Introduction
During the production of dry-aged pork loin ham, proteins and lipids that make up the meat are broken down into free amino acids and free fatty acids by the action of enzymes such as calpain, cathepsin, and lipase (Toldrá et al., 1997). The oxidation of these fatty acids contributes to the flavor formation of meat products. On the other hand, if oxidation occurs in an environment where appropriate temperature and humidity conditions are not met, the risk of meat spoilage and rancidity increases (Morrissey et al., 1998). To ensure storage stability of dried meat products, manufacturers are using oxidation prevention [butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA)] called synthetic antioxidants (Oswell et al., 2018).
Recently, consumer awareness of the potential carcinogenicity of synthetic antioxidants has led to an increase in research to replace BHT and BHA (Karre et al., 2013). Natural additives with antioxidant activity, such as rosemary, berries, and cruciferous plants, have been identified as having antioxidative effects (Lorenzo et al., 2018; Ramirez et al., 2020; Sebranek et al., 2005). In particular, the high phenol and flavonoid content of cruciferous plants has led to research on the possibility of replacing synthetic antioxidants (Ramirez et al., 2020).
Mustard (Brassica juncea), which belongs to the cruciferous, has been reported to have high contents of glucosinolates and phenolic compounds (Nicácio et al., 2021; Sharma et al., 2018). And the plant enzyme myrosinase hydrolyzes the glucosinolates to isothiocyanates (ITC; Barba et al., 2016). ITC, which contribute to the pungency of mustard, are functional additives with anticancer and antimicrobial properties (Lin et al., 2000). The antimicrobial and antioxidant activities of these glucosinolates and phenolic compounds are greatly influenced by the ratio of water to ethanol during the extraction process for additives (Moudache et al., 2016).
Mustard seeds have been widely researched as natural antioxidants due to their proven efficacy. However, studies comparing the storage enhancement effects of different extraction solvents in dried meat products are difficult to find. Therefore, we compared the antioxidant capacities of various ethanol concentrations extracted from ground mustard seeds and selected the treatment group with superior antioxidant capacity for addition during the curing process of dry-aged pork loin ham. Subsequently, we analyzed the storage characteristics of the dry-aged pork loin ham during 6 wk of drying. This research aimed to provide foundational data for understanding the changes in antioxidant capacities of the substances contained in mustard seeds depending on the extraction solvent and their functions within meat.
Materials and Methods
Yellow mustard seeds (B. juncea, bb Royal, India) were ground by grinder (DP-5800BL, Guangdong Xinbao Electrical Appliances Holdings, Guangdong, China) for 5 min at room temperature (23°C). The solvents which used for extraction were distilled water (DW) and ethanol, 5 different ratios (DW:ethanol; 0:100, 25:75, 50:50, 75:25, 100:0, v/v). The ground seeds were mixed with each solvent separately at a ratio of 1:10 and stirred for 24 h at room temperature. After centrifugation at 2,731×g for 30 min (Supra R22, Hanil, Daejeon, Korea), the filtrate of supernatant was stored at –80°C to freeze it before being freeze-dried by a freeze-dryer (FD12008, ilShinBioBase, Dongducheon, Korea). The 5 groups of freeze-dried extracts were dissolved in each solvent to make a stock (20%, w/v) used for experiments and loin ham manufacturing.
The 5 groups of stocks were used in the antioxidant experiment, which were made from extracts of mustard seeds that had been extracted with 25%, 50%, 75%, 100% ethanol, and 100% DW (0% ethanol). The most suitable dilution factor was determined through preliminary experiments conducted in this study, and each extract was finally diluted 100 times and used for experiments (Amarowicz et al., 1996).
To determine TPC, a method using the Folin-Ciocalteu reagent was adapted from Choi et al. (2022). Each stock (40 μL) and 80 μL of 2 N Folin-Ciocalteu reagent were mixed by vortex mixer (SVM-10, SciLab Korea, Seoul, Korea) and incubated for 3 min. Then, 800 μL of 20% Na2CO3 (w/v) was added to the mixture, and incubated for 30 min at 37°C in the dark. The absorbance was measured at 765 nm using multi-mode microplate reader (SpectraMax iD3, Molecular Devices, San Jose, CA, USA). Gallic acid solutions (0–150 μg/mL) were used for the standard curve and the results were expressed as mg gallic acid equivalents (GAE)/g.
