Introduction
Cultured meat technology aims to provide an alternative meat source with lesser ethical and environmental concerns than conventionally produced meat (Bhat et al., 2019). However, this technology remains in its infancy owing to the current limitations in cell line establishment, scaffolds, bioreactors, and media development (Stephens et al., 2018). Although successful cultured meat production has been reported (O’Riordan et al., 2017), the production cost and scalability limit the accessibility and acceptance of cultured meat.
Technological limitations still pose the biggest threat to the industrialization of cultured meat. However, there is progress owing to the increase in the number of start-up companies that are investing in novel methods and advancements for cultivating livestock and seafood. High investments (both from public and private funds) spread across different platforms are being made because of the increasing practicality and scalability of cultivation methods (Swartz, 2023; Zulkosky, 2022). Unfortunately, these advancements remain confidential due to the patentability of this developing technology (Ng et al., 2023).
Considering the current limitations in cultured meat production, the potential of individuals and businesses to commit food fraud could increase. Given that cultured meat is made up of animal cells, differentiating conventional meat from cultured meat becomes a problem, especially, when they are converted into meat products. Thus, identifying the key physical and chemical characteristics of these foods could help validate the innovations in the cultured meat industry.
Incidents of food fraud in the meat industry raise concerns about the authenticity and safety of meat and meat products (Crceva Nikolovska et al., 2019). Cases of adulteration, tampering, simulation, and counterfeiting could also happen in the cultured meat industry. Although meat authenticating tests have been developed for conventionally produced meat and meat products, their applicability to cultured meat should be evaluated. This paper briefly discusses some areas in the cultured meat industry that are vulnerable to food fraud. Specifically, it targets the current meat and meat product authentication tests to emphasize the need for ensuring the traceability of cultured meat.
Meat Standards and Authentication
With increasing meat consumption comes the need for increasing meat production. The meat production in 2020 is four times more than that in 1961 (Ritchie, 2017). However, greater production is accompanied by greater challenges in food safety, quality assurance, and traceability. Countries with developed animal production industries have their own regulatory standards to protect and promote consumer safety and food quality. Some countries develop diplomatic relations in terms of meat quality standards that allow exportation among member countries. For example, countries wanting to export meat or meat products in Europe must have (1) competent authority, (2) animal health standards, (3) hygiene and public health requirements, (4) systems for monitoring livestock and livestock products and ensuring the determination of chemical residues at post-production, (5) certified establishments, (6) valid bovine spongiform encephalopathy status, and (7) clearance from relevant authorities (European Commission, 2018). Similarly, the United States Department of Agriculture Food Safety and Inspection Service (USDA-FSIS) requires eligibility via an equivalence determination process and congruent labelling standards for domestically-produced meat before importation (FSIS, 2023).
However, the standards for novel foods like cultured meat and meat products remain vague. Recently, the Food Standards Australia New Zealand released an article on cell-based meat, stating that the regulation of cell-based meat still falls under the conducts of the Food Standard Code, with considerations on the composition of cultured meat to determine applicable standards for pre-marketing approval (FSANZ, 2021). Meanwhile, the US Food and Drug Administration (FDA) requires a thorough pre-market evaluation and review of the cultured meat production process (right from tissue collection to all processes involved) to evaluate the safety of the meat as food. Furthermore, ensuring via routine inspections that safe and non-adulterated products exit the facilities is essential after pre-marketing approval (FSIS, 2022).
The first commercially available cultured chicken meat by Eat Just (Good Meat), approved by the Singapore Food Agency (SFA) in 2020, marked the beginning of standards-based approval for cultured meat (Waltz, 2021). The decision was based on the novel food regulatory framework that requires proof of conduct of safety assessments (e.g. toxicity, allergenicity, safe food processing, and food chemical exposure tests), followed by a review and scrutiny of food safety and technology by experts comprising the Novel Food Safety Expert Working Group (Yeung, 2023). Meanwhile, in November 2022, the US FDA declared the cultured chicken meat of Upside Foods as safe to eat (Reiley, 2022; Sullivan, 2022). However, before commercialization, Upside Foods needs to get the mark of inspection from the USDA-FSIS (FDA, 2022). The regulatory approval of Eat Just and Upside Foods provides proof that cultured meat is edible and is amenable to the safety requirements for novel foods.
Any form of food fraud endangers the whole production and supply chain. Furthermore, consumer safety is endangered when meat/meat products contain substances that are deemed harmful, such as pathogens, allergens, and toxins (Facts, 2022). Therefore, meat authentication should be conducted for both local and imported meat and meat products to ensure product quality and consumer safety.
