Introduction
Reactive oxygen species (ROS), including hydrogen peroxide, hydroxyl radicals, and superoxide anions, are representative products of cellular oxygen metabolism in all organisms (Wu et al., 2017). ROS play an important role in cellular signaling and homeostasis for physiological functions (Zhao et al., 2014). These ROS are maintained at an appropriate level by the body’s antioxidant defense system, including enzymatic and non-enzymatic antioxidant systems. Catalase, glutathione peroxidase, and superoxide dismutase are enzymatic antioxidant system. Glutathione, ascorbic acid, and carotenes are non-enzymatic system (Zhao et al., 2017). However, when a problem occurs in these systems, ROS are overproduced, causing oxidative stress on various biological molecules (e.g., lipids, proteins, and DNA) in the body, resulting in damage (Qian et al., 2020). ROS are known to be highly associated with the development of various diseases such as cardiovascular diseases, coronary heart diseases, cancer, atherosclerosis, and diabetes (Thetsrimuang et al., 2011; Wang et al., 2016; Wu et al., 2017).
Antioxidants including natural and synthetic antioxidants can neutralize free radicals and inhibit oxidative diffusion to remove overproduced ROS (He et al., 2019). Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are inexpensive with excellent antioxidant activity. However, toxicity and side effects can occur when they are consumed for a long time (André et al., 2010). Therefore, studies are being actively conducted to find a substance that is economical with excellent antioxidant activity from natural sources (Lorenzo et al., 2018). Natural antioxidants include anthocyanin, isoflavone, and bioactive protein and peptides derived from soybean, whey, plant, and eggs (Lorenzo et al., 2018; Pisoschi et al., 2021; Wen et al., 2020). Meanwhile, some researchers have reported a relationship between antioxidant and immune-modulating activity (Ji et al., 2020; Kim et al., 2020a). ROS produced by oxidative stress can influences inflammation by promoting the production of inflammatory mediators such as pro-inflammatory cytokines, anti-inflammatory cytokines and chemokines. Inflammatory response then repeats the production of ROS and causes excessive inflammatory reactions (Saisavoey et al., 2016). Therefore, it is important to study both antioxidant activity and immune-modulating activity (Harikrishnan et al., 2020; Ji et al., 2020).
Hen eggs not only provide excellent nutrition, but also contain various functional components, making them one of the most popular ingredients in the food and pharmaceutical industries as a functional raw material (Moreno-Fernández et al., 2020). In particular, various proteins present in egg white and yolk (ovalbumin, ovotransferrin, ovomucin, IgY, phosvitin, etc.) have been reported to have various functional activities such as antioxidant, anti-inflammatory, immune-enhancing, and anti-biofilm activities (Abeyrathne et al., 2016; Kim et al., 2020b; Kim et al., 2020c; Lee et al., 2018). Egg yolk protein is produced as a by-product after extracting egg yolk lecithin with an organic solvent. Egg yolk lecithin is used in the food and cosmetic industries (Peñaranda-López et al., 2020). Egg yolk protein by-products after lecithin extraction are mostly denatured proteins with limited values and functionalities due to solvents used during the lecithin extraction process (Eckert et al., 2014). Therefore, ethanol and water extraction was performed to extract useful substances from the egg yolk protein by-products, which is a widely used method due to its advantages of low cost and safety as a food grade-solvent (Liau et al., 2017; Liu et al., 2021; Mani-López et al., 2021).
In this study, egg yolk protein extracts were produced using ethanol and water to utilize by-products after extracting egg yolk lecithin. Antioxidant activities of egg yolk protein extracts were investigated by cellular antioxidant capacity (CAC) assay and comet assay. Their immune-modulating activities were investigated by analyzing their effects on inflammatory cytokine production in Balb/c mouse splenocytes.
Materials and Methods
Dulbecco’s Modified Eagle’s Medium (DMEM), Roswell Park Memorial Institute Medium 1640 (RPMI-1640), fetal bovine serum (FBS), penicillin, streptomycin, phosphate-buffered saline (PBS), and Hank’s balanced salt solution (HBSS) were obtained from HyClone Laboratories (Logan, MI, USA). Thiazolyl blue tetrazolium bromide (MTT), lipopolysaccharide (LPS), concanavalin A (ConA), 2′,7′-dichlorofluorescin diacetate (DCF-DA), 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). ELISA kit for analyzing cytokines (IL-2, IL-4, IL-10, and TNF-α) were obtained from BD Biosciences (San Diego, CA, USA), and all other reagents and chemicals used were analytical grade.
