Introduction
The meat consumption in Asian countries such as, Korea and Vietnam shows a continuously stable growth over the years as a result of economic growth, in which Vietnam is among the biggest meat importers (FAO, 2021). Meat quality is an important issue in the meat industry, as consumer demand for high-quality meat is incessantly rising (Taheri-Garavand et al., 2019). Vietnamese yellow cattle (VYC) is a native breed that was originated from a cross between Bos taurus and Bos indicus cattle that migrated from northern China (Tran, 2000). The VYC are characterized by a yellow coat color, small body weight (about 200–250 kg and 250–300 kg for mature female and male, respectively; Nguyen et al., 2010). Before the 2000s, the VYC were mainly used as draft animals, and recently they have been used for beef production purpose due to the rapidly increased beef consumption in the country (Quach et al., 2022). Hanwoo, a type of Korean native cattle classified as B. taurus (Phillips, 1961), is the main beef breed in Korea due to its high performance and unique palatability (Jo et al., 2012). At 25 to 30 months of age, Hanwoo cattle have a body weight of about 600 to 800 kg (Seo et al., 2021). In term of meat quality, Hanwoo beef is characterized by its high marbling, tenderness, juiciness and distinct flavor (Chung et al., 2018).
Muscling (muscle growth) in cattle is strongly affected by genetic factor, finally affecting the beef yield, and is the great interest to the beef industry. During the embryonic development, myogenic precursor cells develop into myoblasts which enter the myogenesis by proliferating and differentiating to form multinucleated myotubes (muscle fiber types; Romanazzo et al., 2015). Myogenesis is accurately controlled by cellular signaling pathways that control cell proliferation and differentiation. The myogenesis and differentiation, can both be regulated by different myogenic regulatory factors (MRF) family such myogenic factor 5 (MYF5), myogenic differentiation (MYOD), myogenic factor 6 (MRF4), and myogenin (MYOG) etc. (Jiwlawat et al., 2018). Another population of myoblasts known as satellite cells, are found in postnatal skeletal muscles and are positioned beneath the basement membrane of the myofibers (Singh et al., 2009). At birth, the number of satellite cells accounts for about 30%–35% of total muscle fiber nuclei in animals (Day et al., 2010). Satellite cells contribute not only to the growth of muscle but also the regeneration processes (Rehfeldt et al., 2000). Isolation and characterization of satellite cells provide us an in vitro test to elucidate the intrinsic and extrinsic factors affecting the muscle growth (Shahini et al., 2018). Until now, the satellite cells have become the most typical candidate cell type to study the mechanism related to the muscle growth in livestock such as, goose, goat, pig and cattle (Han et al., 2022; Li et al., 2015; Wang et al., 2018). Recently, researchers have found that the events during myogenesis plays a crucial role in determining live weight and carcass weight in cattle (Coles et al., 2015).
On the other hand, meat quality is significantly influenced by amount of intramuscular fat (IMF) or marbling degree (Lee et al., 2022). The deposition of IMF in carcass is resulted from the adipogenesis (a process that increase the number and size of mature adipocytes) pathway that is regulated by some key transcription factors such as, peroxisome proliferator-activated receptor gamma (PPARγ; Joo et al., 2017; Nguyen et al., 2021). Until now, the growth performance and meat quality of Hanwoo have widely been studied (Kang et al., 2022; Lee et al., 2021) and compared with other cattle breeds (Holstein, Chikso, Jeju Black cattle) raised in Korea or imported beef cattle (Wagyu and Angus; Jeong et al., 2012; Lee et al., 2019; Van Ba et al., 2013). However, no studies were conducted to compare the differences in myogenic and adipogenic mechanisms related to the body weight and meat quality characteristics (e.g., marbling) between Hanwoo and other breeds. Furthermore, Hanwoo beef has been exported to foreign markets such as Hong Kong since 2015 (Cho et al., 2017), and is planned to export to other countries such as Vietnam (Cho et al., 2022). In this context, providing the consumers in the Hanwoo beef importing countries with the myogenic and adipogenic mechanisms related to its meat quality is necessary to promote the commercialization of Hanwoo beef. Thus, the objective of this study was to compare the myogenic and adipogenic characteristics and meat quality traits between Hanwoo and VYC.
Materials and Methods
For this study, same age (28-months old) Hanwoo and VYC steers (n=10 per breed) were used. Before harvesting, the Hanwoo steers were reared and fattened following the standardized commercial process (Chung et al., 2018) in a commercial cattle farm (Jeonju, Korea). Whilst, the VYC steers were reared following a semi-extensive system in a commercial cattle farm (Thai Nguyen, Vietnam). During the growing phase (6 to 20 months of age), the animals were grazed during daytime on crop fields, and fed ad libitum with rice straw, maize straw, cultivated grass and supplemented with 0.5–1.0 kg maize flour. During the fattening phase (21–28 months of age), the animals were grazed for 2–3 h on crop fields, and fed ad libitum with rice straw, maize straw, cultivated grass, and supplemented with 2–3 kg of mixed rice bran and maize flour (1:1: ratio).
The Hanwoo were slaughtered in a commercial abattoir (Jeonju, Korea) and VYC were slaughtered a commercial slaughterhouse (Thinh Dan commune, Thai Nguyen, Vietnam) following the approved procedure. The averaged live weights at slaughter of Hanwoo and VYC were 728 45 kg and 285 36 kg, respectively. Within 30 min after slaughter, approximately 100 g of muscle tissues were taken from the longissimus lumbrorum (LL) muscle (at 1st lumbar vertebrae) of left carcass side (n=3 per breed) for isolation of myogenic satellite cells. The tissue samples were immediately placed in pre-warm phosphate buffer solution (pH 6.8) containing 1.5% penicillin/streptomycin/neomycin antibiotics solution (Sigma-Aldrich, St. Louis, MO, USA) and transported with a duration of 30 min to laboratories for cells isolation. The carcasses were chilled at 2°C for 24 h in a chilling rooms. Next day, the LL muscles (n=10 per breed) from the left side of carcasses were collected, and transported to the laboratories for meat quality analysis. All the experiments on the Hanwoo and VYC were carried out using the same protocols.
