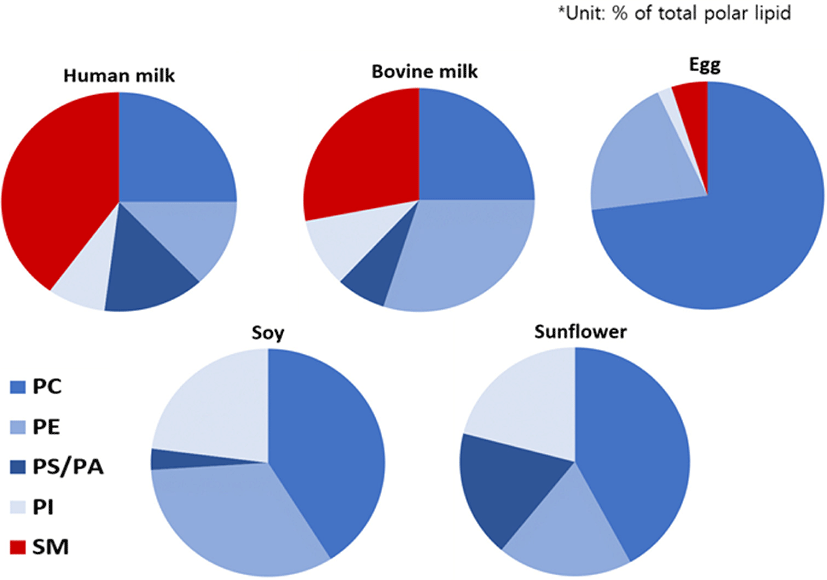
The lipid fraction of MFGM is mainly composed of TAG (56%–62%), polar lipids (26%–46%), and a minor lipid fraction [diacylglycerol, free fatty acids (FFA) and sterols] (Smoczyński et al., 2012). PL in the MFGM acts as an essential nutrient for organ growth. PL is a major constituent of the brain and provides choline, which is required for various fundamental biological metabolisms (Sánchez et al., 2021). The overall PL compositions of human MFGM and bovine MFGM are similar, but the PL derived from plants has a quite different composition, as shown in Fig. 2. Human and bovine MFGM accounts for approximately 40 and 26 wt% of the total polar lipids, respectively, and HM contains more SM but less PE compared to bovine milk (Mathiassen et al., 2015).
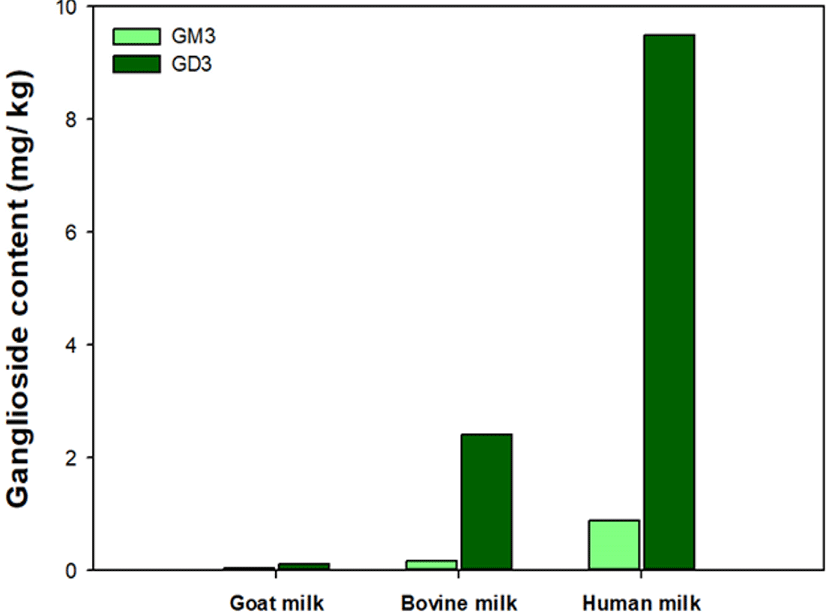
SM accounts for 25% of the total milk polar lipids (MPL) and is complexed with cholesterol in a mass ratio of 3:1 (Dewettinck et al., 2008). The SM content in milk generally increases with fat content but does not have a clear relationship with the size of the MFG (Graves et al., 2007). HM-fed infants obtain about 150 mg SM/day (Nilsson, 2016). Classification of GA is based on the number of sialic acids. GM (monosialylated GAs) and GD (disialylated GAs) are major GA species in HM, and GD3 (Neu5Aca2-8Neu5Aca2-3Galb1-4Glc-Cer) is the most abundant GD in HM (Fig. 3; Ali et al., 2021).