The by-product egg yolk protein produced after extracting the yolk lecithin used in this study was obtained from the Join (Egg processing company, Yongin, Korea). The residue of the lecithin extraction from whole egg yolk extracted using 95% ethanol was provided by the company in a dry state as a by-product. Egg yolk protein powder was extracted with ethanol and water in laboratory. Briefly, the ethanol extracts were added to 100 mL of ethanol (70%) to 5 g of egg yolk protein powder, and extracted for 72 h at room temperature. The water extract was extracted with autoclave (DW-AC-131, Dong Won Scientific, Seoul, Korea) by adding 100 mL of distilled water to 5 g of egg yolk protein powder. After extraction, the ethanol and water extracts were vacuum filtered through Whatman No. 1 filter paper (Whatman, Buckinghamshire, UK). The ethanol extract was evaporated under reduced pressure using rotary evaporator (EYELA N-1000, Tokyo Rikakikai, Tokyo, Japan) at 37°C to remove solvent. Then solvent free extract was freeze dried for 3 days. The water extract was directly freeze dried for 3 days. Samples were classified as EYE-E (egg yolk protein extract-ethanol) and EYE-W (egg yolk protein extract-water).
HepG2 cells were purchased from American Type Culture Collection (Rockville, MD, USA) and were cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin at 37°C in a humidified incubator containing 5% CO2. The effects of EYE-E and EYE-W on HepG2 cell viability were determined using MTT assay (Lee et al., 2017a). HepG2 cells (5×104 cells/well) were seeded on a 96 well-plate and incubated for 24 h. Various concentrations of samples (1, 5, 10, 50, and 100 μg/mL) were treated in each well and incubated for additional 24 h. After then, MTT (2.5 mg/mL in PBS) solution was added to each well for additional 4 h. The supernatant was then removed from each well and dimethyl sulfoxide was added to each well to dissolve the MTT formazan. The absorbance of each well was measured using OPTIMA microplate reader (BMG LABTECH, Ortenberg, Germany) at 570 nm.
HepG2 cells were seeded at a density of 5×104 cells/well on a 96 well-plate to test CAC of egg yolk protein extracts following the method of Song et al. (2018). After 24 h incubation, the medium was replaced with HBSS buffer and EYE-E and EYE-W were added in each well that the final concentration was 1, 5, 10, and 50 μg/mL and incubated for 30 min. After incubation, each well was washed with HBSS buffer and 80 mM AAPH was added to the sample except for the negative control. After an additional 30 min incubation, 40 mM DCFH-DA was added to each well and incubated for 30 min in the dark. The degree of fluorescence was measured at excitation wavelength 485 nm, and emission wavelength 535 nm using OPTIMA microplate reader.
HepG2 cells (2×104 cells/mL) were washed with 1 mL of PBS and used in comet assay. The cells incubated with EYE-E and EYE-W at a concentration of 50 μg/mL at 37°C for 30 min in the dark. To stimulate oxidation stress, 200 μM H2O2 were treated to cells and incubated 5 min at 4°C, then washed with PBS. After incubation, 0.7% low melting agarose were mixed with cells and distributed over slides pre-coated with 1% normal melting agarose. Slides were placed in cold alkali lysis buffer (100 mM Na2EDTA, 2.5 M NaCl, 10 mM Tris, 1% sodium lauryl sarcosine, 1% Triton X-100, and 10% DMSO) for 1 h. Slides were then placed in a gel electrophoresis tank containing fresh electrophoresis buffer (300 mM NaOH, 10 mM Na2EDTA, pH 13.0) for 20 min and subjected to electrophoresis (25 V, 300 mA) for 20 min. After electrophoresis, slides were washed and then immersed in cold ethanol for 5 min and dried. Slides were stained with 20 μg/mL ethidium bromide. Finally, DNA damage image was obtained using fluorescence microscopy (LEICA DMLB, Wetzlar, Germany) and CCD camera (Nikon, Tokyo, Japan), and the image was analyzed using comet image analyzing system (Komet version 5.0, Kinetic Imaging, Liverpool, UK). We quantified head and tail intensity of samples using 50 cells from two replicate slides.
The experimental protocol was approved by the Institutional Animal Care of Kyungnam University (KUIAC-18-02). Male Balb/c mouse (five weeks old) were purchased from Koatech (Pyeongtaek, Korea). The animals were placed in wire mesh bottomed individual cages and housed in climate controlled quarter (23±2°C at 50% relative humidity) with a 12-h light/dark cycle. All animals were acclimatized for seven days before the experiment and were sacrificed by cervical dislocation. Obtained spleens were placed in RPMI 1640 medium. Single cell suspensions were prepared by pressing the tissue through 40 μm cell strainer (Corning, Corning, NY, USA). Cells were centrifuged 450×g, 4°C for 5 min to obtain splenocytes. Splenocytes were maintained in RPMI 1640 medium, supplemented with 10% FBS, 1% penicillin/streptomycin.