The satellite cells were isolated from the longissimus lumbrorum muscles (n=3) of three representative animals per breed. The isolation was carried out in clean benches following the procedure developed by Hindi et al. (2017) with suitable modifications. Prior to the isolation, the muscle tissues were washed with pre-warmed Dullbecco’s Modified Eagle Media (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) containing 1% penicillin/streptomycin/neomycin antibiotics solution for several times. All outer connective tissues were trimmed and discarded using sterile scissor and forceps. The tissues were cut into small pieces, placed in sterile tubes and digested with digestion solution containing DMEM, 1% antibiotics, 2.5% Hepes solution and collagenase D (Roche) for 1 h at 37°C. The digested tissue mixture was filtered through pre-wet 100 μm and then 70 μm nylon cell strainers (Thermo Fisher Scientific), and centrifuged at 76×g for 5 min. The isolated cells were cultured in 25-cm flasks with growth medium containing DMEM, 15% fetal bovine serum (Thermo Fisher Scientific), 1% antibiotics solution and 10 ng/mL basic fibroblast growth factor (Sigma-Aldrich) at 37°C in a pre-set 5% CO2 incubator. The cells were replaced with new growth medium at 2-day intervals. When reaching approximately 70% confluence, the cells were harvested for purification using the adhesion method of Shahini et al. (2018). Firstly, the culture dishes were coated with 0.1 mg/mL type I rat tail collagen (Thermo Fisher Scientific) for 24 h at 4°C. The cells were then plated into the collagen-coated dish for 1 h, non-myogenic cells (mainly fibroblasts) quickly bound to the collagen layer, while the satellite cells remained in the supernatant which was collected for culture. In order to receive the purest satellite cells, this process was repeated three times. The purified satellite cells were cultured and used for cell proliferation, myogenic and adipogenic differentiation assays.
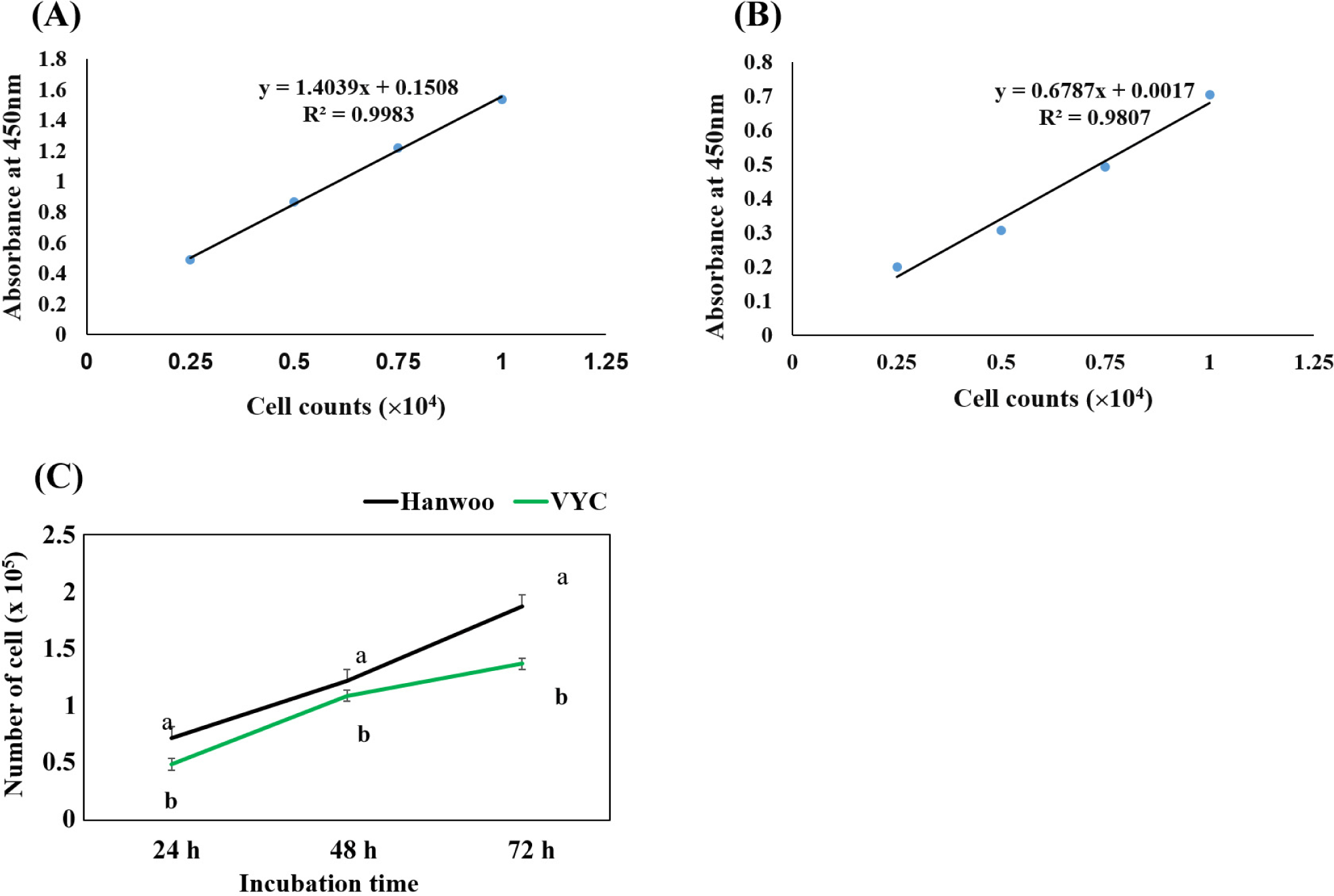
The cell proliferation rate in both the breeds were determined using cell-counting kit (CCK-8, Sigma-Aldrich) following the manufacturer’s instruction. The same number (1×104 cells/well) of cells were seeded in 96-wells plates containing 100 μL growth medium (DMEM, 15% FBS and 1% antibiotics) for 24, 48, and 72 h in a 37°C and 5% CO2 incubator. For each the measuring time, 10 μL of CCK-8 solution was added into each well and incubated for 4 h at 37°C. The absorbance at 450 nm was measured using microplate readers. The number of viable cells were calculated using calibration curves which were made by seeding different cell numbers: 0.25, 0.5, 0.75, 1, and 1.25×104 cells/well for 24 h under the same growing condition and measured with the CCK-8 kit.