MFGM proteins account for 25%–75% of the total mass of the MFGM, and more than 500 MFGM proteins have been identified by proteomic analysis (Reinhardt and Lippolis, 2006). Major MFGM proteins are bound to the membrane structure with different binding strengths. For instance, xanthine dehydrogenase, xanthine oxidase, and mucin-1 are loosely bound and associated with protection against bacterial infection, whereas butyrophilin and adipophilin have relatively high affinities to the membrane structure and are related to protection from multiple sclerosis and fatty acid metabolism (Affolter et al., 2010; Mather, 2000). The affinity of MFGM proteins to the membrane structure also has practical implications for MFGM isolation and extraction (Zheng et al., 2013). Covalently cross-linked butyrophilin-xanthine oxidoreductase may support the physical structure of MFGs by connecting the inner and outer polar lipid layers.
Peroxidases and SM provide protection against oxidation of glycerophospholipids containing PUFA (Coliva et al., 2020; Fong et al., 2007; Oborina and Yappert, 2003). The tight hydrogen bonding between the amide portion of SM and interfacial water molecules interferes with the penetration of oxidizing species in the SM bilayer, resulting in better protection than PC (Oborina and Yappert, 2003). Thus, the extent of lipid peroxidation was inversely correlated with the SM fraction in liposomes (Coliva et al., 2020). Lipases may not have access to the lipid core of MFGs with intact outer polar lipid bilayers in the gut. Non-covalent binding between the outer polar lipid bilayer and inner polar lipid monolayer can be detached by enzymatic reactions in the stomach (Ye et al., 2011). In terms of mechanical property, the HM membrane extract showed greater compressibility than the bovine milk membrane extract due to a greater unsaturated fatty acids content. The greater content of anionic PL in HM means that the HM has superior lipase adsorption activity than bovine milk (Bourlieu et al., 2020).
Fat Digestion in Infants
Fat digestion is an interfacial process, and lipolytic enzymes acting on the surface of emulsified fat droplets determine the rate of fat digestion (Golding and Wooster, 2010). There are significant differences in lipid digestive physiology between infants and adults because of the small size of digestive organs and the differences in the activity of digestive enzymes, gastric pH, gut maturity, and dietary pattern (Abrahamse et al., 2012). In contrast to adults, the role of pancreatic TAG lipase on lipid digestion is not significant in infants while gastric lipase (GL), bile salt-stimulated lipase (BSSL), and pancreatic-lipase related protein 2 (PLRP2) compensate for the insufficient bile salts and pancreatic lipases (Lindquist and Hernell, 2010).
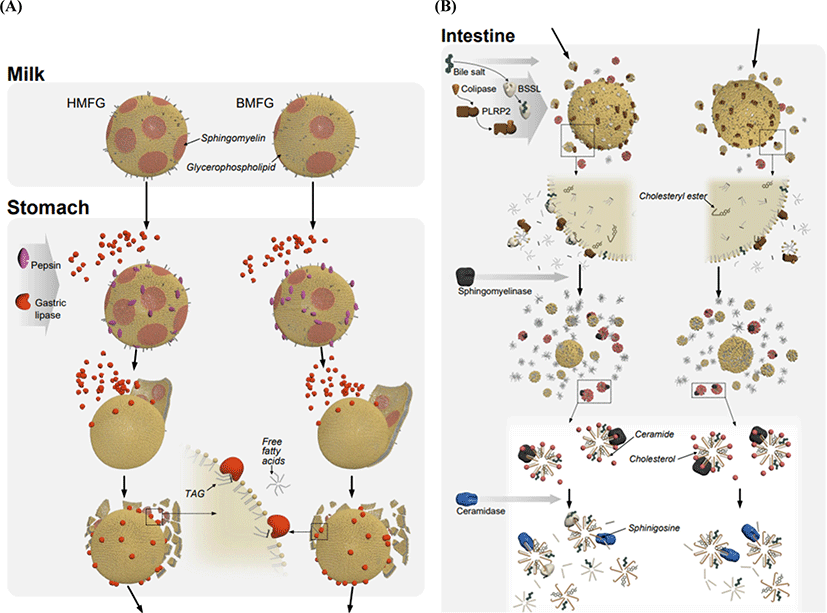