Splenocytes were cultured at 1×106 cells/well in 96 well-plate for cytokines measurement. LPS and ConA were treated to splenocytes to 5 μg/mL of final concentration. Samples were treated to final concentration of 200 μg/mL. After that, splenocytes were incubated 5% CO2 for 24 h at 37°C and cytokines (IL-2, IL-4, IL-10, and TNF-α) were quantitated in supernatants. Cytokines production were conducted using Mouse ELISA kit and were measured at 450 nm.
All analysis was performed in triplicate. Results are presented as mean values±SD. The results were compared by analysis of one-way ANOVA followed by Duncan's multiple-range test using SPSS for Window ver. 20 (IBM, Armonk, NY, USA). Statistical significance was determined at p<0.05.
Results and Discussion
Before determine antioxidant activities of EYE-E and EYE-W, their effects on viabilities of HepG2 cells were determined using MTT assay (data not shown). EYE-W showed no significant (p>0.05) effect on HepG2 cell viability at concentrations of 1 to 100 μg/mL. However, EYE-E at the highest concentration of 100 μg/mL showed significant (p<0.05) cytotoxicity, leading to cell viability of 88.8±4.6% compared to the control (viability of 100%). Therefore, EYE-E and EYE-W were used at concentrations of 1 to 50 μg/mL in subsequent experiments.
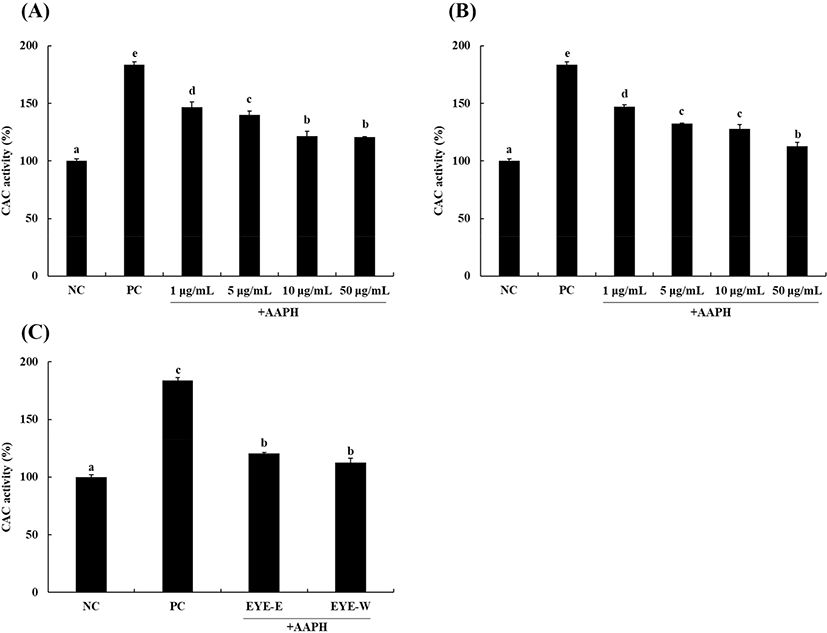
The CAC assay is an experimental method widely used to verify the antioxidant activity of a test sample. The DCFH-DA probe used in this study can be cleaved to DCFH in cells and oxidized to DCF by cellular oxidants such as ROS. When intracellular ROS content is reduced by antioxidants, the oxidation of DCFH to DCF is reduced, allowing the antioxidant activity to be expressed as a quantitative value (Wolfe and Liu, 2007).
Results of cellular antioxidant capacities of EYE-E and EYE-W are shown in Fig. 1. When HepG2 cells were treated with AAPH as an oxidative stress, DCF fluorescence intensity was significantly (p<0.05) increased by 183.7±2.5% compared to the negative control (NC; without treatment). However, after treatment with EYE-E and EYE-W, DCF fluorescence intensity increased by AAPH was decreased significantly (p<0.05) in a dose-dependent manner. After treatment with EYE-E or EYE-W at the highest concentration of 50 μg/mL (Fig. 1C), DCF fluorescence intensity was 120.7±0.6% for the EYE-E group and 112.7±3.5% for the EYE-W group (reduction of 34% and 38%, respectively). These results demonstrate that both EYE-E and EYE-W possess antioxidant activities by scavenging intracellular ROS induced by AAPH treatment, with EYE-W showing a significantly (p<0.05) higher antioxidant activity than EYE-E at a concentration of 50 μg/mL.