Knowing the complexity of the approval process for novel foods, including cultured meat and seafood, and preventing food fraud becomes necessary. Regardless of form or method, meat fraud could potentially harm companies and consumers from unregulated products that tend to get a pass by taking advantage of previously established and approved cultured meat companies. In the formal agreement between the US Department of Health & Human Services and the FDA, a pre-marketing inspection of cultured meat products before exiting premises suggests the importance of following approved standards based on the pre-marketing evaluation of the agency (FDA, 2019).
Like conventional and plant-based meat, cultured meat can be made into easy-to-prepare forms such as sausages, meatballs, bacon, and nuggets. The same goes for conventional and plant-based meat as they are normally processed. Meat authentication includes an assessment of meat origin (species and country of origin), nutritional composition, microbiological quality, chemical residues, and other aspects that could support the identity or form of the product based on how it is presented. Fig. 1 shows the chain of events from the production to the commercialization of both conventional and cultured meat products. It highlights the difference in the processes involved in meat production and the need for the evaluation and approval of cultured meat before commercialization. Additionally, labeling and pre-marketing inspection are warranted for both conventional and cultured meats. Labels can be used as a basis for determining appropriate authentication methods, leading to the verification of compliance with approved procedures and claims.
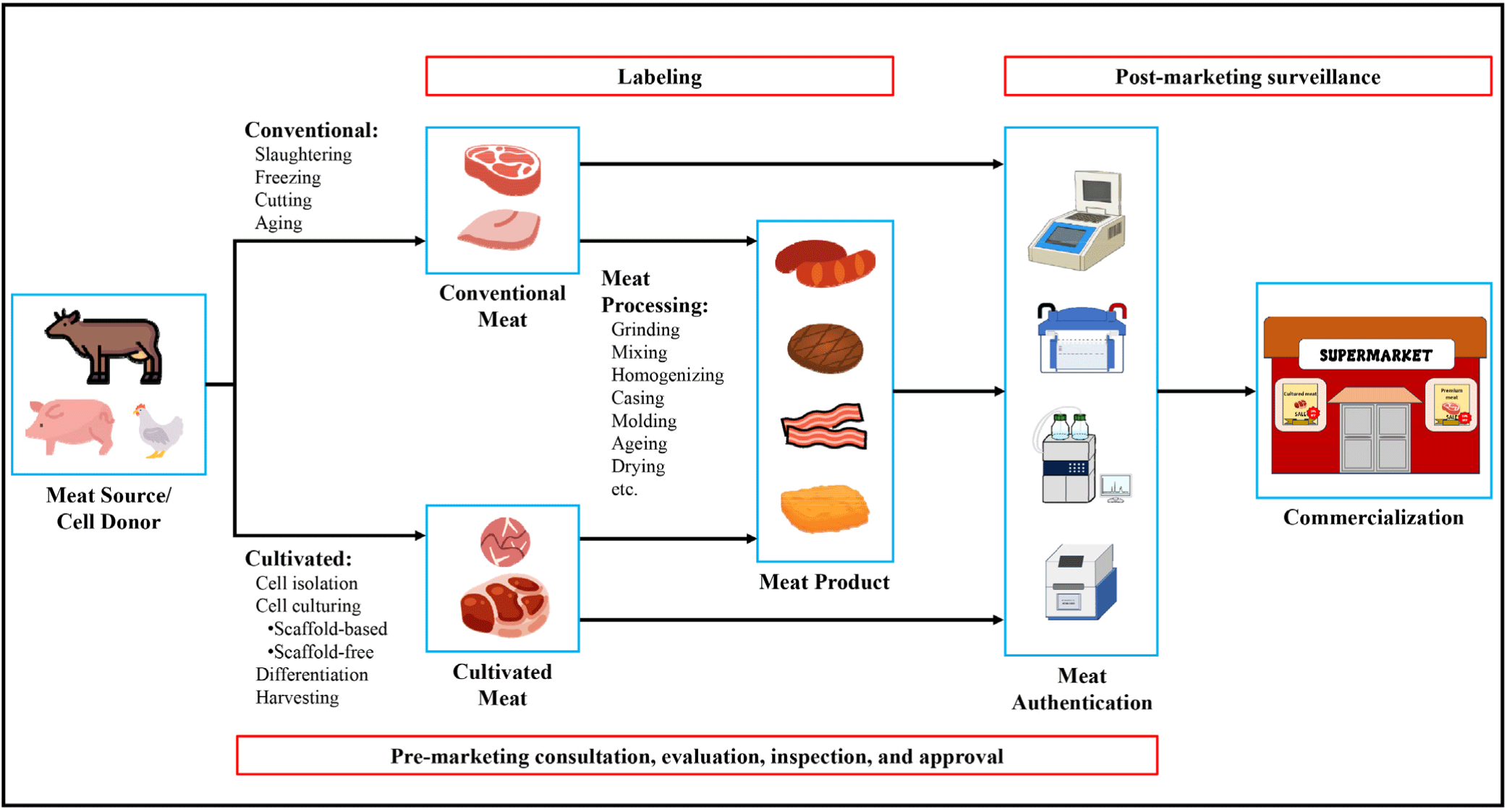
The Food and Agriculture Organization of the United Nations (FAO) defines food fraud as an intentional act of food-related companies or operators taking advantage of consumers by altering the quality and composition of food products (FAO, 2021). Incidents of food fraud in conventional meat products are still being reported, continuously threatening the authenticity of meat products. Thus, establishing standard protocols for meat authentication is essential. Common meat authentication processes include determining meat origin, substitution, processing treatment, and adulterants (Ballin, 2010). The physical and chemical differences between conventional and cultured meat can be used, to some extent, to authenticate meat products. However, it should be noted that the goal of the cultured meat industry is to achieve similar, if not improved, characteristics compared to conventional meat (Fraeye et al., 2020).
Common food fraud types include adulteration, tampering, simulation, and counterfeiting. Multiple types of food fraud can be combined, resulting in a near-authentic form of a particular product. For example, the adulteration of chicken nuggets could be coupled with mislabeling and counterfeiting, to gain more appeal to other food businesses and ultimately, the consumers. The lack of specific cultured meat authentication standards makes the industry vulnerable to food fraud. Table 1 shows potential fraudulent acts in both conventional and cultured meat products. Moreover, it shows some internal and external vulnerable points in the industry. Internal fraudulent acts may include adulteration, unsupported claims, mislabeling, and misdeclaration of methods. Meanwhile, external acts are done by fraudulent companies attempting to counterfeit, tamper, or simulate established cultured meat products.
