Introduction
Edible insects can be used as an important new protein food source in the future (Kim et al., 2019a; Kim et al., 2021). The consumption of edible insect proteins reduces the production of greenhouse gases compared to traditional animal protein sources (Kim et al., 2020a) and can also be an effective response to a shortage in protein supply (Kim et al., 2019a). According to the Ministry of Food and Drug Safety (2020), there are nine types of edible insects allowed as food raw materials in Korea, and consumption of edible insects continues to increase. Edible insects show a high nutritional protein content, but their processing potential is low due to chitin (Kim et al., 2020b). Liu et al. (2020) reported that the existing edible insects cause a phobia phenomenon with consumers due to the appearance of the insects and for this reason their use is limited despite their high nutritional and environmental value. In the case of most edible insects, the problem of their appearance is avoided by rendering the protein through drying or extraction processes (Lee et al., 2020; van Huis, 2013). Thus, various studies have been conducted to increase the utilization of edible insect proteins (Choi et al., 2017; Patel et al., 2019); however, studies of high pressure treatment have yet to be published.
Non-thermal, high pressure processing is a well-known, effective, and eco-friendly method to improve extract yield (Chen et al., 2010). High pressure technology instantly and uniformly transfers pressure to a sample using oil as a pressure medium at 100–500 MPa (Lee et al., 2016). High pressure affects sterilization and extraction by causing a change in the physical biochemical environment of the sample (Farkas and Hoover, 2000). In general, the extraction water is improved because the cell membrane of the sample is destroyed, so that the solvent enters the cell and many components are easily eluted out of the cell (Campus, 2010). We are not aware of any published research on the use of high pressure when extracting protein from edible insects, and there are no studies on the processing potential of edible insect protein when using high hydrostatic pressure.
Therefore, effect of high pressure on protein extracted from edible insect have to be evaluated. Finally, the improvement way to enhance the technical functional properties of edible insect protein could be suggested according to this study.
Materials and Methods
Freeze-dried larval Protaetia brevitarsis seulensis (protein: 51.1%; fat: 21.2%) were procured from an edible insect Farm (Jeongeup, Korea). The ground P. brevitarsis seulensis matter was dispersed in n-hexane (1:5, w/v). The fat dissolved in the hexane was removed. Hexane residue in the defatted P. brevitarsis seulensis was then volatilized at overnight (20°C), after which it was stored at −20°C (Kim et al., 2021).
The non-thermal high pressure treatments were conducted in a high pressure system (maximum pressure: 600 MPa, R–SCS, Chemre System, Anyang, Korea). The high pressure treatment was set at 100, 200, 300, 400, and 500 MPa, and the pressure vessel temperature increased up to 35°C (Lee et al., 2019).
Proteins were extracted from the defatted P. brevitarsis seulensis material using high hydrostatic pressure; the extraction procedure was carried out in 0.58 M saline solution (2°C). The sample and each of the buffers were homogenized at 1:2 (w/v) at 10,000 rpm, and filtered with medical gauze. The filtrate was centrifuged for 30 min (15,000 g, 2°C), and the then supernatant was considered to be an extracted protein solution (Kim et al., 2019b).
The protein solubility of P. brevitarsis seulensis material treated with high pressure (hereafter, “pressure-treated P. brevitarsis seulensis”) was determined by the biuret method.
The amino acid content of the pressure-treated P. brevitarsis seulensis was measured with an L-8800 amino acid analyzer (Hitachi, Tokyo, Japan) with an ion exchange resin column (Kim et al., 2020b). The standard amino acid contents were procured from Sigma-Aldrich (St. Louis, MO, USA). The EAAI was calculated according to FAO/WHO/UNU (1985).
SDS-PAGE was executed as described by Kim et al. (2020a). Simplify, the protein concentration of the pressure-treated P. brevitarsis seulensis was calculated using Bradford reagent. A 20 μg sample of the pressure-treated P. brevitarsis seulensis and the sample buffer (Bio-Rad Laboratories, Irvine, CA, USA) were mixed at a 1:1 ratio. The mixtures were then heated at 100°C (5 min) and parted using 10% SDS-PAGE. The stained protein bands were identified by molecular weight.
The pH value of the pressure-treated P. brevitarsis seulensis was determined using a pH meter. The instrumental color of the pressure-treated P. brevitarsis seulensis was measured using a colorimeter (CR-410, Minolta, Osaka, Japan).
The protein concentration of the pressure-treated P. brevitarsis seulensis was adjusted to 1% (w/v). Each sample of pressure-treated P. brevitarsis seulensis was homogenized at 12,000 rpm to produce foam (2 min). Foam stability was obtained by recording the volume of the foaming solution of the pressure-treated P. brevitarsis seulensis protein for 2, 5, 10, 20, 30, and 60 min after homogenization (Kim et al., 2020a; Mishyna et al., 2019).
