Introduction
The most common pathogens contaminating poultry meat products are Escherichia coli and Salmonella. The chicken carcasses have been observed to have high contamination rates for E. coli (77.5%) and Salmonella (85%) (Yulistiani and Praseptiangga, 2019). E. coli are a gram-negative, facultatively anaerobic bacillus of the Enterobacteriaceae family (Lenhard-Vidal et al., 2011). Salmonella are rod-shaped, gram-negative bacteria that are one of the most prevalent causes of foodborne illness globally (Ha et al., 2020). These pathogens may contaminate meat and meat products through hands of workers in slaughterhouses and during processing. Thus, thermal processing of poultry meat products is performed before its use in food.
Thermal processing enhances the shelf life of food products and ensures microbiological safety, but it may alter the structural-chemical composition, modify heat-labile components, affect the functional properties of food products, and change meat color. When consumed raw ready-to-eat meat such as Yukhoe, the meat color is the important index for consumer’s acceptance. Thus, minimizing change in meat color is a requirement for a non-thermal decontamination process.
High hydrostatic pressure (HHP) processing is a non-destructive food preservation technology that efficiently eliminates food spoilage microorganisms without using chemicals (Kim et al., 2016). According to Garriga et al. (2004), HHP has the potential to be useful in the meat industry. HHP destroys microorganisms regardless of product geometry (Kim et al., 2016), along with increases in the safety of raw meat products and shelf life (Kruk et al., 2014).
Ultraviolet light-emitting diodes (LED) are considered one of the most influential alternatives to UV lamps because of their numerous advantages. Although antibacterial efficacy of LED is not comparable to that of UV light, significant antibacterial effects by LED irradiation has recently been reported (Li et al., 2018). Previous studies have demonstrated significant microbial inactivation of LED on smoked salmon and papaya (Kim et al., 2017; Li et al., 2018). Furthermore, LED are cost-effective and induce material degradation to a low extent (Li et al., 2018). In addition, LED are environmentally friendly, and simple equipment is needed for LED lighting (Aoyagi et al., 2011).
Therefore, this study examined the antibacterial effect of non-thermal decontamination processes, which are equivalent to that of the thermal process, on raw ground chicken.
Materials and Methods
To evaluate E. coli and Salmonella contamination levels in raw ground chickens in Korea, six raw ground chicken samples (1 kg) were purchased from grocery shop, and 25 g portions of raw ground chicken were transferred aseptically into sterile filter bags (3M, St. Paul, MN, USA). The filter bags were filled with 225 mL of 0.1% buffered peptone water (BPW; Becton Dickinson and Company, Sparks, MD, USA). The samples were macerated using a pummeler (BagMixer, Interscience, St. Nom, France) for 1 min. One milliliter of the homogenate was put into a PetrifilmTME. coli/Coliform count plate (3M). The parafilm was used to seal the plate, which was then incubated for 24 h at 37°C. Typical blue colonies with obvious gas bubbles were carefully counted. In addition, for qualitative analysis of E. coli, 1 mL of the homogenate was added to E. coli broth (Becton Dickinson and Company) including Durham tube (Kisan Biotech, Seoul, Korea) and cultured for 24–48 h at 44.5°C. Following streaking, the aliquot in the gas-producing tube was then streaked onto eosin methylene blue agar (EMB; Becton Dickinson and Company) and incubated for 24–48 h at 37°C. Formation of typical colonies with luminescent metal color confirmed the presence of E. coli. One milliliter of the homogenate was spread out on the xylose lysine deoxycholate (XLD; Becton Dickinson and Company) agar, and the plate was sealed using parafilm before being incubated for 24 h at 37°C to count Salmonella. Colonies were carefully counted on XLD agar using typical black colonies. In addition, for qualitative analysis of Salmonella, the homogenate was incubated for 24 h at 37°C. Further, aliquots containing 100 μL were added to 10 mL Rappaport-Vassiliadis enrichment broth (Becton Dickinson and Company) and incubated for 24 h at 42°C. The cultures were streaked onto XLD agar and then incubated for 24 h at 37°C. Formation of typical black colonies confirmed the presence of Salmonella. The colonies on EMB and XLD agar were identified by 16S rRNA sequencing following amplification of the primers 27F (5' AGA GTT TGA TCM TGG CTC AG 3') and 1492R (5' TAC GGY TAC CTT GTT ACG ACT T 3'). The PCR was performed using an EF-Taq (Solgent, Daejeon, Korea), 20 ng genomic DNA was used as the template in a 30 μL reaction mixture. The following PCR conditions used: initial denaturation for 2 min at 95°C; 35 cycles of amplification (95°C for 1 min, 55°C for 1 min, and 72°C for 1 min), and ending with 72°C for 10 min. A multiscreen filter plate was used to purify the enhanced products (Millipore, Bedford, MA, USA). The extension product-containing DNA sample was mixed with Hi-DiTM formamide (Applied Biosystems, Foster City, CA, USA), and then incubated for 5 min at 95°C and for 5 min on ice before being examined with a DNA analyzer (Applied Biosystems).