Although huge technological gaps need to be overcome before achieving the complex structure of conventional meat, the final form of both cultured and conventional meat in meat products can be physically indistinguishable because the meat is homogenized with other product components during processing. Taking advantage of this lack of physical difference, fraudulent companies could potentially use this to label their products as cultured meat products.
Applicability of Conventional Meat Authentication Techniques to Cultured Meat
Cultured meat technology has a promising future as an alternative animal protein source for consumers. However, it is also a potential business target for fraudulent companies prying on the novelty of cultured meat technologies of different companies and the differences in regulatory standards among countries and regulatory agencies. Meat authentication is part of product traceability and has been used to prevent fraudulent products from entering commercial spaces. The establishment of reliable physical and chemical fingerprints based on DNA, proteins, metabolites, and other relevant profiles will increase the stringency of existing authentication techniques, thereby, becoming more discriminating towards fraudulent products. However, authentication standards for cultured meat are yet to be established.
DNA-Based Authentication
Polymerase chain reaction (PCR) technology has led to the development of a sequence-based method for identifying and authenticating meat and meat products (Jonker et al., 2008). The high thermal stability of DNA and its persistence in processed meat makes DNA-based methods ideal for meat authentication (Kaltenbrunner et al., 2018). Li et al. (2020) highlighted that PCR techniques such as direct PCR, real-time PCR, loop-mediated isothermal amplification, droplet digital PCR, and DNA barcoding have high specificity and wide applicability across species and, therefore, are suitable for meat authentication. In these methods, DNA sequences are extracted, purified, and quantified from meats and meat products to obtain the necessary data for validation using genomic databases. For example, the mitochondrial DNA cytochrome b gene has been used as a genetic marker for conventional meat authentication of livestock and game species through PCR methods with varying detection limits (Adenuga and Montowska, 2023).
Cultured meat and meat products are composed of animal cells that have been proliferated and differentiated to reach a structure similar to that of muscles (Swartz, 2023). In principle, cultured meat possesses biological markers that could be used to trace back its animal origins. However, the use of serums in culture media can result in the detection of the animal species that served as the serum or plasma source (Mohd Kashim et al., 2022). Nevertheless, the successful immortalization (induced or spontaneous) of muscle cells, as reported recently, is promising for generating cell lines with increased proliferation and stability, allowing serum-free production of cultured meat (Pasitka et al., 2023; Stout et al., 2023). Like conventional meat, cultured meat also contains DNA, thereby, allowing the identification of the animal source of the cells.