Milk fat digestion in infants is a sequential and balanced process, as shown in Fig. 4. The secretion and role of lingual lipase in fat digestion are uncertain, and actual fat digestion in infants is initiated in the stomach. As the pH drops below 5.5, the MFGM structure becomes less stable, and leads to coagulation of the fat globules (Lopez et al., 2017). Lipid digestion by GL in the stomach facilitates colipase adsorption at the interface and stimulates the action of BSSL and PLRP2 (Bernbäck et al., 1989; Johnson et al., 2013). GL has high activity over a broad pH range (optimum pH: 5.4–5.8) and hydrolyzes ester bonds at sn-3 of TAG. The extent of TAG hydrolysis by GL is up to 10%–30% and 60% in adults and infants, respectively (Abrahamse et al., 2012). The accumulation of liberated fatty acids at the emulsion surface inhibits GL (Pafumi et al., 2002). The 1,2-diacylglycerols produced by GL are further digested by PLRP2 and BSSL. BSSL shows high activity in the neonatal period and hydrolyzes TAG without positional specificity. BSSL is able to hydrolyze TAG, diacylglycerols, and PL and plays an important role in PL digestion in infants with low bile acid content (He et al., 2020). BSSL is an endogenous abundant lipase present in HM but has not been detected in bovine milk. BSSL readily hydrolyzes cholesterol ester, PL, and ceramide (Hernell and Bläckberg, 1994). BSSL is activated upon contact with bile salt but loses its activity upon pasteurization of HM. Alternatively, high-pressure treatment has been suggested as a way to minimize the heat-induced inactivation of BSSL (Singh and Gallier, 2017). Supplementation of recombinant BSSL to pasteurized HM and IF improved the absorption of long-chain fatty acids and the mean growth velocity of preterm infants (n=63) (Casper et al., 2014). BSSL has greater hydrolytic activity on medium and long TAG compared to PLRP2 (Xiao et al., 2011). These two enzymes are also important in PL digestion because PL is barely digested in the infant’s stomach. Dietary SM is hydrolyzed in the intestinal mucosa by alkaline sphingomyelinase (optimum pH: 9.0) to produce phosphocholine and ceramide. In a subsequent step, neutral ceramidase (optimum pH: 7.2) hydrolyzes ceramide to fatty acid and sphingosine. Released sphingosine is adsorbed, phosphorylated to sphingosine-1-phosphate (S1P) and converted to palmitic acid via S1P-lyase in the gut mucosa (Nilsson, 2016). Daily consumption of HM provides approximately 13 and 62 mg SM for preterm (170 mL) and full-term infants (800 mL) infants, respectively (Garcia et al., 2012). Intact MFGM and the presence of BSSL in HM are probably responsible for the difference in lipid absorption between HM-fed and IF-fed infants (Singh and Gallier, 2017).
Gut microbiota can also contribute to fat digestion and absorption by modulation of the gene expression of lipid digestion enzymes. Martinez-Guryn et al. (2018) demonstrated that small intestinal microbiota regulates host adaptation in response to dietary lipids. This might suggest a possible synergic interaction of probiotics with the MFGM in IF for efficient lipid digestion.
Factors Affecting Fat Digestion and Absorption
MFGM components stabilize MFG from coalescence and flocculation by providing electrostatic and steric repulsive forces (Singh, 2019). The physicochemical characteristics of fat globules in HM and bovine milk (or IF) are indicated in Table 1. The average fat globule size of HM (~6 μm) is more than 10 times greater than that of IF (~0.6 μm). IF shows a much higher ζ-potential (–36) than HM (–8) because the membrane PL is mostly replaced by casein and whey proteins during homogenization (Yuan et al., 2020).
