Introduction
While many research groups and companies have recently announced that they have developed prototypes and are undertaking efforts to develop cultured meat, there have been no successful cases of industrialization. One of the most important challenges to developing cultured meat products is lowering the production cost; however, it is believed that the technology is not sufficiently developed to achieve such lowering. Reducing or replacing media, such as fetal bovine serum (FBS), may be the most important goal for the commercialization of cultured meat. Importantly, FBS is the most widely used growth supplement in cell culture technology and is rich in growth factors, hormones, amino acids, proteins, vitamins, inorganic salts, and antibodies (Castells-Sala et al., 2017; Chelladurai et al., 2021). FBS is obtained from the fetus of slaughtered pregnant cows, and more than two million bovine fetuses are used worldwide to produce approximately 800,000 L of FBS (Brindley et al., 2012; Chelladurai et al., 2021). FBS is a key material for cell culture, which is not only expensive but also difficult to completely replace with other materials. Moreover, there are ethical issues associated with its use, because it is obtained from slaughtered cattle during pregnancy. Therefore, many scientists worldwide are conducting research to reduce or replace FBS usage. The components in FBS have not been fully identified, and the effects on the composition and cell culture vary depending on the individual fetus. Previously, the safety of cell cultures using FBS has been consistently stated (Gstraunthaler et al., 2013; Manukyan et al., 2020). Cell culture using FBS may contain viruses, mycoplasma, endotoxin and prion proteins, and these controversies have not been clearly resolved (Gstraunthaler et al., 2013; Manukyan et al., 2020; Merten, 2002). For this reason, the production of cultured meat using FBS has not been verified for human safety for repeated production and ingestion of the culture. Therefore, more in-depth study of the components of FBS is needed to replace FBS or reduce the amount of FBS used in cultured meat. This review aims to summarize the main components and controversies of FBS and the current technologies of serum-free cell culture including cultured meat.
Composition of Blood and Fetal Bovine Serum (FBS) in Animals
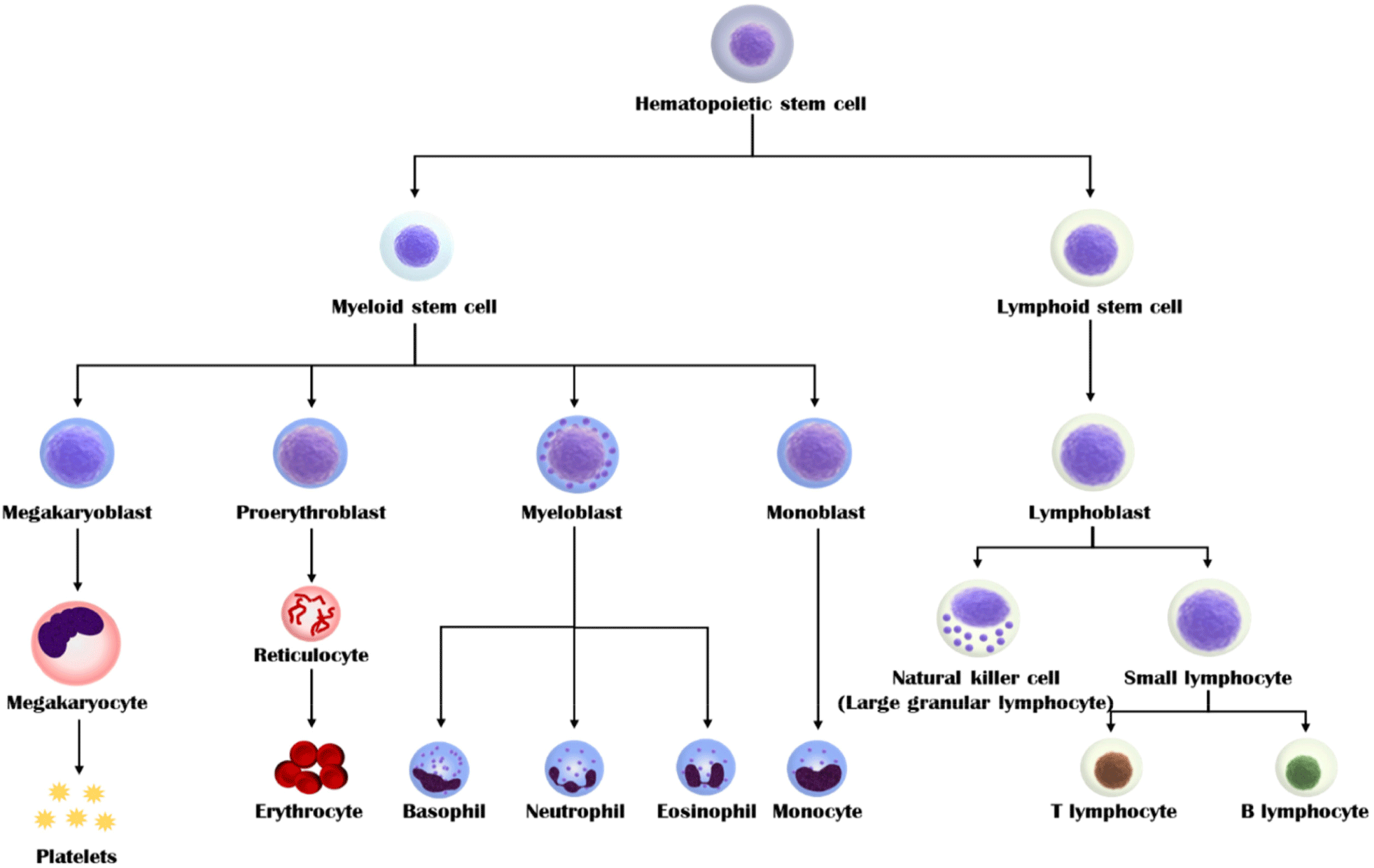
Blood derived from adult animal is produced through the self-replication and differentiation of hematopoietic stem cells in the bone marrow (Fig. 1). Hematopoietic stem cells differentiate into various blood cells (e.g., red blood cells, platelets, neutrophils, eosinophils, basophils, monocytes, and T/B lymphocytes). Although the overall composition of blood has not yet been clearly identified, it can be determined according to various factors, including species, sex, feed, drinking water for livestock, pregnancy, and disease. The composition of blood can be confirmed by dividing anticoagulant-treated blood into blood cells and plasma. Approximately 20%–40% of the blood in the vertebrates is composed of blood corpuscles, which comprise red blood cells, white blood cells, and platelets. Red blood cells do not have a nucleus but instead contain hemoglobin, which oxygenates tissues and removes carbon dioxide and waste products. Hemoglobin is a protein that contains iron (heme-iron). When iron ions in hemoglobin combine with oxygen and oxidize, red blood cells appear red. White blood cells (leukocytes), which account for approximately 1% of the total blood cells, are immune system cells and are nucleated. Leukocytes are divided into granular leukocytes and agranulocytes, according to the presence of specific cytoplasmic granules. Granular leukocytes comprise eosinophils, basophils, neutrophils, whereas agranular leukocytes comprise lymphocytes and monocytes. Finally, platelets play a role in coagulating blood by aggregating fibrin when blood vessels are damaged. The rest of the blood is plasma, which is a yellowish liquid obtained after treatment with an anticoagulant. Approximately 90% of plasma is water and it contains 6%–8% protein, carbohydrates, blood clotting factors, minerals, and hormones. Plasma serves as a matrix for blood cells and transports oxygen, carbon dioxide, and waste products. Most of the proteins in plasma are produced by the liver. Among them, albumin can non-specifically bind to substances, such as bilirubin, bile salts, and penicillin, that are poorly soluble in plasma. Globulin is divided into α, β, and γ, and specifically binds to and transports fat-soluble substances, such as hormones and cholesterol. These components can affect cell growth and adhesion.
Blood is a by-product generated during animal slaughter, and the representative major livestock that can secure a sufficient volume of blood for commercial use are cattle, pigs, and poultry. Livestock blood is generally discarded with livestock wastewater; therefore, there are few studies on its use as a resource. Table 1 details the blood components of representative livestock, i.e., cattle, pigs, and poultry. Although the blood composition of livestock has been analyzed in various studies, the main components were similar, and various components were identified depending on whether a separate component analysis was performed (Table 1). The blood of all species commonly contains the basic blood components: Red blood cells, white blood cells (basophils, neutrophils, eosinophils, monocytes, T lymphocytes, and B lymphocytes), and platelets (thrombocytes). Blood contains hemoglobin, which is present in red blood cells, and protein components, such as various types of albumin and globulin, classified according to the degree of solubility in blood fluids. In addition, various carbohydrates and lipids are present; however, research has not yet been conducted to classify these clearly. In addition to the three essential nutrients mentioned above, blood contains various components, including minerals and vitamins, such as calcium, magnesium, and sodium. Because these blood components are greatly affected by the components of drinking water and feed supplied to the livestock or the living environment, the types and quantities of blood components can vary. In addition, metabolites such as creatine, urea, and bilirubin may be dissolved in the blood, and their quantities are also greatly affected by the species, diet, and environment of the livestock (Jin and Zhou, 2022; Zhang et al., 2022).