Meanwhile, genetically modified cell lines can be traced based on the specificity of the event, focusing either on the edited DNA fragment or the expressed protein (Miraglia et al., 2004). Numerous cultured meat companies use the term “non-GMO” in their advertisements, suggesting the favored use of primary isolation or spontaneous immortalization of cells for cultured meat production. The theoretical traceability of genetically modified cell lines in cultured meat could potentially be used for non-genetically modified cell lines by establishing a unique detectable DNA fragment to validate the cultured nature of the product. Ong et al. (2021) theorized that cells can be designed to have unique physicochemical properties outside of the conventional properties of meat. The development of detectable genetic markers would facilitate the identification of cultured meat.
Protein-Based Authentication
Meat is composed of proteins, providing an array of potential protein biomarkers for meat authentication. Protein-based meat authentication could be generally categorized into electrophoretic, immunoassay-based, or mass spectrophotometric (Li et al., 2020). However, only immunoassays and spectrophotometric analysis are commonly used methods for protein-based meat authentication due to their high specificity (Li et al., 2018; Orduna et al., 2017; Seddaoui and Amine, 2020). The specificity of these methods depends on the protein biomarkers specific to each animal species. Thus, the selected biomarkers must (1) have distinguishable differences among species, (2) be highly detectable in both meat and meat products, and (3) remain stable during processing (e.g., heating and addition of food additives; Zvereva et al., 2015). The dependence of cultured meat production on growth hormones and other protein-based media and scaffold components should be considered for the detection of contaminating proteins from other species or material sources.
The detection of species-specific proteins or the difference in expression of meat proteins (e.g. MYL, TPM, MB, GADPH, ACTAI, PKM, PGAM, and ENO3) still has limitations that could result in inaccurate meat authentication. For example, horse and beef myoglobins have a high homology that could hinder the identification of the meat species (Vostrikova and Chernukha, 2018). These limitations warrant the detection of other protein biomarkers to authenticate a raw meat sample. Protein-based authentication methods are appropriate only for raw meat specimens because the thermal stability of proteins is lower than that of DNA.
In contrast, the use of genetic or epigenetic modifications could induce the expression of novel products (Ong et al., 2021). However, these novel products may not be fit to be used as a reference for cultured meat authentication owing to the different culture conditions, components, and cell sources used by different cultured meat companies. Thus, the establishment of cultured meat protein markers relies on selecting stable proteins that are expressed regardless of modifications during meat cultivation.
Metabolite-Based Authentication
Meat can be characterized based on the metabolome profile resulting from differences in the phenotypic expressions of different animal breeds and species (Muroya et al., 2020). Metabolites are products of cellular metabolic reactions (Siddique et al., 2022). Understanding the differences in metabolome profiles of conventional and cultured meats will increase the sensitivity of the current metabolomic techniques for meat authentication. Conventional meat authentication techniques based on the metabolome had been reported and could be considered for cultured meat authentication. The use of nuclear magnetic resonance spectroscopy is an effective technique to determine complex chemical compositions that could be used to identify potential markers for fraud detection (Consonni and Cagliani, 2019). Differences in the elemental isotope concentrations could be used to determine the geographical origin of beef using gas chromatography and an elemental analyzer (Heaton et al., 2008). Origin estimation based on trace elements in beef (B, Yb, and Zn) and poultry (As, Na, Rb, and Tl) meat that are significantly different across countries can be done using inductively coupled plasma high-resolution spectrometry (Franke et al., 2008). Another method is the detection of terpenes in animal fat to discriminate the dietary background of the meat using mass spectrometry (Priolo et al., 2004). Additionally, Alfaia et al. (2009) analyzed the fatty acid composition of beef to detect chemical discriminators to confirm the impact of feeding regimen on intramuscular fat using a combination of gas chromatography-flame ionization detection and high-performance liquid chromatography (HPLC). However, the unavailability of cultured meat for analysis limits our knowledge of the differences in the metabolic reactions during and after cultured meat production (Hocquette, 2016).