The method proposed by Woisky and Salatino (1998) was chosen for measuring the TFC. In this process, 100 μL of 1 N NaOH and 1 mL of diethylene glycol were mixed with 100 μL of each stock respectively. The mixture was then vortexed using a vortex mixer (SVM-10, SciLab Korea) and incubated in a darkroom at 37°C for 1 h. The absorbance was measured at 420 nm (SpectraMax iD3, Molecular Devices). The standard curve was generated using naringin (0–150 μL/mL), and the results were expressed as mg naringin acid equivalents (NE)/g.
The method described by Choi et al. (2022) was chosen for measuring the DPPH free radical scavenging activity. This involved mixing each stock (500 μL) with an equal volume of DW, followed by the addition of 1 mL of 0.2 mM DPPH solution. The mixture was reacted in a darkroom for 30 min at 23°C. The absorbance was measured at 517 nm (SpectraMax iD3, Molecular Devices). For the standard curve, Trolox (0–600 μg/mL) was used, and the results were expressed as mg Trolox equivalents (TE)/g.
Pork loin (M. longissimus dorsi) was obtained 24 h after slaughter from I-homemeat (Seoul, Korea). The excess fat and connective tissues of the pork loins were removed, and the loins were cut into portions of approximately 500 g each. The portions were then randomly divided into 4 groups. For each group, 1% (w/v) of mustard seed extract stock, extracted using different ethanol concentrations (25%, 50%, and 75%), was added to the curing solution to create the experimental groups (MS25, MS50, MS75). Pork loins cured without added antioxidants served as the control. Each pork loin was weighed and packed in a polyethylene bag (WJpackage, Seoul, Korea) before being immersed in a 100% curing solution (w/w) containing 3.5% salt and 2% sugar (Table 1). After 7 d of curing at 4°C, the pork loins were placed on a tray to allow for a 2 h period of exudate release. To ensure uniform distribution of the curing solution, the polyethylene bags containing the loins were flipped once a day during the curing process. Finally, pork loins were dried in a dry-aging refrigerator (DA-45, Korea Alesso, Seoul, Korea) for 6 wk at 12°C with a relative humidity of 60%–70%.
Aerobic bacteria (AB), Staphylococcus spp. (ST), and Escherichia coli (EC) were selected to evaluate the microbial population. Dry-aged pork loin ham sample (25 g) was mixed with 50 mL of sterile saline in a sterile bag and then homogenized. This homogenate was diluted by adding 1 mL of it to 9 mL of sterile saline, and further dilutions were made as required. The diluted solution was then plated onto Tryptic Soy Agar (TSA) for AB, Mannitol Salt Agar (MSA) for ST, and 3MTM Petrifilm (3M, Saint Paul, MN, USA) for EC and incubated (37°C, 24 h). Cultured colonies were counted and their numbers were expressed as Log CFU/g.
The dry-aged pork loin ham samples were cut in half and allowed to bloom for 30 min prior to color measurement. The measurements were performed using a colorimeter (CR-10, Minolta, Tokyo, Japan), calibrated with a white standard plate (CIE L*: +97.83, CIE a*: –0.43, and CIE b*: +1.98) under an 8-lx illumination angle.
The proximate compositions of dry-aged pork loin ham samples were analyzed as per the guidelines set forth by association of official analytical chemists (AOAC, 2010). Each content was measured through the following methods:
Each dry-aged pork loin ham sample was weighed following the respective aging periods (wk 2, 4, and 6). All aging loss measurements were expressed as a percentage of the weight before aging (wk 0), and the percentage was calculated using the following formula:
For the pH analysis, dry-aged pork loin ham samples were mixed with DW (1:4, v/v). The mixture was then homogenized. After homogenization, a pH meter (Model S220, Mettler-Toledo, Schwerzenbach, Switzerland) was utilized to determine the pH of the samples.
The aw was carried out at 25°C with a LabMaster-aw neo instrument (Novasina AG, Lachen, Switzerland). Measurement results are expressed in terms of %.