E. coli strains (NCCP14037, NCCP14038, NCCP14039, NCCP15661, and ATCC43888) were cultured for 24 h in 10 mL tryptic soy broth (TSB; Becton Dickinson and Company) at 37°C. The aliquots of these cultures (100 μL) were transferred to 10 mL fresh TSB and sub-cultured at 37°C for 24 h. The subcultures were combined and centrifuged at 1,912×g for 15 min at 4°C. The cell pellets were washed twice with 1× phosphate buffered saline (PBS; pH 7.4; 1.5 g/L Na2HPO4·7H2O, 0.2 g/L KH2PO4, 0.2 g/L KCl, and 8.0 g/L NaCl) after removing the supernatants to obtain inoculum containing 8–9 Log CFU/mL.
Salmonella strains (NCCP12231, NCCP12236, NCCP12243, NCCP14544, and NCCP10140) were cultured in 10 mL TSB at 37°C for 24 h. The aliquots of these cultures (100 μL) were transferred to 10 mL fresh TSB and sub-cultured at 37°C for 24 h. The subcultures were combined and centrifuged at 1,912×g for 15 min at 4°C. The cell pellets were washed twice with 1× PBS after removing the supernatants to obtain inoculum containing 8–9 Log CFU/mL.
To obtain 6–7 Log CFU/g on samples, aliquots containing 100 μL of E. coli or Salmonella inoculum were inoculated on 25 g raw ground chicken samples and left for 15 min to allow attachment of the bacterial cells. The inoculated raw ground chicken samples were aseptically transferred to polyethylene pouches and vacuum-packaged (Postech, Haman, Korea). The vacuum-packaged samples were stored at 4°C until further treatment.
The sample pouches were placed on a water bath rack and immediately immersed in circulating water bath at 70°C and 90°C for 1, 15, 30, and 60 min and steam heated in an autoclave at 121°C for 1, 4, 7, and 15 min. After thermal treatment, the samples were aseptically transferred to filter bags containing 50 mL BPW and macerated with a pummeler. The homogenates were serially diluted with 9 mL BPW; 1 mL aliquots of the diluents were put into a Petrifilm™ E. coli/Coliform count plate for determination of E. coli cell counts, and 0.1 mL of the diluent was spread-plated onto XLD agar to enumerate Salmonella cells. The parafilm was used to seal the plate,which then incubated for 24 h at 37°C. Followed by incubation, the bacterial colonies were carefully counted.
The inoculated samples were loaded in a pressure chamber in an HP 300 high pressure machine (Hyperbaric, Burgos, Spain), and pressure was increased to 500 MPa within 3 min. The temperature of the pre-cooled samples increased up to 25°C owing to adiabatic heating during pressure build-up. The samples were pressurized at 500 MPa for 1, 3, 5, and 7 min. The pressure was immediately released to 0.1 MPa post treatment, and all samples were placed on ice until analysis. The samples were macerated with a pummeler after being aseptically transferred to filter bags containing 50 mL BPW. The homogenates were serially diluted with 9 mL BPW; 1 mL aliquots of the diluents were put into a Petrifilm™ E. coli/Coliform count plate for determination of E. coli cell counts, and 0.1 mL of the diluent was spread-plated onto XLD agar to count Salmonella cells. The plate was sealed using parafilm and incubated for 24 h at 37°C, and the bacterial colonies were carefully counted.
LED lamps (12 W, SWL-V2650, Sunwave, Suwon, Korea) with proportional integral derivative controller (ITC-100 VH, INKBIRD Tech, Shenzhen, China) were used. Five lamps were installed in a refrigerator (600 mm×600 mm). The raw ground chicken samples were placed at the distance of 50 mm from the lamps, and they were treated at 405 nm for 30, 60, 90, and 120 min. UV intensity was measured using a spectrometer (StellarNet BLK-C, Stellar Net, Tampa, FL, USA). An optical probe of a spectrometer was placed at distance of 50 mm from the lamps, and the light intensity was then integrated using a auto digitizer. UV intensity was calculated as 2.8, 5.6, 8.4, and 11.2 J/cm2 under LED irradiation for 30, 60, 90, and 120 min, respectively. After LED irradiation, the samples were aseptically transferred to a filter bag containing 50 mL BPW and macerated using a pummeler. The homogenates were serially diluted with 9 mL BPW; 1 mL aliquots of the diluents were put into a Petrifilm™ E. coli/Coliform count plate for determination of E. coli cell counts, and 0.1 mL of the diluent was spread-plated onto XLD agar to count Salmonella cells. The plate was sealed with parafilm and incubated at 37°C for 24 h. Followed by incubation, the bacterial colonies were carefully counted.