| Characteristics | Human milk | Bovine milk/IF | References |
|---|---|---|---|
| Fat globules size (μm) | 0.35–13 | 1.0–10 (milk) 0.3–0.8 (IF) | Lopez and Ménard (2011), Ménard et al. (2010), Lopez et al. (2015) |
| Surface area (m2/g fat) | 2 | 2.6±0.2 (milk) 26.4±2.7 (UHT milk) 20–40 (IF) | Lopez et al. (2015) |
| Zeta potential (mV) | −7.85 | −9.4 (milk) −34 to −38 (IF) | Ménard et al. (2010) Yuan et al. (2020) |
| Total polar lipids (mg/100 mL) | 20.4±2.8 | 19.2±0.8 (milk) | Zou et al. (2013) |
| Concentration of polar lipids (mg/100 mL) | |||
| SM | 3–14 | 2.3±0.2 | Zheng et al. (2019) Sánchez-Juanes et al. (2009) |
| PC | 2–11 | 3.3±0.2 | |
| PE | 0.2–9 | 2.9±0.2 | |
| PI | 0.2–3.3 | 1.4±0.1 (PS/PI) | |
| PS | 0.8–4.5 | ||
| Cholesterol (mg/L) | 90–150 | 300 (milk) 0–4 (IF) | Koletzko (2016) Jensen et al. (1990) |
Smaller-sized fat globules have greater digestion rates than large-sized ones because of increased surface area for lipase adsorption; however, the composition and structure of the interfacial layer surrounding the fat globules also influence the lipid digestion rate (Bourlieu and Michalski, 2015). In accordance with the relationship between surface area and digestion rate, the GL digestion of bovine milk (smaller-sized fat globules) is faster than that of yak milk. However, this trend is reversed when the fat globules in yak milk are adjusted to sizes similar to that observed in bovine milk due to the structural characteristics of yak milk, such as its higher proportion of short-chain fatty acids and SM-rich ordered domain (Luo et al., 2018; Luo et al., 2020). The presence of MPL improves the hydrolytic action of GL by 2.5-fold compared to soy lecithin (Mathiassen et al., 2015). Similarly, the interfacial composition of an emulsion critically influences the rate and extent of lipolysis by pancreatic lipase. The presence of emulsifiers, such as proteins, PL, and surfactant (Tween 20), interact with lipase or released fatty acids and promote/inhibit adsorption of lipase on the emulsion surface (Mun et al., 2007).
Zhao et al. (2019) observed the changes in the size and interfacial composition of MFG following homogenization and combined homogenization-pasteurization (HTST vs. UHT). Homogenization recruited milk proteins (caseins and whey proteins) to the surface of MFG but expelled glycosylated proteins due to disintegration of the MFGM. These process-induced changes modulate digestion rate. Ultimately, thermal processing of homogenized milk slowed the initial rate of lipid digestion and the overall extent of intestinal digestion in vitro (Liang et al., 2017). The thermal treatment was suggested to limit the ability of lipase to adsorb to the fat globules by promoting increased fat globule flocculation or changes in the structure of the interfacial layer surrounding the fat globules, such as protein crosslinking and denaturation. Although these results were consistent with Tunick et al. (2016), they contrasted with those of Bourlieu and Michalski (2015) that thermal processing could increase the extent of lipid digestion under simulated small intestinal conditions. These discrepancies across studies may be caused by differences in the characteristics of the milk samples, processing conditions, or simulated digestion models used. It is important to consider these factors in the interpretation of the findings. Nonetheless, manipulation of the interfacial structure or aggregation state of MFG would be expected to affect milk fat digestion.
In an independent study, mature HM showed a greater in vitro gastrointestinal rate than commercial IF despite a larger fat globule size, perhaps, in part, due to thinner MFGM interfacial layer surrounding the fat globules, which regulates lipase-mediated lipolysis (Cheong et al., 2018). According to Pan et al. (2022), IF prepared from MFGM lacks interface PL associated with the fat globule structure, has a small particle size (0.38 μm) and a thicker interface layer (and interacts with casein micelles), most of the MFGM exists in the aqueous phase in free-form, and IF does not form a fat globule structure like HM. The homogenization-mediated thick interfacial protein layer formed on the fat globule surface of IF possibly impairs the hydrolytic action of lipase. A novel IF concept mimicking the HM fat globule structure has been suggested (Gallier et al., 2015).
Bläckberg et al. (1981) reported that pancreatic lipase hardly hydrolyzes fat globules covered with MFGM in HM but fat globules are readily hydrolyzed with the aid of colipase and phospholipase A2. The result of that study implied a protective function of the PL surface against the digestive action of pancreatic lipase. Recently, Lu et al. (2021) demonstrated that the addition of PL (soy lecithin; 0.5%, w/v) to milk before homogenization delayed liberation of FFA from fat globules during simulated in vitro intestinal digestion. In addition, the stability of fat globules during storage was increased in PL-added milk by suppressing the adsorption of whey proteins on the fat globule surface.
The effect of PL on fat digestion varies depending on digestion stage (stomach vs small intestine) and type of PL. The activity of GL on emulsions coated with milk PL increased noticeably compared to a soy lecithin-stabilized emulsion with similar size (Mathiassen et al., 2015). The major difference between milk and soy PL is SM. The lipid-ordered SM-cholesterol domain in MFGM might facilitate lipase adsorption to the emulsion surface. Conversely, the opposite effect was observed in the rate of pancreatic lipase hydrolysis, but only when the milk PL-stabilized emulsion was pretreated with GL the emulsion has greater pancreatic lipase activity compared to its soy PL counterpart, a result that was confirmed in vivo in mice (Mathiassen et al., 2015). IF does not contain BSSL, which accounts for about 1/3 of the lipase activity in infants. Thus, the strategic design of the lipid droplet surface layer in IF to maximize GL activity could improve the total fatty acid absorption, benefiting IF-fed infants especially pre-term infants.