TBARS were measured by the method described by Jeong et al. (2022). Dry-aged pork loin ham sample (10 g) was homogenized with 97.5 mL of DW and 200 μL of 0.3% BHT. The homogenized sample was then transferred into a round-bottom flask and 2.5 mL of 4N HCl, 1 mL of anti-foaming agent, 3 boiling stones were added and the homogenized sample was steam-distilled. Following this, the distillate was combined with an equal volume of 0.02 M TBA solution and then heated at 100°C for 35 min. The absorbance was measured at 538 nm. 1,1,3,3-Trethoxypropane was used for preparing a standard curve to calculate the amount of malondialdehyde (MDA). The TBARS value was expressed as mg MDA/kg.
VBN was determined using the method of Choi et al. (2018). Dry-aged pork loin ham sample (10 g) and 30 mL of DW were homogenized. Then, brought to a final volume of 100 mL with DW and filtered, and 1 mL of filtrate was filled to the outer compartment of the Conway dish and 1 mL of 0.01 N H3BO3 was filled to the inner compartment. Then the inner compartment was added with 100 μL of Conway reagent, while the outer compartment added 1 mL of 50% K2CO3 and the Conway dish was sealed. The sealed dish was incubated at a temperature of 37°C for 2 h. After incubation, H3BO3 in the inner compartment underwent titration with 0.02 N H2SO4 and the resulting data was then processed by the subsequent formula:
X, volume of sulfuric acid consumed for the sample titration (μL); Y, volume of sulfuric acid consumed for the blank titration (μL); f, factor of reagent; N, normality; d, dilution factor; S, sample weight (g).
Each experiment was conducted a minimum of 3 times to collect the data. All data were presented as the mean value and SD, and processed using the General Linear Models procedure for one-way analysis of variance (ANOVA) in the SAS software (version 9.4 for Windows, SAS Institute, Cary, NC, USA). One-way ANOVA was performed separately for each of the two factors: the presence of mustard seed extract and the dry-aging period. To discern significant differences among the data, Duncan’s multiple range test was utilized with a significance level of p<0.05.
Results and Discussion
Table 2 shows the extraction yield, TPC, TFC, and DPPH free radical-scavenging activity of mustard seed extracts with different extraction solvents. The highest extraction yield of the mustard seed was in 0% ethanol (100% DW) at 24.28% (p<0.05), and the yield then decreased significantly with an increase in ethanol concentration in the solvent. The lowest extraction yield was 11.43% in 100% ethanol (p<0.05). The extraction yield of mustard seed depends upon the polarity of its constituents (Nawaz et al., 2020). Mustard seeds contain a variety of substances such as glucosinolates, phenolic compounds, and other polar compounds (Szydłowska-Czerniak et al., 2015). The high extraction yield observed in 100% DW is owing to the increased solubility of these polar substances in DW, which is a highly polar solvent. This indicates that mustard seeds contain a high proportion of hydrophilic substances.
The TPC, TFC and DPPH free radical scavenging activity of the mustard seed extracts increased with an increase in ethanol concentration in the extraction solvent from 0% to 75% (p<0.05), but the lowest phenol and flavonoid content and DPPH radical-scavenging capacity was obtained at 100% ethanol concentration (p<0.05). DW and ethanol are mainly used to extract antioxidants such as phenol and flavonoids (Hikmawanti et al., 2021). We believe that higher TPCs obtained in mixed solvents as phenolic compounds are generally hydrophilic but the main phenolic compound in mustard seeds is sinapic acid (Nicácio et al., 2021), which is soluble in both water and ethanol (Shakeel et al., 2016). Flavonoids are known to exhibit a higher extraction efficacy in mixed solvents than in pure ethanol or DW, indicating that they are both hydrophilic and hydrophobic (Moudache et al., 2016). The DPPH is proportional to TPC (Muzolf-Panek and Waśkiewicz, 2022), and we observed similar results in this experiment. Therefore, the antioxidant capacity of the extract does not always match the extraction yield, and the extraction efficacy of antioxidants can be reduced when DW is used for extraction (Moudache et al., 2016). Szydłowska-Czerniak et al. (2015) reported that mustard seed’s antioxidants showed a high extraction efficacy in mixed solvents, as observed in this study. We observed that mustard seed extracts in 25%, 50%, and 75% ethanol had a higher antioxidant capacity than those in 0% and 100% ethanol. Therefore, we selected 25%, 50%, and 75% ethanolic extracts in the pork loin-manufacturing process in the current study.