These data were modified from George et al. (2010), Mohri et al. (2007), Ognik et al. (2016), Panousis et al. (2018), and Sorapukdee and Narunatsopanon (2017).
Here, we focus on FBS, as it is essential not only for cultured meat but also for in vitro culture of cells for other experimental analyses. FBS is prepared after removing clotting factors (cruor), such as clotted fibrin, along with blood corpuscles from clotted blood. The blood composition is also greatly affected by the diet and environment of the pregnant female. Therefore, although it is difficult to accurately state the ingredients and contents, many studies have been conducted on the growth factors, hormones, proteins, cofactors, and minerals that can affect cell culture. The identified components of FBS are listed in Table 2. The interaction between FBS components and cell growth, proliferation, maturation, and differentiation is still being investigated. In adherent cells, such as bovine muscle-derived satellite cells used for cultured meat production, the secure attachment of cells to culture dishes is an important factor for early cell growth. FBS factors affect cell adhesion include serum spreading factors including fibronectin and laminin (Koblinski et al., 2005). Albumin, one of the non-adhesive proteins present in serum, activates adhesion and spreading factors, and affects the degree of cell adhesion and is dependent on the amount of non-adhesive protein (Koblinski et al., 2005). In addition, FBS has a very low level of immunoglobulin generated during the growth of livestock, and a high level of growth factors; therefore, it is useful for cell culture of various species because the immune response caused by the antibody is minimized (Chelladurai et al., 2021; Zheng et al., 2006). Although its influence differs depending on the type of cell grown, growth factors present in the serum play an important role in cell growth and proliferation. Further, while the effect of adding a growth factor to the serum alone without the addition of a separate growth factor is evident, the effect may not be uniform, depending on the type and content of the growth factor. FBS factors that effectively induce cell growth include growth factors and cytokines (Table 2). These factors sometimes affect differentiation, such as epidermal growth factor, insulin-like growth factor (IGF), and platelet-derived growth factor (Lee et al., 2022). This effect can shorten the cell culture period and reduce the costs of parenting production or experimental analysis. The composition of enzymes and hormones in serum varies depending on the slaughter period of the animal (e.g., age and reproduction; Mohri et al., 2007). These serum cocktail components contain most of the factors necessary for cell proliferation and maintenance; therefore, it is recommended to use an adequate amount depending on the purpose of cell culture. A definitive study on the effect of each ingredient has not yet been successfully conducted. Similar to the components of whole blood from adult animal, various nutrients can facilitate cell growth in serum. Saccharides or amino acids can support the energy required for cell proliferation and metabolism (Table 2). Trace amounts of vitamins and minerals are present in FBS and even these amounts can affect the growth of cells (Table 2). Many studies have been conducted to determine the positive/negative effects of vitamins or minerals on adhesion, proliferation or differentiation in various type of cell (Miron et al., 2017; Rao et al., 1994; Rembe et al., 2018; Samuel and Sitrin, 2008). In addition, the hormone factors and transport proteins (carrying the hormones, minerals, trace elements, and lipids) are necessary components to maintain the changing pH during cell culture, and they also affect protease inhibition. These effects are very important for maintaining cell viability (Brunner et al., 2010; Chelladurai et al., 2021).
These data were modified from those of Brunner et al. (2010), Chelladurai et al. (2021), Honn et al. (1975), and Zheng et al. (2006).
Fetal Bovine Serum (FBS) for Cell Culture
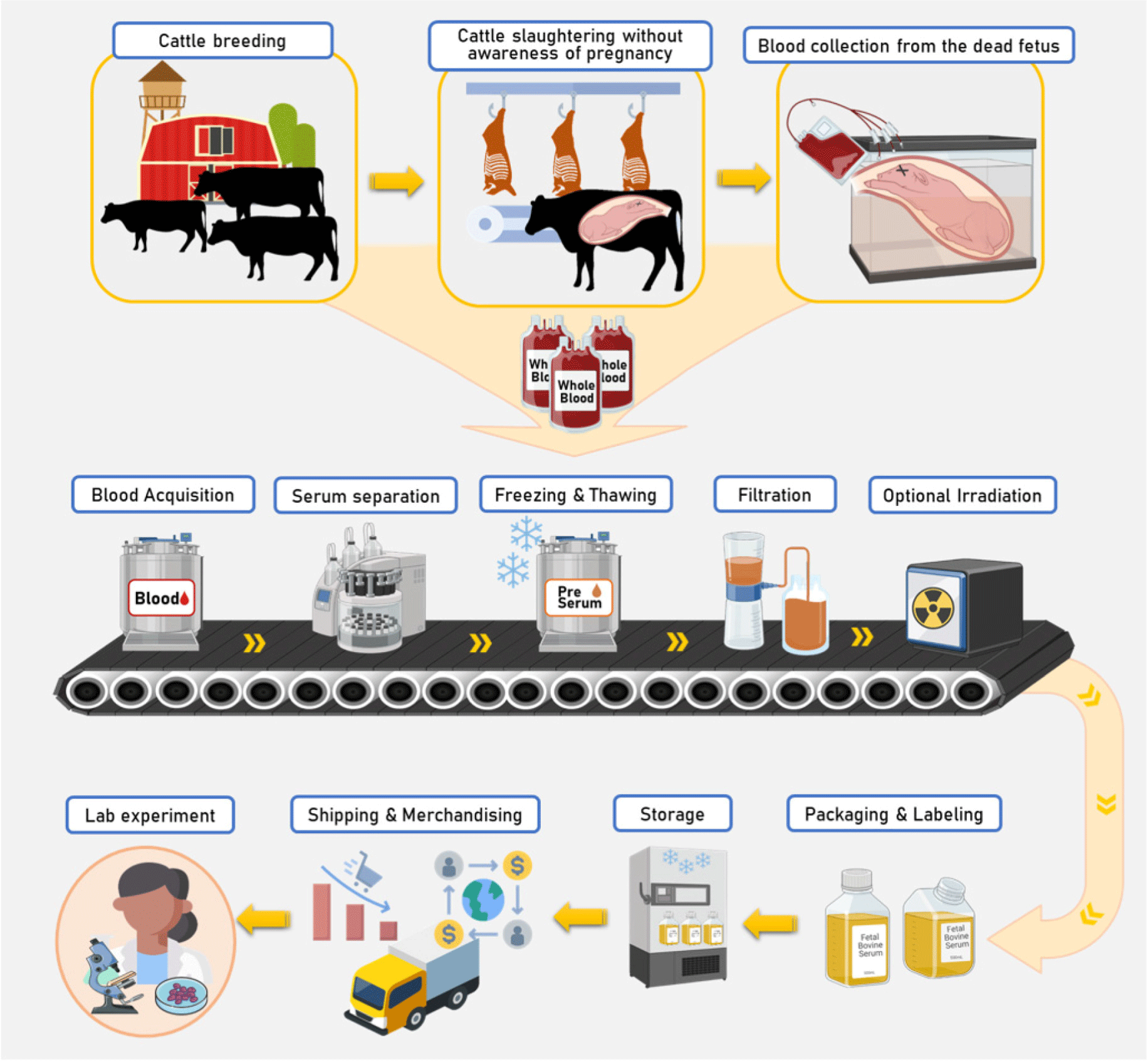
FBS is a by-product of blood drawn from a bovine fetus via a closed collection system at a slaughter house. In the 1950s, when FBS was introduced into cell culture, it was thought that FBS would only stimulate cell growth (Puck et al., 1958). Later, it was identified that FBS contains essential components required for cell attachment, proliferation, and maintenance, such as serum albumin, fetuin, hormones, vitamins, trace elements, and growth factors (Chelladurai et al., 2021). FBS is rich in growth factors and contains low levels of γ-globulins, which have cell growth inhibitory activity (Yao and Asayama, 2017). Thus, it is suitable for cells that are difficult to proliferate in culture and for cloning (Yao and Asayama, 2017). However, the ethics of using serum from fetal calves has always been problematic, and two scientific societies published a plea in 2013 for all cell biologists to stop using FBS and switch to a substitute; the plea was renewed in 2018 (Piletz et al., 2018). FBS can be obtained from the fetus when female livestock is accidentally slaughtered during pregnancy. Therefore, not only are all pregnancy and breeding system are strictly controlled, but in countries such as Korea, where pregnant animals cannot be slaughtered, researchers are not allowed to obtain FBS. In addition, with the development of pregnancy diagnosis technology, opportunities to accidentally acquire FBS are gradually decreasing; therefore, it is important to develop a material that can replace FBS. Moreover, there are also many opinions that FBS contaminated with viruses and various microorganisms is a potential health risk (Piletz et al., 2018). Another problem is that the process of acquiring FBS is not regular or uniform, which may create disadvantages, such as an unstable supply and an increase in the price of FBS. Although FBS contains various growth factors, hormones, and nutrients, it also possesses a high endotoxin content and can be a potential source of microbial contaminants, including fungi, bacteria, viruses, and prions (Tancharoen et al., 2019). Therefore, research on alternatives to FBS for usage in cell experiments and cultured meat production is critical, and future research on ethical FBS alternatives is urgently needed.