Excess ROS can attack biological molecules, resulting in cell damage (Qian et al., 2020). Therefore, many studies involving antioxidants have focused on scavenging or controlling ROS levels in cells (He et al., 2019; Yi et al., 2017). Previous studies have reported that pretreatment with antioxidant materials obtained from various sources such as hen egg protein (Yi et al., 2017) and duck egg protein (He et al., 2019) can reduce the production of ROS induced by H2O2 stimulation in cells. Results of the present study suggest that EYE-E and EYE-W can reduce oxidative stress by scavenging intracellular ROS induced by H2O2 and consequently protect HepG2 cells. Next, comet assay was performed to determine whether EYE-E and EYE-W with ability of decrease oxidative stress by scavenging ROS might be effective in protecting against DNA damage. The comet assay, also known as a single cell electrophoresis assay, is an assay that can analyze the level of DNA damage induced by oxidative stress in eukaryotic cells (Liao et al., 2009). Decrease of DNA damage in HepG2 cells due to antioxidant activity of egg yolk protein extract was measured. Results are shown in Fig. 2. Tail DNA intensity was 3.9±0.5% for the NC and 48.7±1.7% for the positive control (PC), meaning that DNA of HepG2 cells was damaged by oxidative stress due to H2O2 treatment. When egg yolk protein extract treated at a concentration 50 μg/mL, the tail intensity was significantly (p<0.05) decreased to 36.2±0.3% for the EYE-E group and 31.8±1.7% for the EYE-W group than that in the PC.
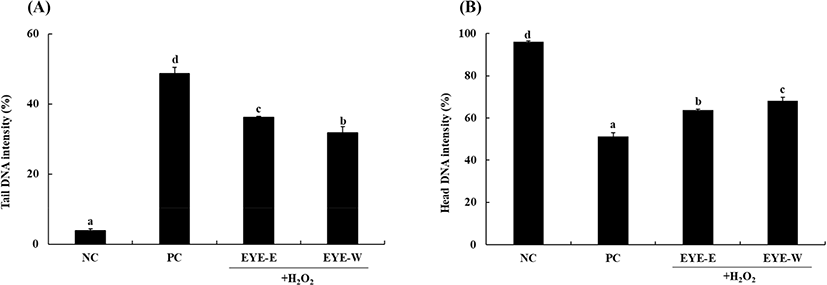
DNA damage induced by ROS can threaten genome stability and plays a role in the pathogenesis of many diseases such as cancer and coronary heart disease. It also affects ageing (Odongo et al., 2019). Many antioxidant substances have been reported to be effective in protecting against DNA damage by scavenging ROS (Lee et al., 2017b; Pukalskienė et al., 2018). Many egg proteins (such as ovotransferrin, ovalbumin, ovomucin) and egg yolk protein hydrolysates also have antioxidant activities by scavenging ROS (Lee and Paik, 2019; Liu et al., 2020). Among egg proteins, phosvitin can effectively protect human leukocytes stimulated by H2O2 against DNA damage due to its excellent antioxidant activity (Moon et al., 2014). Results of this study suggest that various egg yolk proteins with antioxidant activities present in EYE-E and EYE-W could effectively scavenge ROS and possess protective effect against cellular DNA damage. In addition, a significant difference in antioxidant activity between the EYE-E and EYE-W samples was confirmed (p<0.05; Fig. 2). This is considered to be due to the difference in the components according to the extraction solvent of the two extracts. The protein contents of EYE-E and EYE-W were 1,762.4±8.0 mg/100 g and 2,761.2±28.8 mg/100 g. And the total phenol contents of EYE-E and EYE-W were 55.9±1.2 mg GAE (gallic acid equivalent)/100 g and 79.9±0.9 mg GAE/100 g, and the total flavonoid contents of EYE-E and EYE-W were 6.8 mg±0.0 mg QE (quercetin equivalent)/100 g and 5.8±0.0 mg QE/100 g (data not shown). In previous studies, it was reported that the antioxidants flavonoids and polyphenols were present in egg yolk, and it was reported that there was a difference in the content depending on the feed used for poultry breeding (Iskender et al., 2017; Omri et al., 2019). This suggests that the difference in the extraction of active ingredients according to the extraction solvent contributed to the difference in the antioxidant activity of EYE-E and EYE-W.