Table 3 shows the AB and ST as well as color measurement results at 0, 2, 4, and 6 wk. We did not detect EC in both mustard seed extract-treated and control samples. AB and ST increased significantly in the control samples during the dry-aging period (p<0.05). The mustard seed extract-treated samples showed a significant increase in AB and ST until wk 4 (p<0.05) but no significant change between wks 4 and 6. We observed significantly higher levels of AB and ST at all wk in control samples compared to those in treated samples, except at wk 0 (p<0.05). At wk 6, MS50 and MS75 had lower AB levels than MS25. ST was lower in MS50 and MS75 than in MS25 from wk 4 onwards (p<0.05). When the cell membrane of mustard seed collapses due to physical shock, glucosinolates are released, which are hydrolyzed into ITC by myrosinase (Barba et al., 2016). ITC is known to inhibit the growth of pathogenic microorganisms, including S. aureus and EC, by disrupting cellular respiration, collapsing cell membranes, and inhibiting enzymatic activity (Lin et al., 2000). Mustard has a high glucosinolate content that can be useful for producing ITC (Sharma et al., 2018). The polarity of the extraction solvent influences the extraction efficiency of glucosinolates (Nawaz et al., 2020), as these have a higher extraction efficacy in mixed solvents (i.e., solvents with higher ethanol ratio; Doheny-Adams et al., 2017). Therefore, MS50 and MS75 exhibited a superior bactericidal capacity compared to MS25 owing to their higher extraction efficacy.
Across all samples, there was a significant decrease in CIE L*, CIE a*, and CIE b* during the dry-aging period (p<0.05). However, no significant difference was observed in CIE L* between control and the mustard seed extract-treated samples throughout the dry-aging period. The decrease in CIE L*, seen during the dry-aging process, was owing to reduced light scattered by the meat surface due to a decrease in moisture (Hughes et al., 2020). CIE a* and CIE b* did not differ significantly between the control and treated samples at wk 0. However, when compared to the control, a higher CIE a* in all treated samples after wk 2 and a lower CIE b* in all treated samples after wk 4 (p<0.05) were observed. Reduction in CIE a* is associated with the production of metmyoglobin, which exhibits brownness due to oxidation of myoglobin (Wang et al., 2021). Myoglobin is known to form ferrylmyoglobin, which appears green, due to ferryl oxidation and reaction with hydrogen peroxide, causing a decrease in CIE a* but increase in CIE b* (Reeder et al., 2002). This result suggests that the more modest decline in CIE a* within the treatment groups, as compared to the control groups, results from the enhanced antioxidant activity by the addition of mustard seed extract. This increased activity is thought to have decelerated the CIE a* reduction and impeded the CIE b* increase. Furthermore, green sulfmyoglobin produced by the reaction of myoglobin and hydrogen sulfide generated during proteolysis by microorganisms increases the CIE b* of meat (Liu et al., 2022). The increased bacterial count seen in the control groups, compared to that in treatment groups, is consistent with the above observations. The addition of mustard seed extract improves pork colors, such as increasing CIE a* of dry-aged pork loin ham and decreasing CIE b*, by promoting antioxidant and antibacterial activity.
Table 4 shows the proximate compositions of dry-aged pork loin ham at wk 0, 2, 4, and 6. All the samples demonstrated a significant decrease in moisture content (p<0.05), while their protein, fat, and ash content displayed a significant increase during the dry-aging period (p<0.05). We did not observe any significant differences between treated and control samples over the dry-aging period. Moisture content was negatively correlated with protein, fat, and ash content. This is mostly due to a relative increase in the dry-matter content resulting from decreased moisture content (Seong et al., 2015). Kim and Lee (2003) demonstrated that fat and water contents of meat are inversely proportional, as observed in the present study as well.