FBS is the most commonly used supplement in animal cell culture media. FBS used in cell cultures contains more growth factors than other animal serum media and has the advantage of having very low levels of antibodies (Hawkes, 2015). FBS is rich in complex protein components, trace nutrients, and hormones required for the adhesion, growth, and proliferation of various cells (Honn et al., 1975; van der Valk et al., 2010). FBS preparation includes removing coagulation factors that inhibit cell growth; however, with unknown compositions, there may be potential immunogenicity and spread of prions. Nevertheless, FBS is a universal cell medium additive that can be used for almost all cells, and because it is not affected by most chemicals, it is less likely to adversely affect cell culture. The process of obtaining FBS is divided into several stages. The main process for producing FBS is as follows (Fig. 2): 1) Usually, in slaughterhouses for meat production, the source is the fetus of an individual slaughtered without knowledge of pregnancy. This indicates that serum is a by-product of the meat industries. 2) The fetus is then sterilized in a sterile space, and blood is collected by inserting a needle into the fetus. This process can only be performed in government-approved facilities to prevent uncontrolled slaughter of fetuses. 3) The collected blood is stored in sterile packaging and refrigerated to promote coagulation to obtain high-purity serum. The clear yellow serum can then be separated by centrifugation in a refrigerator. This removes cellular components and clotting factors from the clotted blood. 4) The serum can be frozen and stored until the subsequent steps are performed. 5) Serum is filtered through a filtration chain to be used for cell culture (usually 0.1 μm triple filtration). A similar process to remove potential bacterial contamination is essential but cannot completely eliminate the virus. Serum may be further sterilized by irradiation (gamma rays) if necessary. 6) Next, sterile packaging and labeling are carried out, and the samples are frozen until use in the laboratory. This process reduces the degradation of biologically active ingredients that help cell growth and could significantly impact the final quality of FBS. FBS may have undesirable effects on cell cultures because its components have not yet been clearly defined. FBS may have undesirable effects on cell cultures because its components have not yet been clearly defined. In addition, since the composition of FBS differs depending on the origin of the individual from which it is acquired, it is necessary to develop a cell medium or additive with similar effects for the reproducibility of cell culture results.
In the last six decades, researchers have attempted to replace FBS with different sources, including hormones, pituitary extracts, chick embryo extracts, bovine milk fractions, bovine colostrum or platelet lysates, bovine ocular fluid, fish serum, plant origin proteins, sericin protein, and other protein fractions from plant extracts, notably vegetal serum and earthworm coelomic fluid (Chelladurai et al., 2021). Serum-free media have been developed to avoid the use of animals as cell culture media and have achieved great success, especially in the production of some proteins for medical use (Cruz et al., 1998; Hawkes, 2015). However, pharmaceutical companies, diagnostic laboratories, and researchers still depend heavily on FBS for most cell culture needs (Hawkes, 2015). In a recent review, Mckee and Komarova (2017) reported that 73,200 papers contained the search term “culture medium” in 2016, including 31,300 papers with Dulbecco’s modified Eagle’s medium (DMEM), 24,700 with RPMI 1640, 6,480 with MEM, 480 with Alpha modification, and 1,650 with M199. Thus, these four media formulations were used in approximately 90% of published in vitro studies (McKee and Komarova, 2017). Each basal medium was designed for each case based on cell type, origin (i.e., animal species), and purpose of culturing (Yao and Asayama, 2017).
Culture media can be classified as serum-free, protein-free, animal-derived component-free, or chemically defined media (van der Valk et al., 2010). Serum-free media do not require supplementation with serum; protein-free media do not contain high molecular weight proteins or protein fractions. Animal-derived component-free media contain no components of animal or human origin, whereas chemically defined media do not contain proteins, hydrolysates, or any other components of unknown composition (van der Valk et al., 2010). We investigated the developed media using these different definitions, and their origins were based on serum replacement or serum-free media. Information on serum-free media studies based on the species used for cell culture is presented in Table 3.
| Species | Cell type | Media | Additives for replace of FBS | Results | References |
|---|---|---|---|---|---|
| Bovine | Chondrocytes | Chemically defined medium (CDM) | Transforming growth factor (TGF)-β3 | The treatment group to which TGF-β3 was added without the addition of FBS showed excellent cell growth. | Byers et al. (2008) |
| Dulbecco’s modified Eagle’s medium (DMEM) | - | The spontaneous calcium signaling in chondrocytes is affected by FBS exposure. | Zhou et al. (2015) | ||
| High glucose DMEM | - | Cartilage tissue was formed through a three-dimensional culture system in a medium without serum and growth factors. | Ahmed et al. (2014) | ||
| Corneal endothelium cells | DMEM/F12 | Platelet releasates | Human platelet releasates used for ex vivo expansion of corneal endothelial cells. | Chou et al. (2014) | |
| Cumulus-oocyte complexes | Modified synthetic oviduct fluid (mSOF) | Insulin, transferrin, and selenium (ITS), bovine serum albumin (BSA) | Investigation of the necessity and possible interactions of serum, cumulus cells, and oil during cell culture. | Goovaerts et al. (2012) | |
| Follicle | McCoy’s 5a medium | Insulin-like growth factor (IGF)-I (hrIGF-I) | Investigation of culture and development of follicles through regulation of IGF-I bioavailability. | Thomas et al. (2007) | |
| Granulosa cells | Modified alpha-minimum essential medium (MEM α) | 0.1% polyvinyl alcohol | Stimulation of progesterone production in granulosa cells through the addition of norepinephrine. | Piccinato et al. (2012) | |
| Keratinocytes (Comea) | DMEM/F12 | IGF-II and collagen expression in keratocytes. | Kane et al. (2009) | ||
| Nucleus pulposus cells | CDM | TGF-β3 | Comparison of cell development in serum-free/serum-supplemented media containing TGF-β3. | Reza and Nicoll (2010) | |
| Oocyte | KSOM–PVA (potassium-supplemented simplex optimized medium-polyvinyl-alcohol) | Glucose, fructose | Effect of the addition of glucose and fructose to the supplemented serum-free medium on oocyte development. | Bhuiyan et al. (2007) | |
| McCoy’s 5a | Activin, follicle-stimulating hormone | Identification of ingredients that help the growth and activation of oocytes. | McLaughlin and Telfer (2010) | ||
| Primary bovine satellite cells (BSCs) | Beefy-9 | - | Confirmation of the effect of reducing the cost of cultured meat production by developing a serum-free medium. | Stout et al. (2022) | |
| Primary bovine myoblasts | Seven commercial serum-free media | LipoGroTM, XerumFreeTM | Comparison of cell growth effects of 7 commercial serum-free media and 3 additives. | Kolkmann et al. (2020) | |
| Umbilical cord Wharton’s jelly cells | Stemline® mesenchymal stem cell expansion medium | - | Comparison of cell growth and morphology in commercial serum-free medium. | Cardoso et al. (2012) | |
| Whole embryo | HEPES buffered TCM 199 medium | Growth factors and cytokines (GFs-CYKs; IGF-I, IGF-II, bFGF, LIF, GM-CSF, TGF), recombinant albumin (RA), hyaluronan (HA) | Development of fetal calf serum or BSA free medium using various growth factors and cytokines or other molecules with surfactant and embryotrophic properties. | Moreno et al. (2015) | |
| Two-step defined culture system (C1/C2 medium) | - | Development of a defined culture medium that supported improved in vitro bovine embryo development and calving rate after embryo transfer. | Lim et al. (2007) | ||
| - | Evaluation of the developmental competence of bovine embryos in the serum-free medium at early or later embryonic stages. | Park et al. (2010) | |||
| mSOF | CDM, BSA | Comparison of blastocyst development in embryos cultured in serum-supplemented and serum-free media. | Jang et al. (2011) | ||
| Dulbecco’s phosphate buffered saline-sericin (cryopreservation) | Silk protein sericin | Effect of silk protein sericin in serum-free freezing of bovine embryos. | Isobe et al. (2013) | ||
| TCM-199 medium | Albumin, ITS | Evaluation of the development of bovine embryos and blastocyst quality in serum-free conditions. | Wydooghe et al. (2014) | ||
| Porcine | Bone marrow-derived mesenchymal stromal cells | Ultra-CULTURE | Epithelial growth factor (EGF) | Confirmation of substitutability of the serum-free medium through the addition of growth factors. | Wang et al. (2013) |