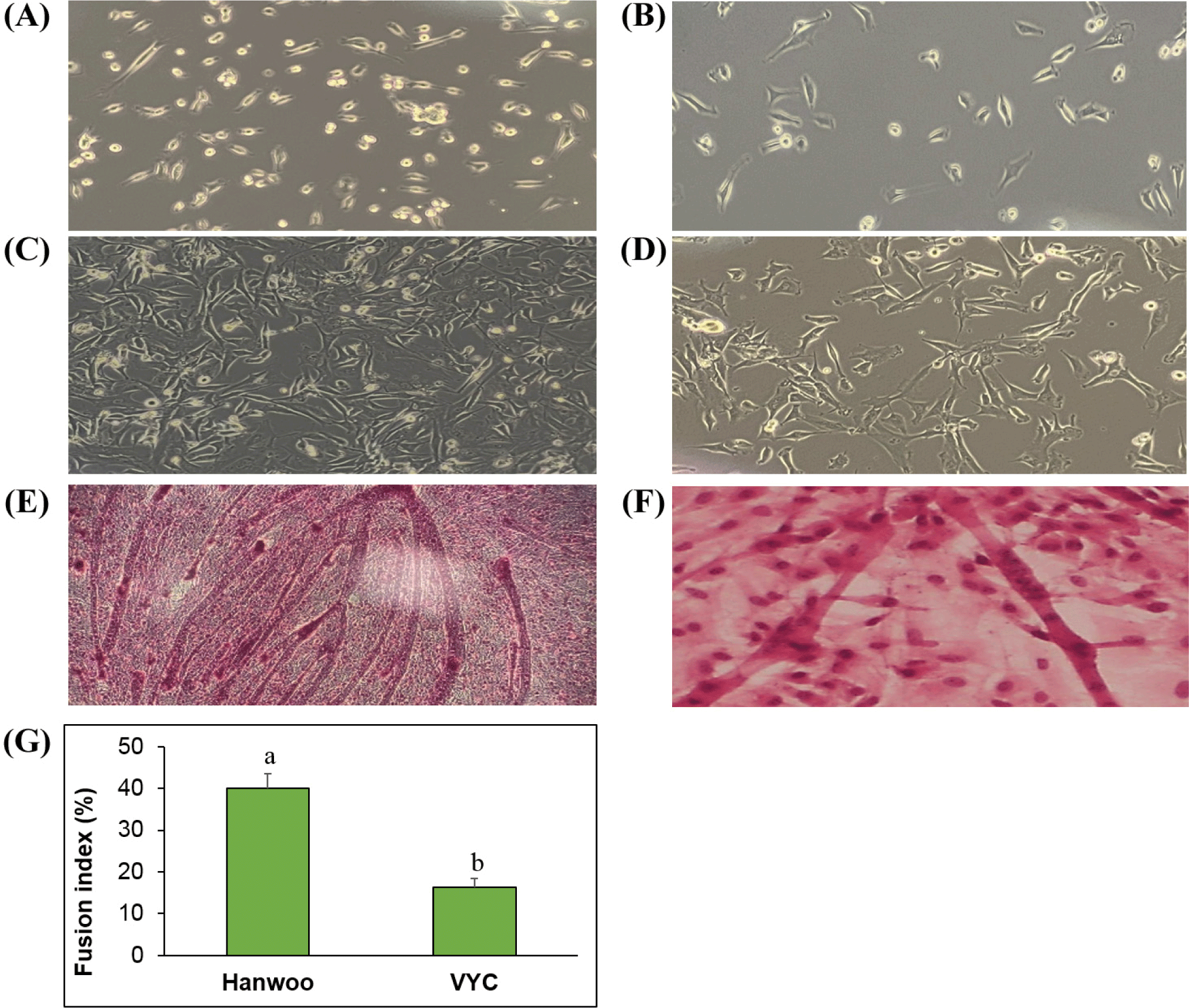
Myogenic differentiation rate of satellite cells was assessed for both the cattle breeds. The same number (106 cells/well) of Hanwoo and VYC satellite cells were cultured in 6-well plates containing growth medium until they reached about 90% confluence, thereafter, the growth medium was removed and replaced with myogenic differentiation medium containing DMEM, 2% horse serum (Thermo Fisher Scientific) and 1% penicillin/streptomycin/neomycin antibiotics solution. The cells were differentiated for 4 days and the formed myotubes (multinucleated cells) were stained with hematoxylin and eosin solution (Sigma-Aldrich) for 5 min. The number of multinucleated cells were counted, and the fusion index was calculated and expressed as a percentage of multinucleated cells to total number of nuclei counted.
Triglyceride, as the product produced by mature adipocytes in the glycolytic pathway, is the important indicator of adipogenesis of pre-adipocytes into mature adipocytes. For the triglyceride assay, the same number (106 cells/well) of Hanwoo and VYC satellite cells seeded in 12-well plate and incubated with growth medium (DMEM containing 15% FBS and 1% antibiotics) until 80%–90% confluence were used. After removing the growth medium, the cells were replaced with an adipogenic differentiation medium containing DMEM, 2% horse serum, 0.5 mM methyl-isobutylxanthine; 0.1 μM dexamethasone; 100 μM indomethacin; 10 μg/mL insulin and 65 μg/mL rosiglitazone (Sanna et al., 2009). The cells were differentiated for 7 days, and the medium was renewed at 2-day intervals. The differentiated cells were harvested, homogenized with lysis buffer, heated at 100°C for 2 min to solubilize all triglycerides and centrifuged at 10,000×g for 2 min. The supernatant was collected for determining the triglyceride content. The triglyceride content was measured using a triglyceride assay kit (Abcam, Cambridge, UK) following the procedure of manufacturer. The concentration of triglyceride (mM/L) in the cells was calculated by using the standard curve drawn from different concentrations of the standard provided.