An MPL-stabilized emulsion showed enhanced in vitro intestinal digestion and postprandial lipid metabolism in mice compared to an emulsion stabilized by soy PL (Lecomte et al., 2015). The proposed reason for these results was that both gastric emptying and intestinal absorption were promoted in the MPL emulsion. More in-depth clinical research is required to confirm whether postprandial lipid metabolism can be modulated by the selection of the type of PL or lipid emulsifiers. The estimated SM intake of HM-fed infants (4 wks old) is about 18–84 mg/day, whereas that of standard IF-fed infants is 9.6–20.4 mg/day (assumed 600 mL consumption/day; Zheng et al., 2019). This difference possibly causes critical difference in fat digestion and the subsequent utilization of fat. Generally, the SM content of commercial milk-based IF is less than that in HM and even less in soy-based IF because soy-based IF does not have SM (Cilla et al., 2016).
Liang et al. (2018) examined the effect of emulsifier type (sodium caseinate, lactoferrin, whey protein isolate, and MPL) and emulsion droplet size on gastrointestinal digestion in vitro. They could not find any significant difference on overall lipid digestion profiles depending on emulsifier type. However, the MPL-stabilized emulsion did not show the transient lag phase observed during digestion of the protein-stabilized emulsions. In a recent study by Liu et al. (2021), the fatty acid release was inversely correlated with the particle size of MPL-stabilized emulsions. Furthermore, the overall gastrointestinal FFA release from MPL-stabilized emulsions was comparable to that of HM. This implies that the particle size of IF does not have to be close to HM as long as an appropriate amount of MPL is incorporated into IF.
The fatty acids of the TAG in HM have preferential region-distribution. Palmitic acid (C16:0), the primary saturated fatty acid (20%–25%), is esterified at the sn-2 position mainly (>70%), whereas the sn-1,3 positions of TAG are occupied by unsaturated fatty acids (Innis, 2011). Fatty acid region-distribution affects fat digestion kinetics because GL shows stereospecificity for the sn-3. Medium chain fatty acids (C8–C12) are mainly located in the sn-3 position of TAG and are rapidly utilized in the liver after absorption via the portal vein (Gómez-Cortés et al., 2018). Fatty acids esterified at the sn-2 position of TAG are regarded as a hot spot because about 70% of the fatty acids absorbed as sn-2 monoacylglycerols are absorbed across the enterocytes and conserved in the original position during re-esterification into TAGs for secretion into the plasma as chylomicrons (Zhang et al., 2018). The improved absorption of at sn-2 palmitate compared to the one esterified to TAG 1,3 positions in HM and IF is well known (Innis et al., 1994; Tomarelli et al., 1968). The absorption of fatty acids decreases as the fatty acid chain length or unsaturation degree increases in HM. The decreased long chain saturated fatty acid and fatty acid-calcium soap formation are associated with constipation and decreased mineral bioavailability (Li et al., 2010). Moreover, the accumulation of long chain fatty acids at the interface limits lipase access to other TAG (Guo et al., 2017).
Hageman et al. (2019) used an in vitro digestion assay to compare the FFA release from IF prepared from only vegetable fat with a milk and vegetable fat combination (67:33). There was no significant difference in the total fatty acid release, but greater amounts of short (C4:0) and medium chain fatty acid (C6:0, C8:0, and C10:0) were released from the IF containing combined fat compared to IF containing vegetable fat alone. The delivery of butyric acid (C4:0) possibly contributes to the development of gut maturation. Differences in TAG composition effect the self-assembly behavior of the lipid species during gastrointestinal digestion, with increasing evidence suggesting that the more copious fatty acid digestion products guide the self-assembly process. Pham et al. (2020) demonstrated that not all IF form the same micellar cubic phase that HM forms during lipid digestion. Inverse micellar cubic phases were formed from milk liberating more long chain unsaturated fatty acids (C18:1 and C18:2) by contrast to hexagonal and inverse bicontinuous cubic phases of lower interfacial curvature from milk liberating more long chain saturated fatty acids (C14:0, C16:0, and C18:0). Although the actual function of specific structures is still uncertain, it could modulate lipase adsorption at the interface, with interesting possibilities for designing IF that effectively mimic the nanoscale structures seen in HM and, in turn, improve the nutritional outcomes of IF-fed infants.