| Chondrocytes | Ham F12 | Autologous serum, fibroblast growth factor—basic (FGF-basic), IGF-1 | Indentification of serum replacement effect of autologous serum and growth factors in serum-free medium. | Kamil et al. (2007) | |
| Hepatocytes | DMEM/199 medium | Dimethyl sulfoxide (DMSO), dexamethasone | Verification of cell monolayer formation and maintenance in serum-free medium condition. | Caperna et al. (2011) | |
| Williams E medium | - | Comparison of phenotype-induced changes in cells cultured in serum-free/serum-supplemented media. | Rasmussen (2017) | ||
| Oocyte | Modified porcine oocyte medium (mPOM) | - | Defined system for in vitro production of porcine embryos. | Yoshioka et al. (2008) | |
| In vitro production studies of pig embryos in a defined serum-free medium. | Akaki et al. (2009) | ||||
| - | In vitro-derived blastocyst quality improvement study in a defined serum-free medium. | Koike et al. (2010) | |||
| - | Study of interaction between oocytes during in vitro maturation in a defined serum-free medium. | Appeltant et al. (2015) | |||
| Modified medium 199 | - | Developmental capacity of selected oocytes to mature in a defined serum-free medium. | Ishizaki et al. (2009) | ||
| North Carolina State University-23 (NCSU-23) Modified NCSU-23 medium | - | Effect of amino acid addition on maturation, fertilization, and preimplantation development of oocytes. | Hong and Lee (2007) | ||
| Whole embryo | Porcine zygote medium (PZM)-5 | Defined system for in vitro production of porcine embryos. | Yoshioka et al. (2008) | ||
| PZM-4 | Subsequent embryo development in a defined serum-free medium using selected oocytes. | Ishizaki et al. (2009) | |||
| PZM-3/4/5 | Effects of the CDM on the early development of porcine embryos. | Cao et al. (2012) | |||
| Glucose, glycine | The effects of glucose and/or glycine on the in vitro development of porcine blastocysts. | Mito et al. (2012) | |||
| Porcine blastocyst medium, PZM-5 | Birth of piglets from in vitro-produced porcine blastocysts vitrified and warmed in a chemically defined medium. | Mito et al. (2015) | |||
| TCM-199–HEPES (TCM) | Polyvinyl alcohol | Vitrification and warming of in vivo-derived porcine embryos in a chemically defined medium. | Sanchez-Osorio et al. (2010) | ||
| Chicken | PBS-12SF (original CHCC-OU2 line) | OptiPROTM SFM | - | Evaluation of immortalized chick embryo cell lines for vaccine production in serum-free conditions. | Coussens et al. (2011) |
| Duck | AGE1.CR, AGE1. CR.pIX | SFM-G, HyQ®SFM4MegaVirTM | - | Evaluation of the growth of avian designer cell lines in serum-free media for use in vaccine manufacturing. | Lohr et al. (2009) |
| Sheep | Oocyte | TCM 199 | Sericin, polyvinyl alcohol | Confirmation of FBS replacement effect of sericin. | Hajarian et al. (2017) |
| HEPES buffered TCM 199 | Growth factor, hormones | Comparison of oocyte development in serum-free medium supplemented with growth factors and hormones. | Arunakumari et al. (2010) | ||
| Goat | Whole embryo | mSOF, DI / II | Bovine serum albumin, polyvinyl alcohol | Identification of optimal medium conditions and cryotolerance levels for culturing goat embryos. | Choi et al. (2016) |
Byers et al. (2008) reported that the maturation response of bovine chondrocytes treated with transforming growth factor (TGF)-β3 in a serum-free medium was superior to that of the serum-containing temporary TGF-β3-added treatment group or the treatment group continuously supplemented with growth factors. Zhou et al. (2015) found that adding FBS dampened the intensity of spontaneous calcium signaling in cells and a chemically defined serum-free medium is beneficial in long-term cultures. In a study by Ahmed et al. (2014), chondrocytes were cultured with a high glucose content media supplemented with insulin and dexamethasone on membranes coated with 3D collagen type II. Chondrocytes sufficiently expressed chondrogenic genes without the addition of serum or growth factors and accumulated in the extracellular matrix, confirming the possibility of promoting the formation of hyaline-like cartilage tissues. Corneal endothelial cells grown in a medium supplemented with different concentrations of human platelet releasates and without FBS, maintained the expression of membrane markers (Chou et al., 2014). In a study on culturing cumulus-oocyte complex cells (Goovaerts et al., 2012), the growth effect of embryos was verified by adding a mixture of insulin, transferrin, selenium, and bovine serum albumin (BSA) to a serum-free medium. However, based on the blastocyst rate, a sufficient replacement effect was not observed (Goovaerts et al., 2012). The study by Thomas et al. (2007) on the culture and development of bovine follicles involved using a culture where IGF-I was added to the medium as a control. Further, Piccinato et al. (2012) added 0.1% polyvinyl alcohol to a serum-free medium to avoid the side effects of serum substitutes in the growth of granulosa cells. After the addition of norepinephrine, stable and sufficient cells were grown based on progesterone production by granulosa cells. In another study, Kane et al. (2009) conducted an experiment to determine whether keratocytes synthesize collagen and IGF-II in a serum-free medium. It was confirmed that bovine keratocytes, which did not synthesize collagen and IGF-II on their own in serum-free medium, deposited collagen under the medium conditions controlled by rabbit keratocytes. Therefore, IGF produced in rabbit keratinocytes may affect the growth of other cells (i.e., bovine keratinocytes; Kane et al., 2009). Additionally, cells grown in serum-free and serum-supplemented medium conditions containing growth factor (TGF-β3) were compared (Reza and Nicoll, 2010). In this study, the glycosaminoglycan and type II collagen levels were higher in the serum-free medium than those in the serum-supplemented medium, and the final cell-matrix development was indentified to be suitable (Reza and Nicoll, 2010). Bhuiyan et al. (2007) added glucose and fructose to a protein-free serum-free medium for oocytes and determined the ratio of the medium required to optimize the development of bovine transgenic cloned embryos. Studies have also been conducted to reveal that oocyte growth and activation can be induced by the addition of activin or follicle-stimulating hormone, even without serum (McLaughlin and Telfer, 2010). Kolkmann et al. (2020) conducted a study to confirm the effects of seven commercially available serum-free media on primary bovine myoblast growth and differentiation. According to the study, some commercial serum-free media supported the proliferation of myoblasts but induced a lower level of proliferation compared to treatment with 20% FBS. However, the two types of serum-free medium showed continuous myoblast proliferation similar to proliferation effect of myoblast by using medium containing 10% FBS (Kolkmann et al., 2020). Recently, Cardoso et al. (2012) obtained Wharton’s jelly cells from bovine umbilical cord and differentiated them into osteocytes, chondrocytes, adipocytes, and neural-like cells in a serum-free medium. They verified the passage growth limit of umbilical cord Wharton’s jelly cells in serum-free medium along with marker expression, morphology, immune profile, and growth conditions. Moreno et al. (2015) studied the optimal composition of media additives for growing bovine embryonic cells without the addition of fetal calf serum or BSA. In this study, the medium additive composed of growth factors, cytokine, recombinant albumin, and hyaluronan facilitated embryo development, and the quality and viability of the embryos were similar to those of the control group using a medium containing BSA. Lim et al. (2007) used a two-step culture medium composition without FBS to confirm an increase in the efficiency of calf production using in vitro bovine embryos. Subsequently, in a study evaluating the development of bovine embryos using an additive (i.e., activin; Park et al., 2010), the serum-free medium used by Lim et al. (2007) was used as a basal medium for culturing cells after fertilization. Jang et al. (2011) cultured embryos by adding chemically defined medium (CDM) or BSA to modified synthetic oviduct fluid (mSOF) and verified that there was no difference in the effect on embryo development between the two medium compositions. In addition, because there was no difference in the number of blastocyst cells cultured in the CDM and mSOF, it was found that serum-free CDM was capable of culturing cells at a level similar to that of mSOF containing BSA (Jang et al., 2011). In another study, sericin, a silk protein, was used as a substitute for FBS when cryopreserving bovine embryos (Isobe et al., 2013). Sericin is a safe substance added to drugs, foods, cosmetics, and health foods and is sufficient for replacing the 20% FBS normally used for freezing embryonic cells. A study by Wydooghe et al. (2014) confirmed that serum clearance during embryonic culture might be the main contributing factor. The addition of insulin, transferrin, and selenium (ITS) under serum-free medium conditions contributed to the synergistic effect of ITS in high blastocyst production (Wydooghe et al., 2014).