For both cattle breeds, the same initial cell number (5×106 cells) was used and cultured in 75-cm2 Corning cell culture flasks. To assess the mRNA expression of MRF such as, MYF5 and MYOD1, the cells cultured in the growth medium for 24, 48, and 72 h was used. Whilst, the cells cultured in the myogenic and adipogenic differentiation media were used to assess the mRNA expressions of differentiation-regulating genes such as, MYOG, MRF4 and peroxisome proliferator activated receptor gamma (PPARγ). Total mRNA was extracted from the satellite cells using a TRIzol reagent (Sigma-Aldrich) according to the manufacturer’s instructions. The quantity of RNA samples was estimated at 260 nm using a spectrophotometer. The first-strand cDNA was synthesized from 1 μg of the total RNA using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Real-time polymerase chain reaction (RT-PCR) was carried out using SsoFastTM EvaGreen® Supermix (Bio-Rad Laboratories), cDNA and primers as listed in Table 1. The primers were designed using a free online designing tool (National Library of Medicine, 2022). The thermal conditions for amplification of genes used were similar to those described by Yang et al. (2013). All data was normalized to housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase, GAPDH) using the 2-ΔCt method (Schmittgen and Livak, 2008).
The ultimate pH values of meat were measured in triplicates using the protocol of Ji et al. (2010). Briefly, duplicate aliquots (3 g) of each sample were homogenized with 27 mL of DW in 50-mL conical tubes for 30s at 3,388×g. Thereafter, the pH values were measured by placing the probe of pH meter into the homogenates. The color traits of meat were measured using a color meter (Konica, Milton Keynes, UK). For each the sample, five measurements were taken on fresh cut surface after 30 min blooming at 4°C. The color values were expressed as CIE L*, CIE a*, and CIE b*. The cooking loss was done by placing the samples (in steak form with weight of about 200 g) in plastic bags and cooked in a 72°C pre-heated water bath until the core temperature reached 70°C. After cooling in ice water for 30 min, the weight of samples was recorded to determine cooking loss.
Moisture content was measured using a moisture analyzer (HR73, Mettler-Toledo, Schwerzenbach, Switzerland). IMF content was determined using the Soxhlet method as described by Ji et al. (2010). Briefly, duplicate aliquots (5 g) of each sample was taken and placed into extraction thimbles which were then dried at 102°C for 5 h. Thereafter, the samples were placed into a Soxhlet extractor and kept in petroleum ether for 6 h to extract the fat which was then kept in a dry oven at 102°C for 1 h. The weight % of wet muscle tissue was used to calculate the IMF content. Protein content was determined using total nitrogen content that was measured with a nitrogen analyzer (Rapid N cube, Germany). The total nitrogen content was then converted to protein using the N×6.25 equation (N: nitrogen content from the sample; 6.25: conversion factor). Water holding capacity (WHC) of meat sample was measured using the centrifugal method of Laakkonen et al. (1970). The WHC percentage was calculated as a ratio of moisture to the water loss.
The method of Folch et al. (1957) was used to extract the lipids in beef samples from both breeds. Duplicate aliquots (10 g) of each samples were weighed and homogenized with 150 mL of chloroform: methanol (2:1, v/v) solvent mixture. The extracted fatty acids composition in the VYC beef was determined using gas chromatography (GC) and mass selective (MS) detector (Agilent Technologies, Santa Clara, CA, USA), and connected to a column (30 m×0.25 mm×0.25 μm film thickness; Supelco, Bellefonte, PA, USA). The GC/MS conditions used for separation of fatty acids were same as those described by Cho et al. (2009). While, the fatty acids composition in Hanwoo beef was determined using a gas chromatography/flame ionization detector (GC-FID, Varian Technologies, Palo Alto, CA, USA) equipped with a capillary column (30 m×0.25 mm×0.25 μm film thickness; Supelco). The GC-FID conditions used for separation of fatty acids were same as those described by Cho et al. (2020). Fatty acids were identified by comparing their retention times with those obtained from standard fatty acids. Individual fatty acids were expressed as relative percent (%) of total fatty acids.
Data was analyzed using the SAS Enterprise software (version 7.1; SAS Institute, Cary, NC, USA). In the statistical model, the General Linear Model procedure was used in which the cattle breed was considered as a fixed effect, and the obtained data were considered as dependent variables. The mean difference was compared using Duncan’s Multiple Range Test. Significant differences were set at a 5% level. Data was presented as means±SD. To obtain the trend of relationship between the cattle breed with myogenic, adipogenic and meat quality traits, the Principle Component (PC) Analysis was carried out using the XLSTAT statistical software 2023.5.1 (Addinsoft, New York, NY, USA).
Results and Discussion
The proliferation rate of primary satellite cells from both breeds were assessed during the in vitro culture and the result is shown in Fig. 1. The cells showed a continuous growth pattern with increased culturing time in both the breeds. However, the proliferation rate was higher in Hanwoo cattle, compared with VYC throughout the measuring times (24, 48, and 72 h; p<0.05). Similar to our results, Coles et al. (2015) observed that breed significantly affected the proliferation of skeletal muscle cells, with a higher rate in Angus cattle compared with Hereford and Wagyu. Furthermore, the myogenic differentiation of satellite cells was also investigated for both breeds. Our results (Fig. 2) showed that the fusion index (ratio of number of multinucleated myotubes to total nuclei counted) was almost 3 times higher in Hanwoo (42.17%), compared with the VYC (14.93%). It is known that the muscle growth determines growth rate, and ultimately affects the beef yield in cattle. In the present study, both the cattle breeds were slaughtered at the same age (28 months old), however, the Hanwoo had a higher body weight (728 45 kg) compared to VYC (285 36 kg). Thus, the higher rate of proliferation and fusion index of satellite cells in the Hanwoo could be the possible mechanism underlying the higher body weight of this cattle breed compared to the VYC.