The food matrix refers to the specific organization of food constituents in space or the presence of nutrients that critically influence the release and absorption of nutrients during digestion in the gastrointestinal tract (Ubbink et al., 2008). Lamothe et al. (2017) compared the rate of fatty acid release from various dairy matrices (milk, yogurt, and cheese). Fatty acid release from the solid matrix was delayed compared to the liquid and semi-solid dairy matrices.
The supramolecular structure of the MFG influences the rate of lipid digestion (Michalski et al., 2006). In a dietary intervention study involving 49 men and women, cheese consumption lowered LDL-cholesterol compared with butter intake of equal fat content (Hjerpsted et al., 2011). This indicates that the integrity of the MFGM matrix in the products possibly delivers different health consequences. In addition, the high calcium content of dairy products, such as cheese, is involved in reduced fat absorption by the formation of calcium-fatty acid complexes (Lamothe et al., 2017).
In randomized human clinical trial (n=57), consumption of an equal amount (40 g milk fat/day, 8 wks) of whipping cream (no MFGM) or butter oil (MFGM) led to significant differences in the lipoprotein profile and cholesterol metabolism (Rosqvist et al., 2015). The exact mechanisms for this result still remain unclear. The one possibility is that the SM in the MFGM suppressed intestinal cholesterol uptake via decreasing the thermodynamic activity of cholesterol monomers (Eckhardt et al., 2002). These findings suggest that the manipulation of lipid structure poses different health consequences.
The homogenization-induced increased casein content on the fat globules surface results in gastric coagulation and inhibitory effect on GL. The physical state of milk in the stomach and the protein integrity also affect milk lipids digestibility (Lopez et al., 2015). Lipid digestion in IF was increased when intact proteins were replaced with hydrolyzed proteins (Nguyen et al., 2018). Fondaco et al. (2015) compared the physicochemical properties and rate of in vitro lipid digestion in HM, bovine milk, and IF (4 types). A positive linear relationship between the rate of lipolysis and fat globule surface area was only found in IF. This implies that other matrix components are possibly involved in the rate of lipolysis in HM. In addition, the pH value for maximum viscosity differed (pH 3.0 for HM vs. pH 4.0–5.0 for IF), which could influence the rate of gastric emptying and possibly satiety, but there was no correlation found between viscosity and lipid bioaccessibility.
The physical state of fat (solid fat vs liquid oil) critically affects its digestibility. Whey protein-stabilized emulsions containing increasing levels of solid fat (hydrogenated soybean oil, melting point>37°C) showed decreasing liberation of FFA during intestinal digestion in vitro (Guo et al., 2017).
The Role of Milk Fat Globule Membrane (MFGM) in Infant Nutrition
There is a growing body of evidence regarding the beneficial health effects of MFGM in infants. MFGM supplementation to IF (0.5 g/L) accelerated neurodevelopment and promoted cognitive function in healthy full-term infants with a low incidence of pathogen-associated adverse effects (Li et al., 2019). Infants fed formula enriched with SM (28–71 mg/mL) displayed increased developmental myelination in the brains and later verbal development in the first 2 years of life (O’Muircheartaigh et al., 2014; Schneider et al., 2019). Comparing the effect of SM-fortified milk (20% SM vs. 13% SM) on the neurobehavioral development of low-birth-weight infants revealed a markedly higher serum SM level and neurodevelopment test score in the SM-enriched group than the control group. Dietary SM and its metabolites such as cerebroside, are able to cross the blood-brain barrier and promote myelination (Tanaka et al., 2013). Although the long-term effect of SM fortification is still not clear, SM has great potential in brain development in preterm or low-birth-weight infants.
Healthy 6-month-old infants fed IF fortified with MPL showed an increased serum GD level and improved hand-eye coordination, and general IQ compared to the control group fed standard IF but did not differ in cognitive development score or GD serum level from the reference group of healthy exclusively breastfed infants (Gurnida et al., 2012). On other work, MFGM supplementation improved insulin signaling in hippocampus and cerebral cortex of aged rats, which suggests that MPL possibly improve age-related cognitive decline (Tomé-Carneiro et al., 2018). However, these positive effects should be confirmed in clinical studies.