Fig. 1 illustrates the aging loss of dry-aged pork loin ham samples at wk 2, 4, and 6 compared to the wk 0. The amount of aging loss increased significantly in all the samples throughout the dry-aging period (p<0.05), and the primary reason for this is believed to be the reduction in moisture content. Over the dry-aging period, the control and the mustard seed extract-treated samples showed no significant difference in the amount of aging loss. Similar to the results of the present study, Andrés et al. (2017) reported that adding pomegranate, grape, and tomato extract did not affect the weight loss in lamb patties (longissimus thoracis). While manufacturing dry-aged pork loin ham, high reduction of weight during aging can lead to economic loss and decline in quality (Bonfatti and Carnier, 2020). In this study, mustard seed extracts did not affect the composition change or yield during the dry-aging of pork loin ham. Therefore, we believe that the addition of mustard seed extracts can improve storage without any decline in quality of dry-aged pork loin ham.
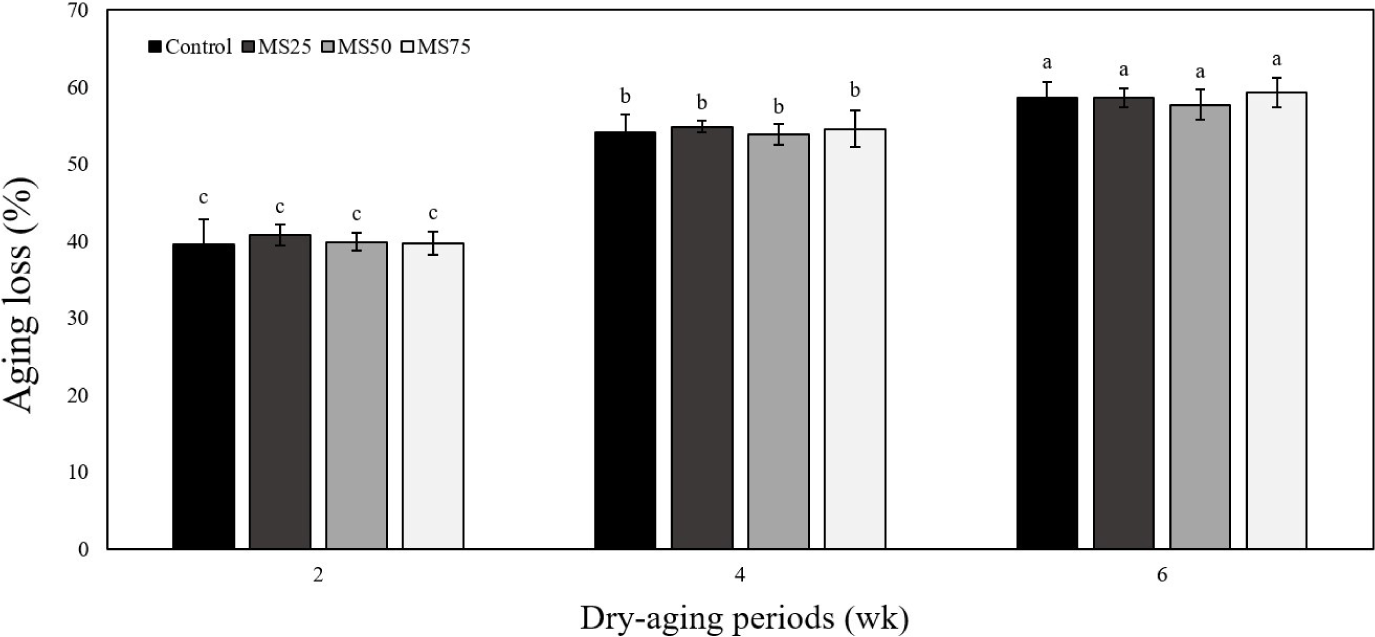
Table 5 shows the pH and aw of dry-aged pork loin ham samples at wk 0, 2, 4, and 6. The pH of the control and MS25 samples tended to increase with the passage of the dry-aging period, and the highest pH value was measured at wk 6 (p<0.05). MS50 and MS75 showed no significant change in pH over all wk. The mustard seed extract-treated samples and control samples did not show a significant difference in pH at wk 0, but significantly higher pH values were observed in control starting from wk 2 (p<0.05). The lowest pH was observed in MS50 and MS75 samples at wk 4 and 6 (p<0.05). Increased pH in meat products signifies decay or growth of pathogenic microorganisms (Sujiwo et al., 2018), which is consistent with the microbial content trends observed in the control and MS25 samples. Conversely, AB tended to increase in MS50 and MS75 over the dry-aging period even though pH decreased. This might be due to the inhibition of pathogenic microorganisms and delayed changes in pH because of the lactic acid produced by lactic acid bacteria (Leroy and De Vuyst, 2004). The curing solution used for preparing the dry-aged pork loin ham in this experiment (Table 1) contained sugar. The lactic acid bacteria present in meat might have used this sugar for metabolism (Gänzle, 2015).