In 2013, a study using the serum-free medium on porcine-derived cells was undertaken. In this study by Wang et al. (2013), bone marrow-derived mesenchymal stem cells were cultured in a serum-free medium with EGF added to the culture. When 10 nM/L EGF was added, the serum-free medium showed the same effect as that of the conventional medium (Wang et al., 2013). Further, Kamil et al. (2007) studied whether FBS could be substituted by adding an autologous serum, fibroblast growth factor, and IGF-1 to the basal media (Ham F12) to culture porcine chondrocytes. They identified the possibility of partially replacing FBS with growth factors (Kamil et al., 2007). In a study by Caperna et al. (2011), the formation and maintenance of hepatocytes were verified in a serum-free medium supplemented with dimethyl sulfoxide and dexamethasone. Cells first cultured in the presence of serum achieved the formation and maintenance of a confluent hepatocyte monolayer in a serum-free medium (Caperna et al., 2011). Rasmussen et al. (2017) determined the loss of phenotype in hepatocytes cultured in a serum-free medium; these cells showed little dedifferentiation, making them more sensitive to CYP (cytochrome p450s) inducers. This study compared the effects of serum on cultured cells rather than supplementing the absence of serum. Another study involving oocytes used a modified porcine oocyte medium (mPOM) as the serum-free medium. In 2009, Akaki et al. (2009) achieved in vitro maturation to produce porcine embryos in a defined serum-free medium (mPOM). Based on this result, Koike et al. (2010) found that the quality of blastocysts derived from the fertilized porcine oocytes was improved in the defined serum-free medium (mPOM). In the above studies, polyvinyl alcohol was added to supplement the serum-free medium, and in a subsequent study on the interaction between oocytes during in vitro maturation (Appeltant et al., 2015), polyvinyl alcohol was used with the POM medium. In general, serum is not added to the medium used at the maturation stage of oocytes in oocyte studies (Akaki et al., 2009; Appeltant et al., 2015; Hong and Lee, 2007; Ishizaki et al., 2009; Koike et al., 2010). Therefore, while the above studies used a serum-free medium, this cannot be considered as the development of serum-free medium to reproduce the effects of a serum-supplemented medium on cell growth, proliferation, and differentiation. This is similarly observed in bovine cell studies (Bhuiyan et al., 2007; McLaughlin and Telfer, 2010). Yoshioka et al. (2008) confirmed the generation of porcine blastocysts in vitro using a serum-free medium supplemented with polyvinyl alcohol. Additionally, Ishizaki et al. (2009) used another serum-free medium (porcine zygote medium-4, PZM-4) in an embryo development experiment that used oocytes cultured in a serum-free medium. In a study by Cao et al. (2012), PZM-3/4/5 was used to culture pig embryos, and PZM-4 efficiently supported the early development of embryos. Mito et al. (2012) found that the addition of glucose and glycine to PZM-5, a serum-free medium, enhanced the development of blastocysts in vitro. In a follow-up study in 2015, the freezing resistance of pig blastocysts using the same serum-free medium (PZM-5) was confirmed (Mito et al., 2015). A similar study found the effect of cryopreserving embryos using TCM-199-HEPES (TCM) medium supplemented with polyvinyl alcohol without using serum (Sanchez-Osorio et al., 2010).
In addition to the two representative livestock species (i.e., bovine and porcine), there have been other cell culture studies using serum-free medium. For example, Coussens et al. (2011) conducted a basic study on vaccine production by growing immortalized chicken embryonic cells in a serum-free medium. Additionally, Lohr et al. (2009) cultured the growth of two types of avian (duck) designer cell lines in a serum-free medium for vaccine production. Cells cultured in various conditions, such as T-flasks, roller bottles, and small-scale bioreactors, have been evaluated for their potential as host cells for vaccine production (Lohr et al., 2009). In a sheep study using a serum-free medium, an experiment was conducted to confirm the serum replacement effect of sericin, a protein derived from cocoons (Hajarian et al., 2017). The addition of sericin was more effective for embryonic development than that of polyvinyl alcohol, which has been widely used in previous serum replacement studies; such a medium was as good as the serum-supplemented medium for development to the blastocyst stage (Hajarian et al., 2017). Hajarian et al. (2017) also evaluated the appropriate concentration of sericin for oocyte competence. In another study, an optimal medium combination was made uo using a serum-free medium to determine the effects of growth factors and hormones on oocyte development (Arunakumari et al., 2010). In a study using a serum-free medium in goats, Choi et al. (2016) confirmed embryo developmental competence and cryotolerance. Many studies using serum-free media have been performed in the field of reproduction, such as in embryos and oocytes.
Many types of media have already been developed to culture various cell types without the addition of serum. For example, Panserin 701 (P04-710701, PAN-Biotech, Aidenbach, Germany) is a serum-free medium used for culturing lymphocytes from whole blood. Airway epithelial cell growth medium (C-21060, PromoCell, Heidelberg, Germany) is a serum-free medium capable of culturing epithelial cells from large air passages and can be used for culturing various mammalian airway epithelial cells. Renal epithelial cell medium (C-26001, PromoCell) was used as a serum-free medium to culture epithelial cells from the kidneys. It was developed to increase the initial cell culture efficiency using renal epithelial cell medium 2 (C-26030, PromoCell) with a small quantity of added serum. Further, fibroblast growth medium (C-23010, PromoCell) is suitable for culturing neonatal and juvenile fibroblasts and can be used for culturing fibroblasts from mammals such as cattle, pigs, and humans. The hepatocyte growth medium (C-25010, PromoCell) is a serum-free medium for culturing human hepatocytes that can also be used for culturing porcine hepatocytes. Keratinocyte growth medium 2 (C-20011, PromoCell) is a serum-free medium used for culturing epidermal keratinocytes without feeder cells. It can also be used to culture human, horse, pig, and mouse keratinocytes. Melanocyte growth medium M2 (C-24010, PromoCell) is a specialized medium for culturing juvenile melanocytes by adding phorbol myristate acetate. Further, OptiPROTM SFM (12309050, Gibco, Thermo Fisher Scientifi, Stockholm, Sweden) is a serum-free, animal-origin-free culture medium for culturing kidney-derived cell lines for the production of viruses or recombinant proteins. Finally, BIO-MPM-1 SFM (05-060-1A, Sartorius, Göttingen, Germany) is a serum-free medium for various adherent cells. This medium does not contain albumin, a major component of serum, and thus, may limit cell adhesion.
In this paper, we reviewed previous studies related to FBS substitution or serum-free culture medium. Although there have been numerous studies on alternatives and serum-free culture media, there are few studies related to serum-free culture media that have effectively replaced FBS or shown superior results compared to FBS. The development of a serum-free medium to completely replace FBS is currently underway. In addition, it has not yet been confirmed whether such FBS can substitute research and serum-free media development can be applied to the production of cultured meat; it is expected that such studies will not be widely applied in the development of cultured meat. In addition, we predict that the development of food-grade culture media will be the goal of future research, as most culture media components reported in previous studies have not been identified for use in food materials.