Cytokines play an important role in hormonal mediation in the host defense system. They are small proteins secreted by a wide range of immune cells (Liu and Lin, 2013). Cytokines can be classified to Th1 and Th2 type cytokines with different roles in the immune system (Ku and Lin, 2013). Th1 type cytokines are recognized as pro-inflammatory cytokines that trigger local inflammation, whereas Th2 type cytokines are recognized as anti-inflammatory cytokines that inhibit the synthesis of Th1 cytokines (Liu and Lin, 2013). Therefore, when determining immunomodulatory activities of test samples, it is important to know their effects on the secretion of both Th1 and Th2 types of cytokines.
Effects of EYE-E and EYE-W on secretion of Th1 cytokines are shown in Fig. 3A and 3B. LPS and ConA were used as mitogens to stimulate splenocytes. The production of TNF-α was significantly (p<0.05) increased to 322.0±11.0 pg/mL after stimulation with LPS compared to the control (197.4±28.8 pg/mL). TNF-α is a representative Th1 cytokine that can stimulate an innate immune response (Thieringer et al., 2000). However, EYE-E and EYE-W treatments significantly decreased TNF-α production levels to 177.4±21.5 and 217.4±28.8 pg/mL, respectively (p<0.05). Levels of IL-2 production were significantly increased to 158.5±40.8 μg/mL in the presence of ConA (p<0.05). EYE-W treatment significantly reduced the level of IL-2 production to 44.9±2.2 μg/mL, whereas EYE-E treatment did not significantly affect IL-2 production in splenocytes. IL-2 is a pro-inflammatory cytokine that can regulate immune cells (e.g., T-cells, B-cells, and macrophages; Ku and Lin, 2013).
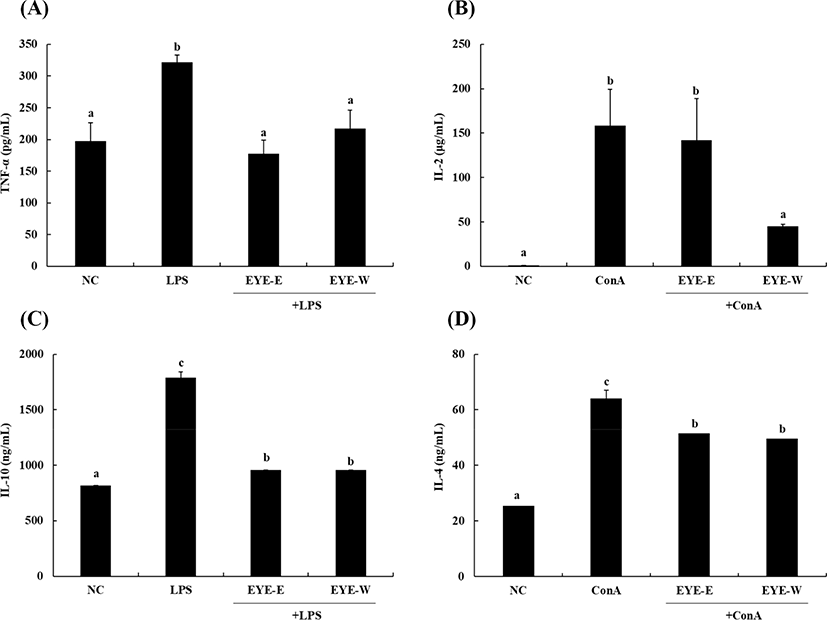
Effects of EYE-E and EYE-W on secretion of Th2 cytokines are shown in Fig. 3C and 3D. In the presence of LPS or ConA, IL-10 and IL-4 secretion levels were significantly increased to 1,788±0.0 ng/mL and 64±3.1 ng/mL (p<0.05), respectively. However, EYE-E and EYE-W treatment significantly (p<0.05) decreased IL-10 and IL-4 secretion levels. IL-10 is produced in the later stages of inflammation. It can reduce the release of ROS with an ability to present antigens of mononuclear phagocytes (Kim et al., 2017). IL-4 is also a representative anti-inflammatory cytokine that can suppress the production of inflammatory cytokines and chemokines (Woodward et al., 2012). Similar to our results, Ku and Lin (2013) have reported that terpenoid compounds can inhibit the production of both IL-10 and IL-2 cytokine simultaneously. It has been suggested that some terpenoid compounds have immunomodulatory effects by modulating Th1/Th2 cytokine production.
In conclusion, this study showed that EYE-E and EYE-W obtained by utilizing by-products of egg proteins after extracting lecithin from egg yolk had significant antioxidant activities. Both EYE-E and EYE-W could protect cells against DNA damage caused by oxidative stress. Furthermore, EYE-E and EYE-W could effectively regulate the secretion of pro- and anti-inflammatory cytokines. Therefore, EYE-E and EYE-W could be used as a functional food ingredient with excellent antioxidant and immunomodulatory activities in the food industry.