The aw of dry-aged pork loin ham samples were decreased significantly during the dry-aging period (p<0.05). The samples did not show a significant difference in aw at wk 0. But at wk 6, significantly lower aw was measured in the mustard seed extract-treated samples compared to the control sample (p<0.05). In the proximate composition analysis, all the samples had 73%–74% moisture at wk 0 and this significantly decreased over time to 26–27% at wk 6, indicating that the major factor in aw reduction was the dry-aging process. Furthermore, the mustard seed extract was added to the pork loin in the form of a stock dissolved in a solvent, including ethanol. Ethanol has been reported to potentially influence microbial metabolism inhibition and the reduction of aw (Hallsworth and Nomura, 1999). This could have had an impact on the aw measurements in this study. aw is an indicator of the moisture level that can be used for growth of microorganisms. Maintaining the aw of the final dried meat product below 70% effectively inhibits the proliferation of harmful bacteria and ensures stability during storage (Syamaladevi et al., 2016). We observed that addition of the mustard seed extracts in dry-aged pork loin ham reduced microorganisms and aw.
Table 6 shows the TBARS and VBN of dry-aged pork loin ham samples at wk 0, 2, 4, and 6. All the samples had significantly higher levels of TBARS at wk 6 than at wk 0 (p<0.05), but there was no significant change in TBARS after wk 2. While there was no significant difference in TBARS between the mustard seed extract-treated samples, they consistently exhibited lower TBARS than the control every wk (p<0.05). Continuous exposure of lipids to oxygen results in accumulation of malondialdehyde in meat due to an oxidative reaction, which is assessed by measuring TBARS levels (Zhao et al., 2020). The lack of significant difference in TBARS levels, observed in the different treatment samples during the experimental duration, is most likely due to the low-fat content of the meat (Fuentes et al., 2014). All the treated samples had lower TBARS levels than the control sample owing to the antioxidant activity of the mustard seed extracts (Nicácio et al., 2021).
VBN increased continuously in all the samples throughout the dry-aging period (p<0.05). The control sample showed significantly higher VBN than all the treated samples continuously from wk 0 to 6 (p<0.05). We observed the lowest VBN in MS50 from wk 0 to 2 (p<0.05). MS50 and MS75 samples had significantly lower VBN compared to the control sample and MS25 from wk 4 onwards (p<0.05). During the dry-aging period, the level of VBN increases due to protein degradation and metabolism of microorganisms (Sujiwo et al., 2018). Mustard seed extract-treated samples had significantly lower VBN levels due to the inhibitory action of mustard seeds against microorganisms (Kanemaru and Miyamoto, 1990). Therefore, levels of VBN tended to be consistent with the results of microbial analysis. In summary, the mustard seed extracts effectively inhibited lipid oxidation and protein deterioration during the dry-aging process of the pork loin, and the greatest effect was observed in MS50 and MS75.
Conclusion
This study evaluated the effects of mustard seed extracts on storage characteristics of dry-aged pork loin ham during the aging period. Based on the study conducted, mustard seed extracts, especially those obtained from 50% and 75% ethanol, positively influenced the physicochemical and storage characteristics of dry-aged pork loin ham. These extracts significantly inhibited bacterial growth, stabilized pH levels, and reduced water activity, contributing to overall improved storage stability. In terms of color attributes, treatments with mustard seed extracts resulted in higher CIE a* and lower CIE b* compared to the control. Additionally, the levels of TBARS and VBN were lower in samples treated with mustard seed extracts. Therefore, these extracts could serve as an effective natural alternative to synthetic antioxidants, promoting enhanced safety, color, and storage longevity of dry-aged pork loin ham. This contributes towards the development of healthier and more naturally preserved dry-aged pork products.