Current Technologies in the Development of Cultured Meat
We review and summarize the key techniques for developing cultured meat over the last 5 years (Table 4). Simsa et al. (2019) found that the addition of myoglobin (Mb) to media promotes the growth of primary bovine satellite cells (BSCs). Mb is a heme protein present in muscle, and the addition of Mb also resulted in the same coloring effect as traditional meat (TM) in the final 3D cultured meat. One of the greatest challenges going forward will be avoiding the use of FBS in the production of cultured meat to replace traditional beef. Kolkmann et al. (2020) confirmed this possibility by culturing bovine myoblasts in a serum-free medium. Cells were cultured by mixing commercially available serum-free media and serum-free additives to supplement the factors lacking in the serum-free media. Although the initial cell adhesion efficiency and cell viability were low during cell culture using a serum-free medium, the cell growth efficiency was improved to a value close to that of the serum-containing medium through partial medium replacement and removal of antibiotics. In another study, Stout et al. (2022) found that cell activity was maintained by subculturing BSCs in a serum-free medium and evaluated the possibility of reducing the medium cost by predicting the optimal amount of medium and additives. In particular, long-term cell expansion was possible when recombinant albumin was added to the developed Beefy-9 medium. Albumin is a protein that accounts for approximately 60% of the serum content, and the addition of recombinant albumin could compensate for the shortcomings of a serum-free medium (Stout et al., 2022). Since then, Joo et al. (2022) compared the composition and taste characteristics of cultured meat tissue (CMT) obtained from chicken and cattle satellite cells with those of TM. Such studies can objectively evaluate the composition and taste characteristics of cultured meat according to the cell culture medium used. Amino acids are one of the factors that affect the taste of meat; therefore, it is important to reproduce the same amino acid composition to maintain the quality of cultured meat. The results showed that the content of all amino acids except valine and tyrosine in CMT significantly differed from that in TM, and the taste value was also lower overall. These results confirm the need to further study the composition of cultured meat medium and additives to improve the taste of CMT. Okamoto et al. (2022) used autotrophic microalgae to promote the growth of bovine myoblasts for producing cultured meat. Chlorella vulgaris was subjected to acid hydrolysis to extract nutrients [Chlorella vulgaris extract (CVE)] that could be used in the medium. CVE contained 14 of the 15 proteinaceous amino acids included in DMEM, a general culture medium for cultured meat, and proteinaceous amino acids not included in DMEM, such as aspartic acid, asparagine, glutamic acid, proline, and alanine. Some nutrients, such as vitamins B1, B6, and B9, glutamine, and tryptophan, were less abundant in CVE than in the conventional medium, but it was confirmed that they could be sufficiently supplemented through extraction and concentration. The CVE-supplemented medium provided the same level of nutrients as the existing medium and showed a positive effect on the proliferation and differentiation of myoblasts, indicating that it could induce myotube formation. These results suggest that non-animal-derived substances can help produce stable cultured meat. The acquisition of raw cells is essential for producing cultured meat, and it is very important to control cell stability parameters. Skrivergaard et al. (2021) studied cell proliferation and differentiation according to the storage period of the bovine muscle tissue for cell acquisition. Satellite cells isolated from tissues stored under refrigeration at 4°C were poorly cultured at the initial cultivation compared to the satellite cells isolated from fresh cultures, but it was found that there was no difference in metabolic activity and DNA concentration. This effect appeared in the satellite cells isolated from tissues stored for 2–5 d. Cell culture techniques based on the regenerative capacity of fish have also been studied (Tsuruwaka and Shimada, 2022). Dedifferentiated Stephanolepis cirrhifer (deSc), a fibroblast-like cell obtained from fish, was cultured up to passage 350 without mutation, and cell differentiation showed using salmon and other mammalian serum. Cell differentiation using salmon serum, SeaGrow, differentiated deSc cells into adipocytes (Tsuruwaka and Shimada, 2022). These deSc cells can be stacked to form a sheet, which contracts when stimulated. The culture was prepared in the form of sashimi, which was sufficient for sensory testing (Tsuruwaka and Shimada, 2022).
| Titles | Cells | Results | Concepts | References |
|---|---|---|---|---|
| Extracellular heme proteins influence bovine myosatellite cell | Primary bovine satellite cells (BSCs) from semitendinosus of Charolaise × Simmental beef cow | The proliferation and metabolic activity of BSCs was significantly increased when myoglobin (Mb) was added. Mb application to bioartificial muscles led to a the development of a color similar to that of the cooked beef. |
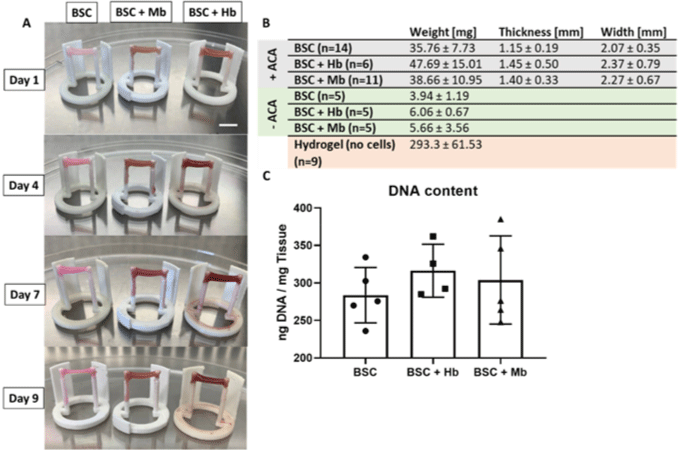
|
Simsa et al. (2019) with CC-BY |
| Serum-free media for the growth of bovine myoblasts | Skeletal muscle cell of cow biceps femoris | Serum-free media stimulate exponential cell expansion, albeit not to the extent of the current growth medium containing up to 30% serum. Further research is needed to investigate whether prolonged cell culture or an adaptation period could further increase cell proliferation. |
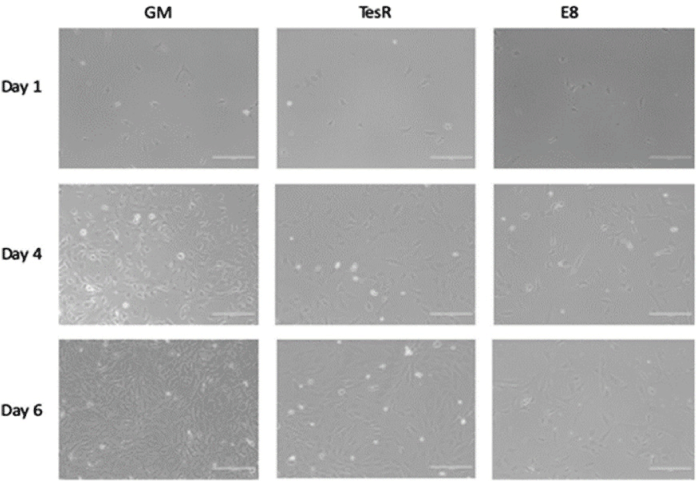
|
Kolkmann et al. (2020) with CC-BY |
| Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cultured meat production | Primary bovine satellite cells | This new media (Beefy-9) maintained robust cell growth over the entire culture period tested (seven passages) with an average growth rate of 39 hours per population doubling. |
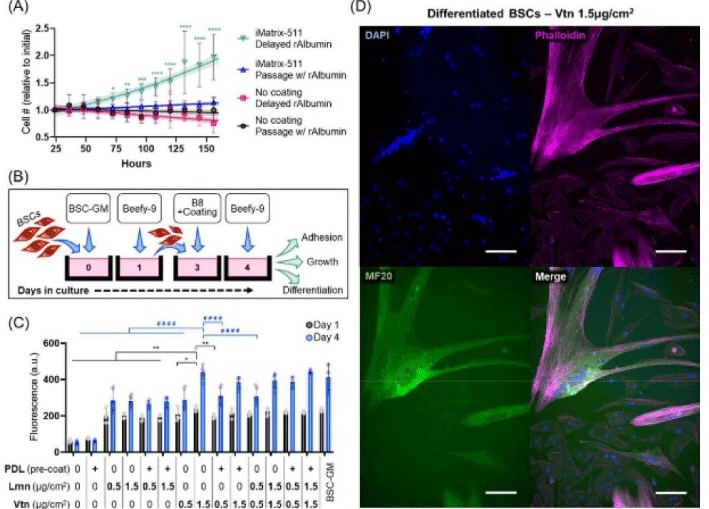
|
Stout et al. (2022) with CC-BY |
| Effect of smooth muscle cells on the quality of cultured meat | Smooth muscle cells of piglet | The addition of basic fibroblast growth factor to the medium significantly increased the growth rate of smooth muscle cells and the expression of extracellular matrix-related genes, especially collagen and elastin. | Zheng et al. (2021) | |
| Taste characteristics of satellite cell cultured meat | Chicken skeletal muscle cell | The content of all amino acids except valine and tyrosine was significantly different between cultured meat and traditional meat. |
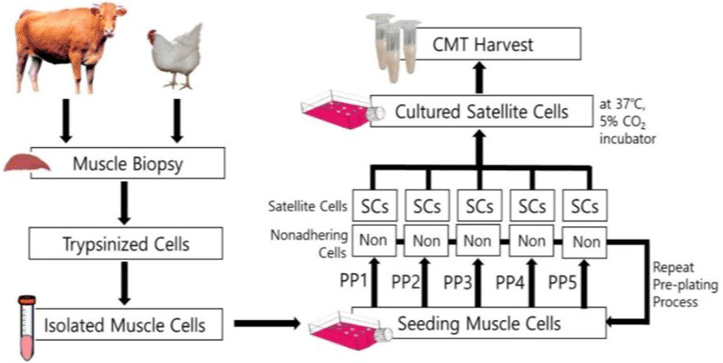
|
Joo et al. (2022) with CC-BY-NC |
| Proliferation and differentiation of bovine myoblasts using Chlorella vulgaris for cultured meat | Primary bovine myoblasts (PBM) | The addition of Chlorella vulgaris extract (CVE) significantly improved PBM viability compared to that in conventional culture medium. Furthermore, by adding horse serum to induce differentiation, the formation of myotubes was confirmed when CVE was used. | Okamoto et al. (2022) | |
| Bovine satellite cell maintains the proliferative myogenic capacity for cultured meat | Satellite cell of Holstein M. semimembranosus | The data indicated a positive trend in terms of myogenic potential after tissue storage. The timeframe in which viable myogenic satellite cells can be isolated and used for cultured meat production can be greatly extended by proper tissue storage. |
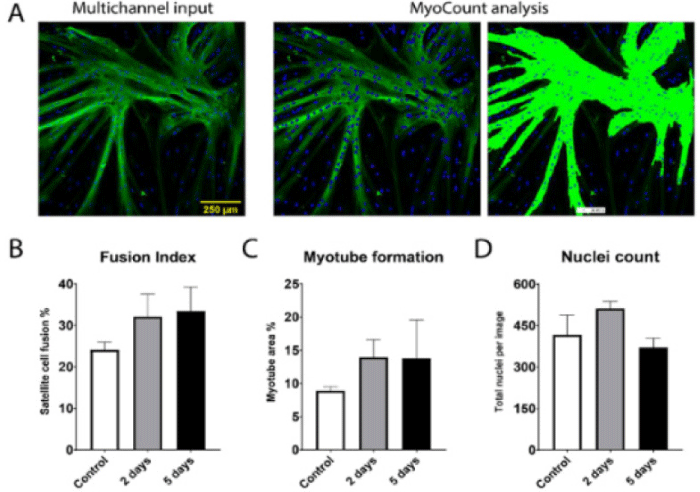
|
Skrivergaard et al. (2021) with CC-BY |
| Develop aquatic clean meat from fish cells | Fibroblast-like cell of the fin of thread-sail filefish (Stephanolepis cirrhifer) | Cell differentiation was regulated by a “simple stimulus” such as medium, serum and extracellular matrix without using a specialized technique. |
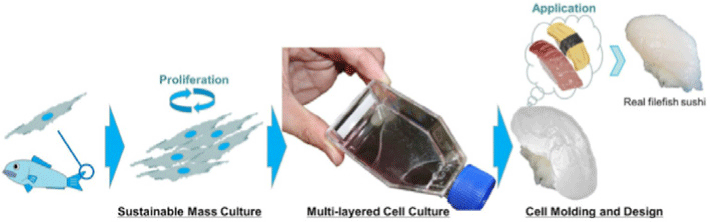
|
Tsuruwaka and Shimada (2022) with CC-BY |
| Multi-layered skeletal muscle tissue by using 3D collagen scaffolds | Rat L6 skeletal muscle myoblasts | 3D micropatterned scaffolds can promote cell alignment and muscle tissue formation. The micro-grooved collagen scaffolds could be used to engineer organized multi-layered muscle tissue. | Chen et al. (2015) | |
| Developing cultured meat scaffolds of vegetable-based proteins | C2C12 skeletal muscle cells | Fibrous growth substrates from extruded plant-based proteins that the cells are able to attach to and grow on. |
|
Krona et al. (2017) |
| Edible scaffold (decellularized spinach) for cultured meat | Bovine satellite cell | After 14 d, primary bovine satellite cells seeded on the decellularized leaf scaffold maintained approximately 99% viability, and approximately 25% of the cells expressed the myosin heavy-chain. |
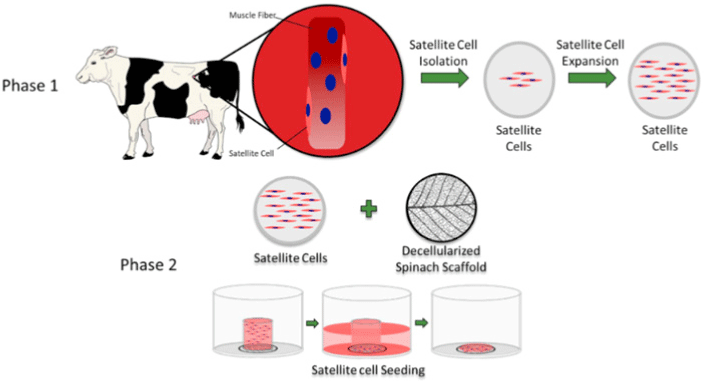
|
Jones et al. (2021) with CC-BY-NC-ND |
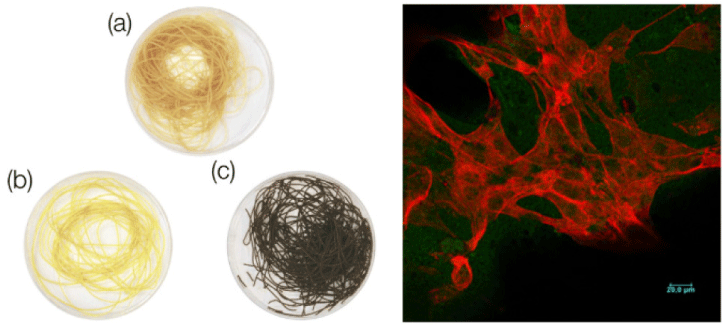
| Nanocellulose from Nata de Coco as a bioscaffold for cell-based meat | Mouse C2C12 myoblast | Nanocellulose bioscaffolds show limited potential as a biocompatible matrix for cell-based meat. |
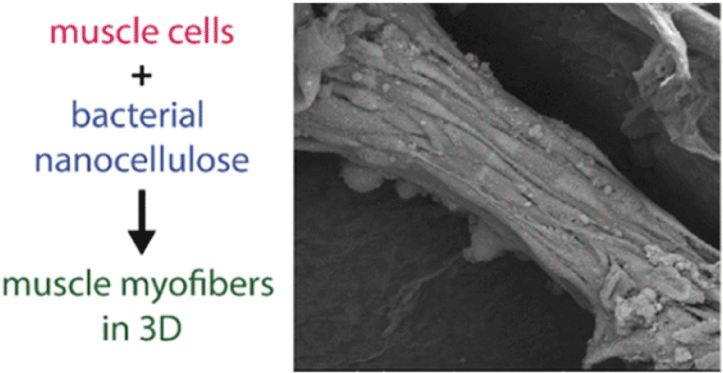
|
Rybchyn et al. (2021) with CC-BY-NC-ND |
| Scaffolds for cultured meat on the basis of polysaccharide hydrogels with plant-based protein | Murine myoblast C2C12 cell | All evaluated polysaccharide-protein blends turned out as potential candidates for cultured meat. |
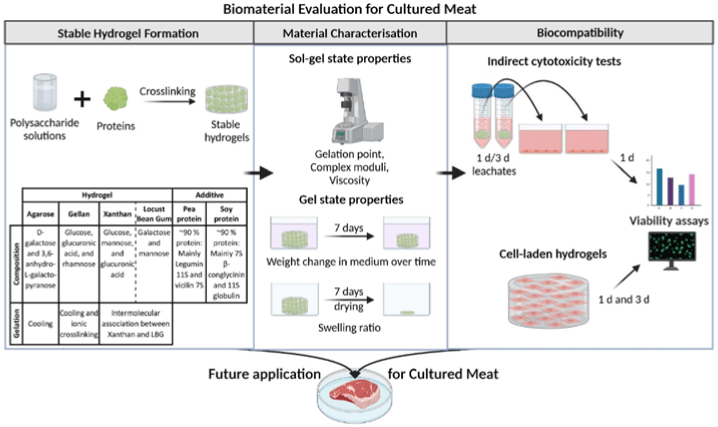
|
Wollschlaeger et al. (2022) with CC-BY |
| It is possible to make protein blends (containing up to 1% of pea and soy protein) with all polysaccharides to increase the nutritional value. | ||||
| Chitosan-collagen hydrogel microparticles for cultured meat | Mouse C2C12 skeletal myoblasts | Cell microcarriers support the attachment and rapid proliferation of mouse skeletal C2C12 myoblasts, rabbit smooth muscle cells, sheep fibroblasts, and bovine umbilical cord mesenchymal stem cells. | Zernov et al. (2022) | |
| Modified cell-electrospinning for 3D myogenesis of C2C12 | C2C12 myoblasts | Loading C2C12s as cellular aggregates and modifying several other electrospinning parameters drastically increased cell viability. C2C12-seeded fibrin/polyethylene oxide microfiber bundles were cultured for up to 7 d. | Guo et al. (2019) | |
| Formation of contractile 3D bovine muscle tissue for construction of millimeter-thick cultured steak | Bovine myocytes of beef cattle | When the myocytes were cultured in the hydrogel for 14 d, fiber-shaped bovine muscle tissue of diameter 295±105 μm was generated, the ends of which were immobilized with pillars, showing that the length of the muscle tissue was equal to the gap between the anchors (7 mm). |
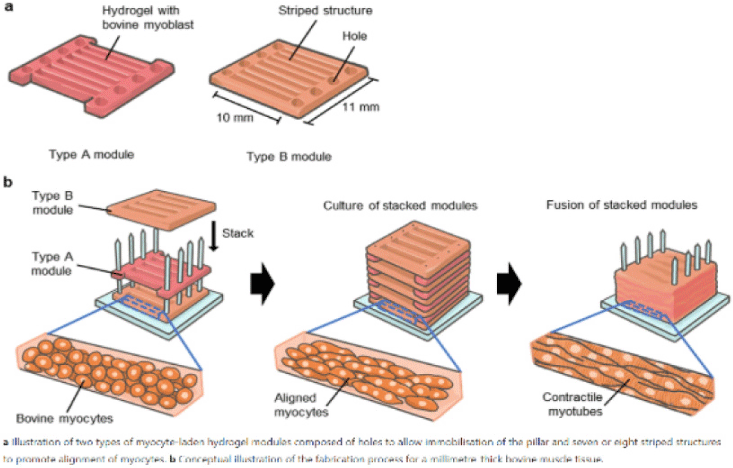
|
Furuhashi et al. (2021) with CC-BY |
| Cultured meat production using 3D printing technology | Newborn pig satellite cell | The 4% sodium alginate-gelatin and gelatin-methacrylate 20% silk fibroin hydrogel demonstrated good performance and was hybridized with porcine skeletal muscle satellite cells for 3D printing. |
|
Li et al. (2021) with CC-BY-NC-ND |
| Muscle-derived fibroadipogenic progenitor (FAP) cell for production of cultured bovine adipose tissue | FAP cells | FAP cells reached a mature level of adipogenic differentiation in three-dimensional, edible hydrogels. The resultant tissue accurately mimics traditional beef fat, and FAP cells thus represent a promising candidate cell type for the production of cultured fat. |
|
Dohmen et al. (2022) with CC-BY |