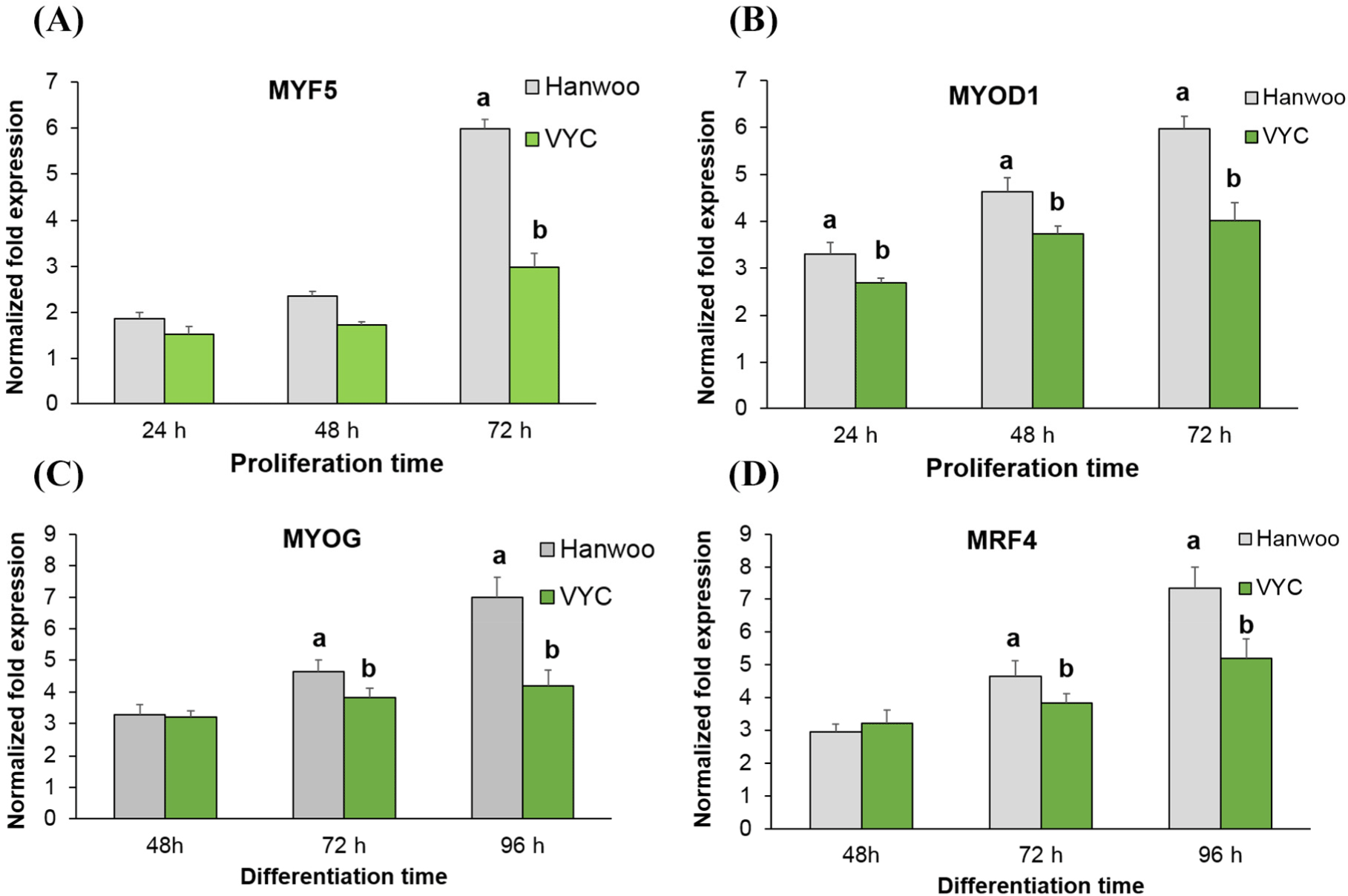
Researchers have found that MYF5, MYOD1, MYOG, and MRF4 etc. are the most important MRF (Braun et al., 1992; Rudnicki et al., 1992; Shahini et al., 2018). To elucidate the differences in the proliferation and fusion index between the breeds, mRNA expression profiles of these major MRF were assessed, and the results are shown in Fig. 3. MYF5 and MYOD1 are known as the major early MRF in myoblasts, while MYOG and MRF4 are two major myogenic differentiation markers of myotubes (Yablonka-Reuveni, 2011; Zanou and Gailly, 2013). It was observed that the expressions of MYF5 and MYOD1 showed an increasing trend with increased culturing time in both the cattle breeds. At 72 h cultured in growth medium, the expression of MYF5 was higher in the Hanwoo, compared to the VYC (p<0.05). Similarly, the Hanwoo cattle showed a higher expression level of MYOD1 on all the examining days compared with the VYC (p<0.05). For the MYOG and MRF4, their expressions were also higher in Hanwoo cattle after 72 and 96 h of induction in the differentiation medium (p<0.05). Thus, the results obtained on the mRNA expression examinations of MRF genes were in line with the results of cell proliferation (Fig. 1) and fusion index (Fig. 2).
It is well recognized that amount of IMF or marbling degree is a key determinant of beef eating quality (e.g., tenderness and juiciness; Lee et al., 2022; Nguyen et al., 2021). Adipogenesis is known as the process that differentiates the pre-adipocytes into mature adipocytes, ultimately resulting in the fat deposition (Schumacher et al., 2022). In animals, the fat deposition occurs in subcutaneous, intermuscular and intramuscular locations (Malgwi et al., 2022). In the present study, the satellite cells were induced in the adipogenic differentiation medium to determine whether they could differentiate into adipocytes. Our results (Fig. 4A) showed that after 5 and 7 days of induction, the satellite cells in both cattle breeds exhibited their ability of adipogenic differentiation. Particularly, the triglyceride content in Hanwoo cattle satellite cells was 28.17 mM/L and 37.62 mM/L after 5 and 7 days of induction, respectively. Compared with Hanwoo, a significantly lower triglyceride content (13.27 and 18.35 mM/L after 5 and 7 days of induction, respectively) was found in the VYC satellite cells (p<0.05).