The effect of MFGM or MFGM/prebiotic combination on stress (maternal separation)-induced microbiota change, visceral hypersensitivity, and brain function was evaluated in rats (O’Mahony et al., 2020). The MFGM supplementation significantly increased the beta-diversity of the cecal microbiome and improved spatial learning in the stress group (O’Mahony et al., 2020). A similar phenomenon might be expected between MFGM and probiotics on cognitive development (Kosmerl et al., 2021). Further studies are needed to examine this possibility and elucidate mechanism of interaction. It may be that the MFGM interacts with the bacterial surface to facilitate the delivery of probiotics to their target site of action or that the MFGM creates a favorable environment in the gastrointestinal tract for the probiotic species, alters their metabolism, and, ultimately, influences the gut microbiome. According to these studies, the MFGM-mediated improved brain function may be associated with modulation of the gut microbiota via the gut-brain axis, and supplementing an MFGM concentrate may modify gut microbial composition and by-products to a profile more comparable to an exclusively breastfed reference group.
Supplementation of IF with MFGM altered metabolic outcomes due to a shift in the preference for protein utilization (e.g., increased level of lactate, succinate and amino acids) to a preference for fat utilization and converted the fecal microbiome to more like that of HM-fed infants (He et al., 2019). The high sensitivity to lipolysis and β-oxidation in early life can help inhibit excessive weight gain in later life and thus decrease the risk of obesity (Lee et al., 2021). MFGM supplementation significantly reduced adipogenesis and body weight gain by promoting brown fat formation in white adipose tissues in rats (Li et al., 2018b). A subsequent study found that administration of MFGM to high-fat diet-fed rats during pregnancy and lactation stimulated brown fat development in male offspring (Li et al., 2020). Recently, Zhang et al. (2021) demonstrated that MFGM supplementation during suckling reduced the risk of maternal high-fat diet-induced nonalcoholic fatty liver disease in mice due, in part, to decreased oxidative stress, and restoration of mitochondrial dysfunction. The same research group evaluated the effect of MFGM supplementation during pregnancy and lactation in obese rats on the skeletal outcomes of male offspring. Maternal MFGM supplementation (400 mg/kg body weight) ameliorated the stunted skeletal growth of male offspring at weaning and protected against high-fat diet-induced bone microstructure degeneration and insulin resistance in adulthood offspring (Han et al., 2021). The enhanced insulin-like growth factor-I activity was suggested as one of the possible reasons for the positive skeletal outcomes. Based on these results, MFGM has a protective role against diet-induced obesity development and obesity-related complications.
SM, the major MFGM constituent, was evaluated for its effect on lipid metabolism. Narita et al. (2016) found that long chain bases of SM were effectively transported into cells via acyl-CoA synthetases and competitively inhibited the uptake of long chain fatty acids. Other research demonstrated the anti-inflammatory activity of SM. Dietary SM reduced systematic inflammation in a diet-induced obese mice model, and milk SM downregulated pro-inflammatory gene expressions, such as tumor necrosis factor-alpha (TNF-α) and C-C motif chemokine ligand 2 (CCL2) in LPS-stimulated RAW 264.7 macrophages (Norris et al., 2017). Interestingly, the long chain sphingosine bases (C16-ceramide and C24-ceramide), exerted a similar anti-inflammatory effect to SM, but dihydroceramide species (sphinganine base) did not display anti-inflammatory activity. Human clinical studies should be undertaken to confirm the beneficial effects of SM on lipid metabolism and immune modulation.
Richard et al. (2017) reported that the administration of a choline mixture (50% PC, 25% free cholines, and 25% glycerophosphocholines) to rat dams during lactation improved the immune system development in offspring and elicited a more effective maternal inflammatory response following mitogenic immune challenge. Similar effects were confirmed when butter milk was used as a choline source (Azarcoya-Barrera et al., 2021).
It is well known that SM and its metabolites present in MFGM involved in the regulation of cell growth, intestinal lipid uptake, and gastrointestinal immune responses (Rohrhofer et al., 2021). They act as signaling molecules and mediates cell proliferation and apoptosis. For example, S1P improves endothelial cell survival and migration of immune cells such as lymphocytes, dendriatic cells and macrophages which are closely related to gut homeostasis (Nilsson, 2016). Moreover, metabolites of dietary sphingolipids influence gut microbiota by modulation of cell attachment of commensal bacteria or pathogens (Rohrhofer et al., 2021). In addition to sphingolipids, GA in MFGM also modulate proinflammatory signaling in the gut. Miklavcic et al. (2012) reported that the decreased GM3 stimulates synthesis of proinflammatory signals and increases susceptibility to pathogens. Conversely, dietary GA consumption alleviate inflammatory symptoms by blocking inflammatory cascade.