Chemical compounds found in meat are not exclusively produced by muscles but are a collective contribution of multiple cell types that could metabolize the nutrients from animal feed (Fraeye et al., 2020). An alternative way of authenticating cultured meat is by determining the absence of such compounds as a result of favored culturing of myogenic cell types. However, the production of cultured meat by co-culturing multiple cell lines for improved extracellular matrix and differentiation could result in cultured meat with higher similarities to conventional meat (Ben-Arye et al., 2020). Moreover, future developments in culture media optimization could supplement the lacking metabolites, resulting in the detection of the same compounds in both conventional and cultured meats (Fraeye et al., 2020). Therefore, it is necessary to monitor the pre-marketing and post-marketing differences during the phases of cultured meat production. Any changes after harvesting to processing must be accounted for to establish the chemical and physical fingerprint of a specific product of a particular company.
Currently, the requirements of regulatory agencies for animal cell-based products is focused on the safety and sanitation of food production, relying on pre-marketing inspections (FDA, 2022). However, the threat of products from fraudulent companies that could enter the market must be anticipated. Thus, authentication methods must be developed and specified for post-market surveillance of commercially-available cultured meat products.
Other Potential Bases for Authentication
Different methods of meat cultivation could result in differences in physical structure and chemical fingerprints. Generally, meat cultivation techniques are categorized into scaffold-based and scaffold-free methods. The components of scaffolds for cultured meat are mainly selected based on their food safety (i.e. toxicity, allergenicity, etc.), sensorial attributes, cost, and scalability (Bomkamp et al., 2022). Scaffolding materials possess diverse chemical components that may affect the resulting chemical composition of cultured meat. Additionally, the use of chemicals such as crosslinking agents, photoinitiators (Oryan et al., 2018), and dissociation reagents (Ong et al., 2021) could hint toward the cultured nature of the product. As part of food safety, it is expected that these chemicals are food-grade, considering their potential to be included in the resulting product (Stephens et al., 2018). Considering the diversity of potential scaffold materials for cultured meat production, establishing a standard across cultured meat products is difficult.
Meanwhile, scaffold-free techniques produce biomass by harvesting self-organizing cell structures in the form of mush from bioreactors or cell sheets from culture dishes (Tanaka et al., 2022). The absence of scaffolding makes it easier to establish physical and chemical fingerprints for scaffold-free cultured meat than for scaffold-based cultured meat. Thus, a categorical classification among cultured meat products could ease the authentication process, which could further result in guided product labeling, providing necessary information for prospective consumers.
Another potential basis for comparison is the detection of chemical and veterinary drug residues. The mere presence of veterinary drug residues in supposedly cultured meat hints toward the nature of meat production involved. For example, the detection of anthelmintic residues in cultured meat questions the overall process of cultivation. Since cultured meat is produced in sterile facilities, the use of veterinary drugs is not warranted. Thus, the detection of veterinary drug residues in purported cultured meat highlights conventional farming as the source of the meat. The main techniques used to screen residues include immunological methods (e.g. enzyme-linked immunosorbent assay, radioimmunoassay, multiarray biosensors) and chromatography (e.g. high-performance thin-layer chromatography, HPLC; Toldrá and Reig, 2006).
Future Perspectives for Cultured Meat Authentication
Currently, the lack of genetic, metabolite, and other relevant physical or chemical profiles of cultured meat, with or without regulatory approval, inhibits the establishment of a common standard for cultured meat authentication. This lack of physical and chemical profile standards is contributing to the vulnerability of the industry to food fraud. Fig. 2 shows an example of how a cultured meat authentication standard could be established. It starts with determining the technique used for cultured meat production, categorized into scaffold-free and scaffold-based production. Regardless of the form to be commercialized, elements such as meat composition, non-meat additives, and microbiological quality should be determined. These analyses target specific discriminating factors in different product components and help in validating the truthfulness of claims and the product’s compliance with approved production methods. Thus, in addition to providing product or industry security, these assessments ensure the quality, safety, and traceability of cultured meat products.