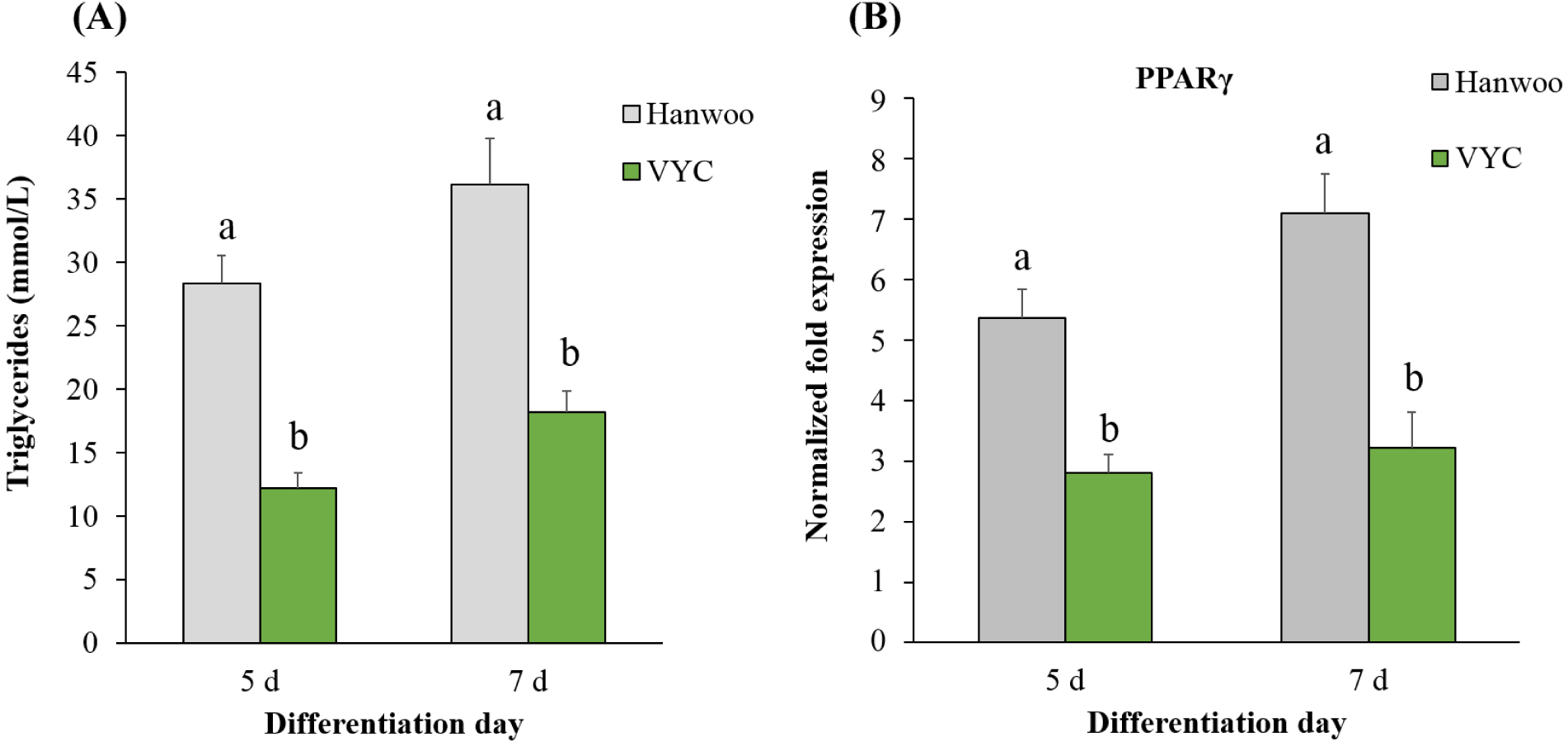
This finding agrees with that of Yang et al. (2013), who showed the adipogenic potential of muscle satellite cells from Hanwoo cattle. Also, this result is coinciding with those in studies of Cassar-Malek et al. (1999) and Kook et al. (2006). Triglycerides are the main storage form of fat, which are primarily produced in adipocytes via the glycolytic pathway (Malgwi et al., 2022). During the adipogenesis, some key transcription factors (e.g., PPARγ) play an important role in controlling the differentiation of pre-adipocytes in to mature adipocytes (Liu et al., 2021; Rosen et al., 2000). Our result (Fig. 4B) showed that the mRNA expression of PPARγ was approximately two times higher in the satellite cells of Hanwoo, compared with the VYC (p<0.05). Similar to the current finding, Duarte et al. (2013), Liu et al. (2021), and Martins et al. (2015) analyzed the mRNA from muscle tissues, and observed that cattle breed affected the expression of adipogenesis-regulating genes in the longissimus dorsi muscle. Thus, the result indicating the higher concentration of triglycerides in the satellite cells of Hanwoo (Fig. 4A) might be resulted from the upregulated expression of PPARγ in this cattle breed.