To evaluate if HHP and LED treatment affect on the meat color. The lightness (L*), redness (a*), and yellowness (b*) in the samples treated with HHP or LED were measured using a colorimeter (CR-10, Konica Minolta Sensing, Osaka, Japan). It had the illuminant of D65, observer angle of 2° and aperture diameter of 8 mm. The total color difference (ΔE) was estimated using the following equation to determine the overall color change compared with that of the control.
where L*0, a*0, and b*0 represent the readings at 0 h, and L*, a*, and b* represent the individual readings after the defined treatments.
Results and Discussion
E. coli were detected in all tested samples at 2.2±0.5 Log CFU/g, whereas Salmonella were not detected in any of the samples. According to the Eyi and Arslan (2012), E. coli were detected in 87.5% of poultry samples at 3.3 Log CFU/g. Eyi and Arslan (2012) reported predominant contamination of carcasses of animals and birds with E. coli and suggested that contamination of meat and meat products with E. coli are likely during preparation and sale also. Although the prevalence of Salmonella in retail chicken in previous studies was reported as 53.3% in Vietnam (Van et al., 2007), 36.5% in Belgium (Uyttendaele et al., 1999), and 42.3% in Korea (Hyeon et al., 2011), the result from our study showed no positive samples for Salmonella. This difference might be caused by washing chicken carcasses with sodium hypochlorite in Korea. MFDS (2019) and Yoon et al. (2014) also showed very low prevalence of Salmonella in chicken. Collectively, these results suggest the importance of decontamination processes in foods containing raw chicken.
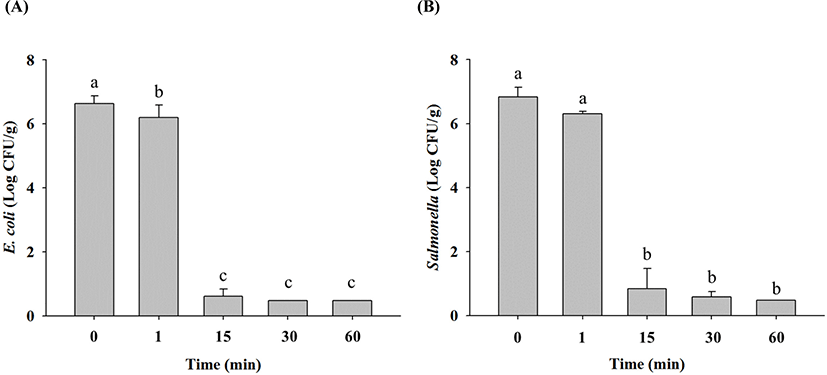
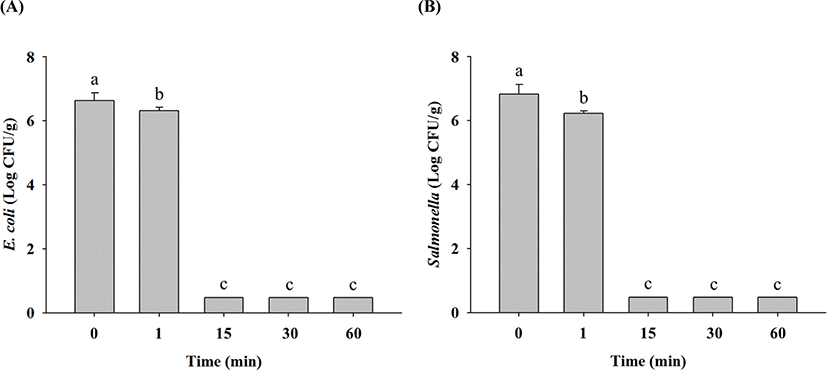
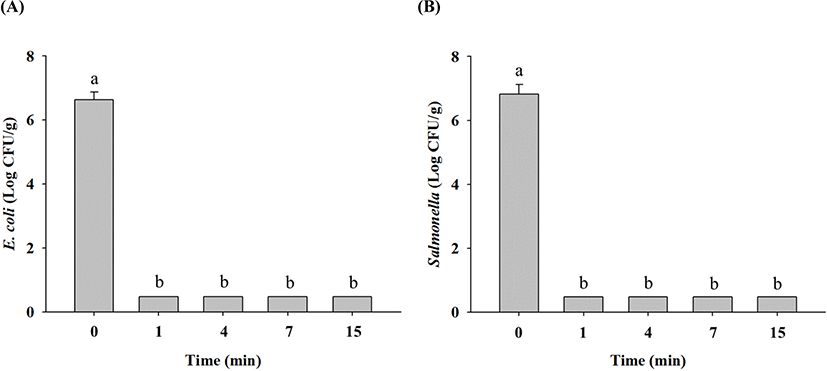
In non-heated raw ground chicken samples, E. coli cell counts were 6.6 Log CFU/g, and these decreased to 6.2 Log CFU/g after 1 min, 0.6 Log CFU/g after 15 min, and limit of detection (LOD; 0.5 Log CFU/g) after 30 min at 70°C (Fig. 1A). Salmonella cell counts in non-heated raw ground chicken samples were 6.8 Log CFU/g, which also decreased