Chen et al. (2015) promoted cell alignment and muscle tissue formation using 3D scaffolds. Scaffolds were prepared by liquid dispensing and lyophilization of collagen. The cell-seeded scaffold induced cells to gather and sort through a structure that mimicked muscle fibers. This facilitated the subsequent contraction of cells, making it possible to form an ordered bundle of cells. These results laid the foundation for the stacking of muscle-tissue layers. Krona et al. (2017) seeded cells on scaffolds extruded from pea, maize, and hemp fibers. Among them, corn protein fibers sterilized with ethanol were most suitable for the growth of skeletal muscle cells. Similar to the study by Krona et al. (2017), developing scaffold materials from plants is being explored. In a recent study, Jones et al. (2021) fabricated a scaffold that aids the attachment of satellite cells using edible raw materials. They decellularized spinach and used it as a scaffold, which has two advantages: The use of leaf veins that can replace the vascular network and the use of inexpensive and edible raw materials. Decellularized spinach leaves provide sufficient elasticity to be applied to the muscle, with high differentiation efficiency during cell seeding. At the same time, bacterial nanocellulose (BNC) has been found to be suitable for the proliferation and differentiation of murine myoblasts and is effective for myotube formation (Rybchyn et al., 2021). As a food safety material, BNC could serve as a suitable scaffold for cultured meat if it supplements sensory properties, such as the texture of the final product (Rybchyn et al., 2021). Wollschlaeger et al. (2022) produced scaffolds for cultured meat in 2022, using a polysaccharide-protein with edible soybean protein added. This study verified the cytotoxicity and optimal blend manufacturing conditions in C2C12 cultures. Thus, the polysaccharide-protein support was confirmed as a potential candidate for cultured meat. In another C2C12 cell culture study, mouse myoblasts were cultured using edible microcarriers (Zernov et al., 2022). This microcarrier was prepared by mixing chitosan and collagen, and the cells attached to the carrier could be used as a material for processed meat products without a separate recovery process (Zernov et al., 2022). Furuhashi et al. (2021) formed bovine muscle tissue using hydrogels and improved the ratio of the root canal and tissue contractility through electrical stimulation. A 1 Hz electric pulse promoted the maturation of myotubes, but since the hydrogel for realizing the texture and shape of the tissue is not edible, it is necessary to develop technology to replace the hydrogel. Further, adherent satellite cells attach only to the outer surface of the scaffold during culture to form a cell-free region. To overcome this limitation, Guo et al. (2019) sorted cells via electrospinning, allowing the cells to effectively proliferate and settle inside the scaffold. The expression of MHC (Myosin skeletal Heavy Chain) and the length and diameter of the myotube were found to increase. In contrast, Li et al. (2021) fabricated scaffolds directly on cell-culture dishes. This was done using 3D bioprinting technology, and the culture was carried out by immersing 4% sodium alginate-gelatin and gelatin-methacrylate-20% silk fibroin hydrogel in the cell-seeded medium. Although this technique facilitates the stacking of scaffolds in which cells can be cultured, cells can only grow within the scaffold if sufficient micropores exist. This result indicates that the 3D formation of bioengineered tissue could be applied to cultured meat production. Since then, Dohmen et al. (2022) have succeeded in growing fibro-adipogenic progenitor (FAP) cells capable of producing fat, another important component of cultured meat. When mononuclear cells from skeletal muscle were isolated and treated with a differentiation medium containing an adipogenesis inducer, the growth of FAP cells, the source of intramuscular fat, was induced. The composition of mature cultured fat was similar to that of traditional animal fat, and the taste was also confirmed to be similar. The success of adipose tissue culture using isolated FAP cells showed the potential for cultured meat production through co-culture with satellite cells.
This review reveals that various studies are being conducted on the development of cultured meat. However, standardized manufacturing methods, such as collecting and culturing cells, must be established to industrialize cultured meat for human consumption. Therefore, more research is needed on developing low-cost culture media that can be safely consumed by humans, inexpensive and mass-producible methods, and standardized cultured meat production technology.
Conclusion
FBS is the most commonly used supplement in animal cell culture media; however, many studies have been conducted to replace serum originating from fetal bovine because of ethical issues related to animal ethics. We reviewed previous studies on FBS and animal blood, FBS replacers or serum-free culture media, and recent development of cultured meat. Our review reveals that not only is there still a lack of research on the ingredients of FBS, but the reasons for fully replacing FBS being problematic are not yet adequately identified. Many companies and research groups have announced that they have developed cultured meat, but a specific description of their development has not been disclosed. Research papers on cultured meat published by many companies and research groups have shown that basic research on the development of cultured meat is sufficient; however, mass production and low-cost production technology have not yet been completely developed. Therefore, we expect that additional technological developments are needed to provide cultured meat that can be safely consumed and produced at a lower price. Nevertheless, this study provides baseline data for the research on culture media that will be necessary to produce and industrialize cultured meat.