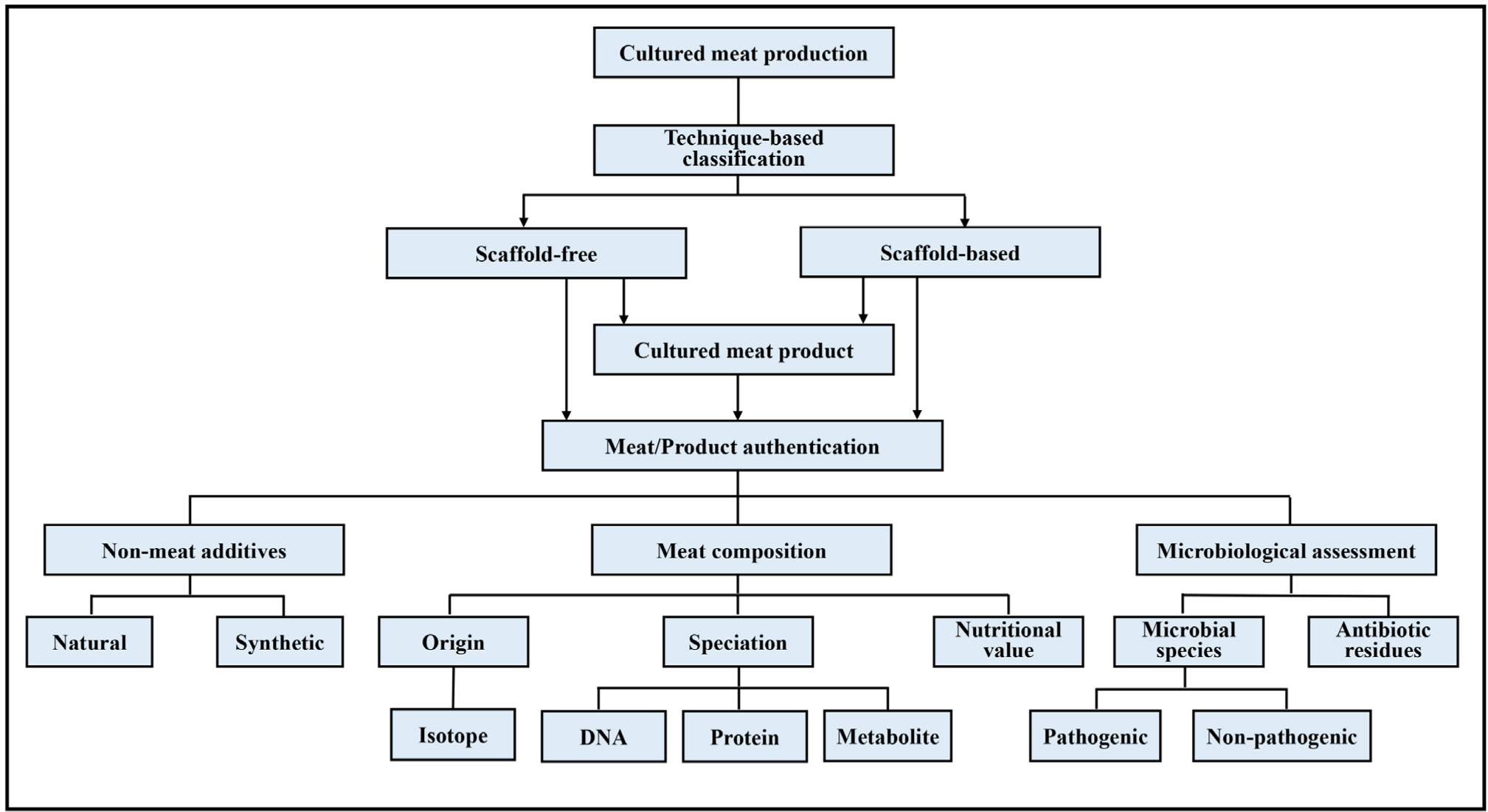
However, additional regulatory requirements tend to hamper the commercialization process owing to the additional costs incurred for conducting authentication tests or procedures. Therefore, the development of stable and high-specificity authentication procedures should be deemed important for strengthening product security and traceability.
Conclusion
The advancement of science has led to the development of cultured meat technology, which is regarded as the future for greener and ethically-sound production of animal protein. Novel technologies for novel foods, such as cultured meat, need a different approach in terms of authentication methods. The increasing production efficiencies of cultured meat companies should be coupled with increasing regulatory support to protect them from cases of sham products, which could threaten the future of the cultured meat industry. Cultured meat authentication is essential and must be considered because, in the future, these gaps may be bridged by technological advancements, increasing the similarities between conventional and cultured meats. Several conventional meat techniques have been cited but the applicability on cultured meat products should be evaluated. A standards-based approach for cultured meat authentication would create a safer future for all stakeholders and help prevent food fraud. This could also lead to the increased acceptability of cultured meat and meat products by validating claims and labels. The development of meat authentication standards for the cultured meat industry would depend on the combined efforts of cultured meat companies, regulatory agencies, and academe. However, additional steps for authentication could increase the production cost. Therefore, strategic, cost-effective, and accurate authentication methods must be developed.