The meat quality traits of LL muscles from Hanwoo and VYC are presented in Table 2. The Hanwoo exhibited a lower (p<0.05) pH value, compared to the VYC, this may be related to a faster rate of post-mortem glycolysis in Hanwoo or a lower level of muscle glycogen in the VYC due to insufficient energy input from the feeding diet. The rate of post-mortem glycolysis is largely influenced by various factors such as, animal species, breeds, feeding regimes and stress before slaughter (Chauhan and England, 2018). Priolo et al. (2001) observed that meat from cattle fed with grass-based diets usually have a higher pH values compared with meat of cattle fed with grain-based diets. In the present study, the feeding with a high ratio of grasses might result in a high ultimate pH value in the VYC beef. Regarding the proximate composition, Hanwoo beef had a higher IMF (approximately 6 times greater), and lower moisture and protein contents, compared with the VYC meat (p<0.05). As earlier-mentioned, the beef quality (e.g., tenderness, juiciness and flavor) is significantly influenced by the IMF (Lee et al., 2022). To meet the consumer’s demand, a lot of attempts such as, castration, genetic improvement and nutritional intervention etc. have been made to improve the IMF deposition in Hanwoo (Chung et al., 2018; Hoa et al., 2022). The higher IMF content in Hanwoo meat could be due to its better potential of fat accumulation, as indicated by a higher triglycerides content (Fig. 4A) and higher expression of adipogenesis-regulating genes (Fig. 4B). Supporting our findings, Duarte et al. (2013) reported that improved marbling score in Wagyu beef was associated with up-regulated expression of PPARγ. Martins et al. (2015) observed that up-regulated expression of PPARγ in Angus cattle led to a higher IMF content compared with Nellore cattle breed. Previous studies have also showed a high potential of fat deposition in Hanwoo cattle compared to the other breeds (Chung et al., 2018; Jo et al., 2012). Additionally, feeding with a high ratio of concentrate during fattening period have resulted in the higher IMF content as seen in concentrate-fed cattle by Gotoh et al. (2009).
| Item | Moisture (%) | Fat (%) | Protein (%) | pH | WHC (%) |
|---|---|---|---|---|---|
| Hanwoo | 59.67±4.09b | 20.17±5.32a | 18.26±1.04a | 5.54±0.06b | 64.44±1.75a |
| VYC | 71.79±1.37a | 3.52±0.53b | 19.47±6.03a | 5.89±0.06a | 60.70±2.28b |
Color is the first quality trait seen by consumer, and is of the utmost importance in meat marketing (Salim et al., 2022). Our results (Table 3) showed that the cattle breed affected all the color traits. The Hanwoo beef had a higher CIE L*, indicating its lighter color while, the VYC meat had a higher CIE a*, indicating its redder color (p<0.05). The differences in color traits could be attributed to the differences in muscle chemical composition in which the lighter color in Hanwoo beef might be due its higher IMF content (Table 2). Corresponding to the current finding, Yim et al. (2015) observed that meat with higher IMF level is lighter in color. Previous studies showed that meat color is largely influenced by both intrinsic (e.g., breed, muscle type etc.) and extrinsic factors (e.g., feeding diet; Salim et al., 2022). In many studies, beef from grass or grass and grain supplement-fed cattle is redder in color due to the presence of natural antioxidants compared to meat from grain-fed cattle (Luciano et al., 2011; Tansawat et al., 2013). In the present study, the used animals were different from breeds and feeding diets, which both might affect the color of beef.
| Item | CIE L* | CIE a* | CIE b* |
|---|---|---|---|
| Hanwoo | 40.71±2.56a | 20.88±1.11b | 16.55±2.34a |
| VYC | 32.74±2.67b | 21.73±3.27a | 13.92±3.19b |
The fatty acid profiles in LL muscles from Hanwoo and VYC are shown in Table 4. A total of fourteen fatty acids was found, in which 5 fatty acids (C18:1n9, C18:1n7, C18:2n6, C18:3n3, and C20:4n6) showed a significant difference between the cattle breeds (p<0.05). Fatty acid composition of meat is of increasing interest due to its effect on organoleptic characteristics of the meat (Dinh et al., 2021). Monounsaturated fatty acids (MUFAs) such as, oleic acid are the most important precursors of meat flavor since they generate a variety of volatile compounds contributing to desirable aromas of cooked meat (Dinh et al., 2021). Results showed that the oleic acid and MUFAs contents were higher in Hanwoo meat, compared with the VYC meat (p<0.05). This may indicate a higher rate of desaturation of C18:0 to C18:1 by the desaturase enzymes in the adipocytes from Hanwoo cattle, compared to the VYC. Similar to the current findings, Hwang and Joo (2017) reported a higher MUFA and oleic acid contents in LL muscles of Hanwoo, compared with American and Australian cross breeds. The high MUFAs content in Hanwoo beef has been resulted from feeding with a high ratio of concentrate (Hwang and Joo, 2017; Jayasena et al., 2014). Contrastingly, the VYC beef exhibited a higher content of polyunsaturated fatty acids (PUFAs) such as, C18:2n6, C18:3n3, and C20:4n6 compared with Hanwoo (p<0.05). The total n3 PUFAs were also higher in the VYC meat, which resulted in a lower n6/n3 ratio in this beef type, compared with Hanwoo. The fatty acids profiles in beef have been reported to be affected by both breed and feeding conditions (Nogoy et al., 2022; Vázquez-Mosquera et al., 2023). Vázquez-Mosquera et al. (2023) observed a higher MUFA and oleic acid contents in LL muscles from the highly-marbled Wagyu compared with Angus-by Charolais under identical raising condition. Waters et al. (2009) reported that feeding cattle with grass-based diet reduces the activity of desaturase enzymes by increased absorption of the linolenic acid. Nogoy et al. (2022) observed that grass-fed beef contains a greater concentration of n3 PUFAs and lower n6/n3 PUFAs ratio, compared to grain-fed beef. While, the breed may affect the de novo fatty acid synthesis pathway which ultimately impacts the fatty acid composition in meat (Schumacher et al., 2022). On the other hand, the fatty acid composition in meat is also used to evaluate its nutritional values (Vargas-Bello-Pérez and Larraín, 2017). According to the recommendations of World Health Organization for the healthy diet, the PUFA/saturated fatty acid (SFA) ratio should be 0.40 or higher, and the n6/n3 fatty acid ratio should be 4.0 or lower (Burlingame et al., 2009). Thus, the VYC meat exhibited a ″healthier″ fatty acid profile, compared with Hanwoo meat.