to 6.3 Log CFU/g after 1 min, 0.8 Log CFU/g after 15 min, 0.6 Log CFU/g after 30 min, and LOD after 60 min at 70°C (Fig. 1B). These results showed that E. coli and Salmonella in raw ground chicken were destroyed within 30 min and 60 min of thermal treatment at 70°C, respectively. Thermal treatment at 90°C resulted in decrease in E. coli cell counts in raw ground chicken samples to 6.3 Log CFU/g after 1 min and LOD after 15 min compared with 6.6 Log CFU/g in untreated samples (Fig. 2A). Similarly, Salmonella cell counts significantly decreased to 6.2 Log CFU/g after 1 min and LOD after 15 min after treatment at 90°C from the initial concentration of 6.8 Log CFU/g (Fig. 2B). These findings showed that E. coli and Salmonella in raw ground chicken were destroyed within 15 min at 90°C. Moreover, both E. coli and Salmonella cell counts in raw ground chicken samples were estimated to reach LOD after 1 min when heated at 121°C (Fig. 3A and 3B). This shows that E. coli and Salmonella in raw ground chicken can be eliminated within 1 min by heating at 121°C.
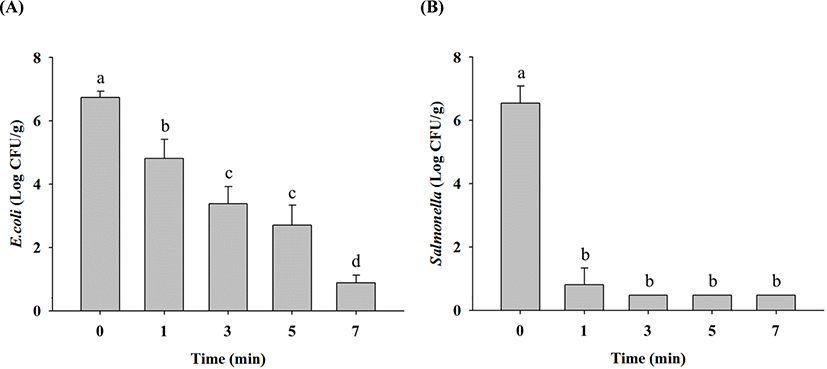
HHP treatment reduced E. coli cell counts in raw ground chicken samples to 4.8 Log CFU/g after 1 min, 3.4 Log CFU/g after 3 min, 2.7 Log CFU/g after 5 min, and 0.9 Log CFU/g after 7 min from the initial concentration of 6.7 Log CFU/g (Fig. 4A). Salmonella cell counts decreased to 0.8 Log CFU/g after 1 min and LOD after 3 min of HHP treatment from the initial concentration of 6.5 Log CFU/g (Fig. 4B). This shows that Salmonella are more sensitive to HHP than E. coli. HHP treatment causes many changes in microbial cell membranes, resulting in lysis, nuclear material alteration, osmotic changes, and other modifications, finally leading to pathogen eradication (Mackey et al., 1994). Jung et al. (2012) and Kruk et al. (2011) reported that HHP of 450–600 MPa almost completely eliminated E. coli and Salmonella. These results indicate that 7-min HHP treatment at 500 MPa can reduce both E. coli and Salmonella cell counts, equivalent to thermal treatment for 60 min at 70°C, 15 min at 90°C, and 1 min at 121°C (p<0.05).