According to Bezirtzoglou et al. (2011), HM-fed infants had doubled the fecal number of Bifidobacterium cells of IF-fed infants. IF feeding increased the Atopobium level and decreased the numbers of Bifidobacterium. In an independent study, supplementation of IF with dairy lipid and MFGM influenced protein digestibility and microbiota composition. Incorporation of milk lipid and MFGM fragments in IF accelerated mucosal immune development, reduced protein digestion, and altered the fecal microbiota composition in neonatal piglets (Le Huёrou-Luron et al., 2018).
MFGM supplementation in IF stimulates epithelial cell proliferation and gut barrier function in rats by strengthening tight junction proteins and modulating the neonatal gut microbiome (Bhinder et al., 2017). MFGM supplementation also improved obesity-associated gut dysbiosis and increased the Bacteroidetes/Firmicutes ratio in high-fat diet-fed mice study (Li et al., 2018a). Following the analysis of fecal samples, Zhao et al. (2022) reported that MFGM components especially lactadherin (milk fat globule-epidermal grow factor-8), sialic acid, and PL, promoted growth of Bifidobacterium and suppressed Veillonella, Escherichia and Shigella in healthy full-term newborns. By contrast, supplementation of MFGM and lactoferrin to IF led to only subtle changes in the stool microbiome and metabolome of healthy full-term infants at 4 mo of age (Chichlowski et al., 2021). The authors of that study reported that the abundance of the microbial community varies primarily depending on infant age rather than the diet.
The MFGM enhanced the gut barrier function and lowered the intestinal permeability in a rat model of short bowel syndrome (Yu et al., 2021). Regulation of the NLRP6 inflammasome pathway contributed to the improvement of gut dysbiosis. According to Levy et al. (2015), microbiota-modulated metabolites (taurine, histamine, and spermine) regulate NLRP6 inflammasome, intestinal IL-18, and downstream antimicrobial peptide (AMP) patterns. Inflammasome deficiency distorts this harmonious AMP environment. Restoration of the metabolite-inflammasome-AMP axis reestablishes a normal microbiota and ameliorates colitis. Zhang et al. (2020) reported that MFGM improved viability of Lactobacillus rhamnosus GG from bile stress both in vitro and in the murine GI tract. The promotion of probiotic survival by MFGM might provide a beneficial effect on gut health.
Safety of Milk Fat Globule Membrane (MFGM) Fortification
GD supplementation to IF at 1.43 mg/100 kcal significantly reduced Escherichia coli in feces of preterm newborn infants at 7 days after birth and substantially increased the level of bifidobacteria in feces at 30 days without any hint of adverse effects (Rueda et al., 1998). Billeaud et al. (2014) reported that the MFGM might have different nutritional and safety consequences depending on the actual composition of the MFGM ingredient. Lipid-rich MFGM supplementation (n=70, age 14 days) for 14 weeks did not show any major safety concerns such as weight gain, morbidity, and metabolic markers, whereas protein-rich MFGM enrichment (n=72) was associated with a higher eczema rate compared to the controls (n=57). However, caution is needed in the interpretation of results because eczema rate assessment was not standardized and was mainly based on the parental report. Moreover, more large-scale clinical data are required to draw solid conclusions about the health benefits and safety of MFGM supplementation (Fontecha et al., 2020).
Conclusion
To date, the importance of the fat globule organization and interfacial composition on infant nutrition has been overlooked compared to the chemical composition of lipid in IF. The lipid species of milk exosomes are close to that of the MFGM, and some of the bioactivity of exosomes is derived from their lipid content (Subra et al., 2010). Although evidence supporting MFGM or its fraction in the formulation of IF are increasing, several issues outlined below should be clarified to proceed to the next clinical stage.
Standardization of the MFGM ingredient is a priority. Currently, MFGM is produced from dairy side streams, such as serum or cream concentrate. Substantial variations in the types and amounts of PL, sialic acid, and membrane protein components are observed depending on the source and processing methods (Brink et al., 2020; Qu et al., 2019). Heterogeneity in the MFGM composition is probably a major reason for the discrepancy in the beneficial effects of MFGM supplementation in children (Ambrożej et al., 2021). The concentration of individual MFGM components still shows large variation, and underlying the molecular mechanisms of MFGM for infant health are still ambiguous. Moreover, the exact and synergistic roles of individual components in MFGM on specific health effects should be clarified to establish a nutritional recommendation. In addition the effective target population (pregnant mothers or infants) should be carefully selected to optimize the health benefits. The final future challenge will be the modulation of lipid digestion and subsequent fat absorption via the formation of a composite structure. Furthermore, a multi-composite emulsion structure that elicits an improved sensory quality will be possible in the future.