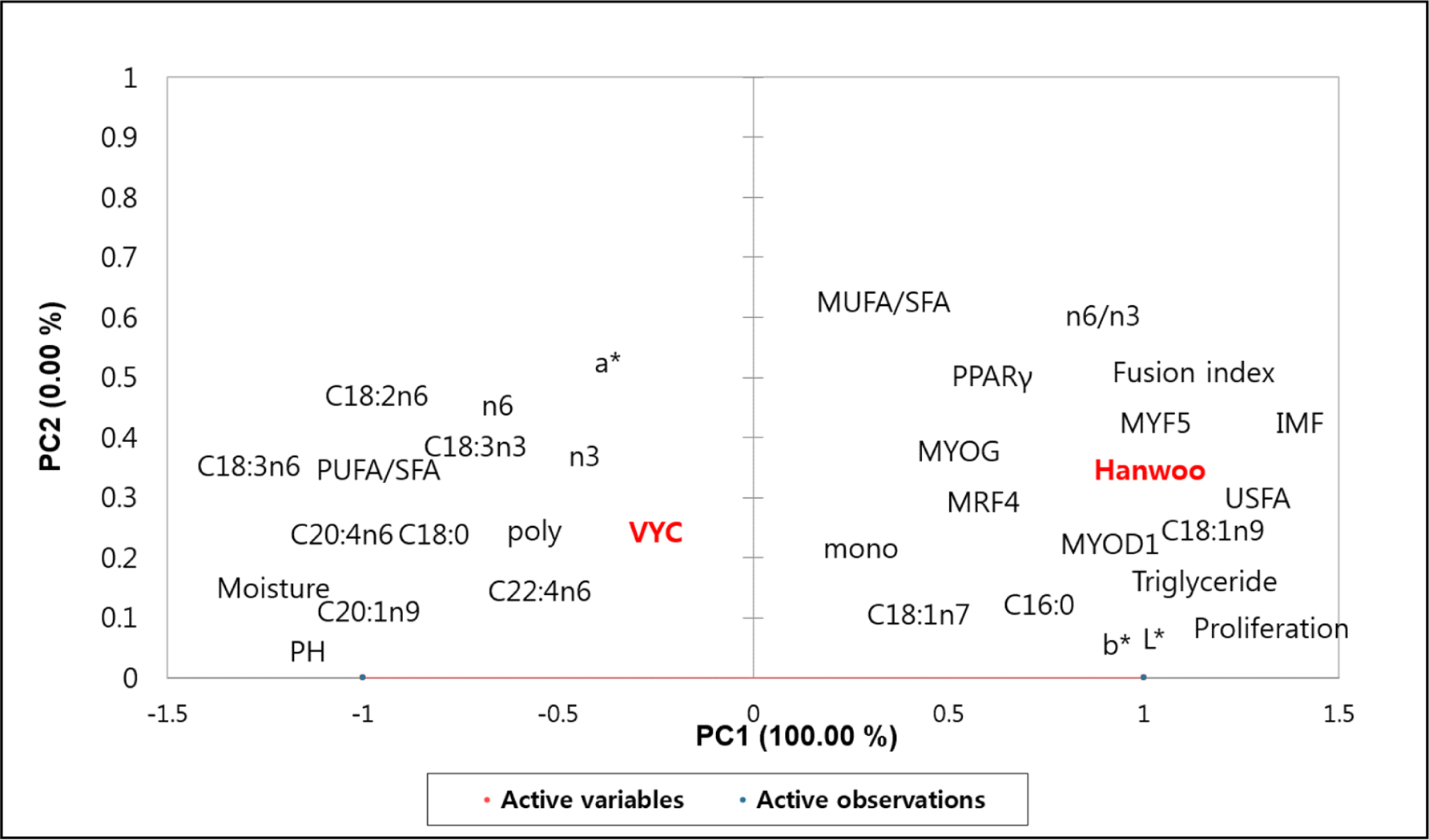
Furthermore, PC analysis was also carried out to obtain a trend of relationship between the variables and observations. As shown in Fig. 5, 100% of variability was explained by the PC1. The VYC was on the negative PC1 axis, therefore, it was related to the CIE a*, pH, moisture, PUFA, C18:26, C18:3n3, and total n3 PUFAs etc. While, Hanwoo cattle was on the positive axis, therefore, it was related to cell proliferation rate, fusion index, myogenesis- and adipogenesis-regulating genes (MYF5, MYOD, MYOG, MRF4, and PPARγ), triglyceride, IMF, C18:1n9, and MUFA etc. The results of multivariate analysis again indicate a significant difference in the myogenic and adipogenic potentials, and meat quality properties between the Hanwoo and VYC.
Conclusion
This study, for the first time, compared the myogenic and adipogenic mechanisms and their associations with meat quality characteristics between Hanwoo and VYC. Apart from the difference in the feeding systems, Hanwoo cattle showed a considerably higher body weight, indicating its higher growth rate than the VYC when harvesting at the same age. By using the in vitro culture model, we found a significant difference in the proliferation rate, fusion index, differentiation, and of mRNA expression patterns of related genes in these processes in the satellite cells between the two breeds. Noticeably, the Hanwoo showed a higher expression level of adipogenesis-regulating gene (PPARγ) in the satellite cells and a greater IMF content in LL muscles, signifying its better ability of IMF accumulation, compared with VYC. The VYC were characterized by a ″healthier″ fatty acid profiles, with a higher PUFAs content and PUFA/SFA ratio while, the Hanwoo meat had higher MUFA content. The findings suggest that the higher proliferation and differentiation rate together with a greater expression of the adipogenesis-regulating factor (e.g., PPARγ) in the satellite cells could be the main reasons responsible for the better muscle growth potential and meat quality (e.g., higher IMF) of Hanwoo cattle in comparison with the foreign cattle breed such as VYC. Furthermore, to improve the muscle growth and meat quality of VYC, particular strategies such as, genetic improvement (e.g., by crossbreeding with commercial beef breeds), and dietary modification (e.g., using a high-concentrate diet) may be needed.