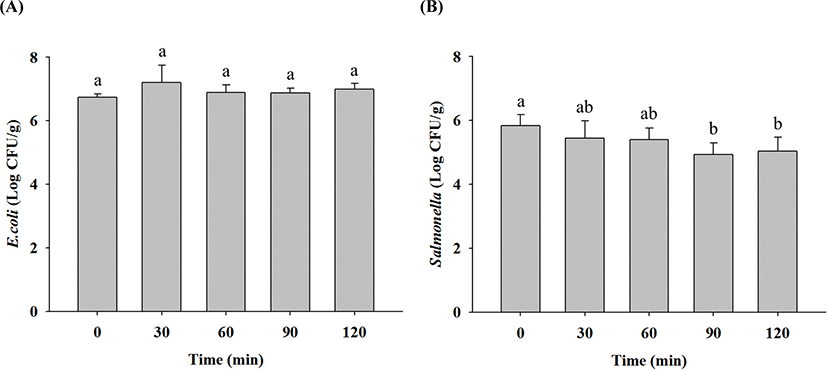
E. coli cell counts in raw ground chicken samples before and after LED irradiation at 405 nm was not significantly different (Fig. 5A). LED irradiation for 90 min reduced Salmonella cell counts in raw ground chicken only by 0.9 Log CFU/g (p<0.05), but it did not reduce E. coli cell counts for 90 min (Fig. 5B). According to Kim et al. (2017), Salmonella cell counts in fresh-cut papaya were reduced by 1–1.2 Log CFU/cm2 when exposed to 405 nm LED for 48 h. Li et al. (2018) suggested that LED irradiation at 405 nm for 8 h reduced a 0.5-Log CFU/cm2 of Salmonella cell counts in ready-to-eat fresh salmon. These results indicate that LED irradiation has no significant effect on microbial reduction compared with thermal treatment, and Salmonella are more sensitive to LED irradiation than E. coli.
The parameters of chicken color (L*, a*, and b*) are shown in Table 1. The L* values were high (p<0.05) in the HHP-treated raw ground chicken samples. The L* value represents the light-dark spectrum with a range from 0 (black) to 100 (white), which is closely related to the browning level of the samples (Pathare et al., 2013). The a* values were low (p<0.05) in the HHP-treated raw ground chicken samples. Wang et al. (2018) reported a* value as the most important color parameter for fresh meat, which is defined as the red-green spectrum with a range of −60 (green) to +60 (red). The alterations in L* and a* values following HHP treatment were caused by the oxidation of ferrous myoglobin to metmyoglobin, which represents the effect of pressure on globin denaturation and structural rearrangement (Carlez et al., 1995; Fraqueza et al., 2019; Szerman et al., 2011). The b* value is defined as the blue-yellow spectrum with a range from −60 (blue) to +60 (yellow) (Wang et al., 2018). In our study, the b* values were not significantly different among the samples. The total color difference (ΔE) represented the magnitude of the overall color difference between the non-HHP and HHP-treated samples. The ΔE values in HHP-treated raw ground chicken samples ranged between 10.00–13.54 (Table 1). According to the study by Kim et al. (2016), ΔE values >12 indicates a significant color difference. Thus, the HHP-treated raw ground chicken samples showed a significant color difference from the non-HHP-treated samples (Table 1).
The L* values decreased in the LED-irradiated raw ground chicken samples, whereas the a* values increased (p<0.05; Table 2). Our findings were comparable to those reported by Chun et al. (2010) that a* values increased in the chicken breast after UV irradiation. The b* values increased (p<0.05) in the LED-irradiated raw ground chicken samples (Table 2). The ΔE values in LED-irradiated raw ground chicken samples were 1.93–2.98 (Table 2). Francis and Clydesdale (1975) suggested that color difference is not clearly distinguished by human eyes when ΔE values <3. Thus, the results indicate that LED irradiation slightly changed the color of raw ground chicken compared with that of the non-irradiated samples.
Conclusion
In this study, HHP treatment at 500 MPa can destroy more than 5 Log CFU/g of E. coli cell counts for 7 min and more than 6 Log CFU/g of Salmonella cell counts for 1 min; these effects are equivalent to thermal treatment of raw ground chicken at 70°C for 60 min, 90°C for 15 min, and 121°C for 1 min. However, LED irradiation at 405 nm does not have the significant antibacterial effects as much as by the thermal treatment. Collectively, although HHP treatment causes color change, HHP treatment at 500 MPa for more than 7 min can be used as a non-thermal decontamination process, equivalent to thermal treatment, to improve the microbiological safety of raw ground chicken.