Ten milliliters sample of 1% (w/v) pressure-treated P. brevitarsis seulensis protein and 1 mL pure olive oil were homogenized at 18,000 rpm (2 min). The emulsion capacity of the pressure-treated P. brevitarsis seulensis protein was the difference between the solution volume before and after homogenization and was calculated as a percentage. To determine the emulsion stability, 50 μL pressure-treated P. brevitarsis seulensis protein was mixed with 10 mL 0.3% (w/v) SDS solution. A spectrophotometer set at 500 nm for 2, 5, 10, 20, 30, and 60 min was used to detect the difference before and after the holding time to measure emulsion stability (Kim et al., 2020a; Pearce and Kinsella, 1978).
Significant differences among the samples of pressure-treated P. brevitarsis seulensis were calculated using one way analysis of variance with Duncan’s multiple range test (p<0.05), calculated with 20.0 version SPSS statistical software (IBM, Armonk, NY, USA). Regardless of the level of pressure (treatment) applied to the P. brevitarsis seulensis sample.
Results and Discussion
In Table 1, we present the protein solubility of P. brevitarsis seulensis treated with different levels of hydrostatic pressure. The protein solubility of the pressure-treated P. brevitarsis seulensis was higher (p<0.05) than that of the controls. The protein solubility of the P. brevitarsis seulensis extracted at 200 MPa was the highest (p<0.05) among the treatment groups, and the protein solubility tended to decrease as the hydrostatic pressure exceeded 200 MPa. These results agree with the findings from a study by Zhang et al. (2017), in which the solubility of myofibrillar protein induced by high pressure increased at 200 MPa and then decreased gradually with increasing pressure (300–500 MPa). This may be because of the quaternary structure being dissociated at moderate pressures (100–200 MPa). Mishyna et al. (2019) reported that protein solubility was affected by rheological properties due to salinity, pH, and temperature with changes in the protein net charge. Marcos et al. (2010) reported high pressure induced changes on protein solubility and that the highest protein concentration was obtained at 200 MPa.
| High pressure (MPa) | Protein concentration (mg/mL) |
|---|---|
| Control1) | 64.09±1.38e |
| 100 | 68.38±1.33c |
| 200 | 73.89±1.11a |
| 300 | 70.99±1.15bc |
| 400 | 71.23±1.21b |
| 500 | 66.49±1.15d |
The amino acid contents and EAAI of the pressure-treated P. brevitarsis seulensis samples are presented in Table 2. The essential amino acid content was the highest (p<0.05) in the P. brevitarsis seulensis sample treated with 400 MPa hydrostatic pressure. The total amino acid content of the control was lower (p<0.05) than that of the P. brevitarsis seulensis treated with 200 MPa hydrostatic pressure. The EAAI of the P. brevitarsis seulensis treated with 100 MPa hydrostatic pressure (p<0.05) was lower than that of the control. The EAAI of control showed lower or similar tendency at high pressure of 200 MPa or higher. Yi et al. (2013) reported that the EAAI could be used as a nutritional index for protein sources. Zhang et al. (2017) reported that the amino acids content of high pressure treated myofibrillar protein showed no significant changes. Kim et al. (2020a) reported changes in amino acid contents of edible insect protein based on the extraction processes. In their study, it was found that the species of edible insect and the extraction processes both had a significant effect on the amino acid composition and EAAI. Furthermore, there was a significant interaction between the edible insect species and the extraction processes. In general, the hydrophobic amino acid composition plays a substantial role in the emulsion capacity of the protein (Li et al., 2019). Thus, the pressure-treated P. brevitarsis seulensis was expected to have the greatest protein functionality.
| High pressure (MPa) | FAO/WHO/UNU (1985) | ||||||
|---|---|---|---|---|---|---|---|
| Control1) | 100 | 200 | 300 | 400 | 500 | ||
| Amino acid profile (mg/g) | |||||||
| Essential amino acid (EAA) | |||||||
| His | 7.80±0.28 | 6.25±0.07 | 7.65±0.49 | 7.30±0.28 | 7.05±0.35 | 7.45±0.64 | 15 |
| Ile | 10.60±0.14b | 9.45±0.21c | 11.75±0.07a | 11.25±0.49ab | 11.65±0.21a | 11.50±0.57a | 30 |
| Leu | 18.25±0.35ab | 16.05±0.49c | 19.20±0.57a | 17.55±1.06b | 19.25±0.49a | 18.50±0.42ab | 59 |
| Lys | 12.95±0.07bc | 10.30±0.28d | 14.00±0.14a | 12.80±0.28c | 13.35±0.21b | 12.75±0.21c | 45 |
| Met+Cys | 1.35±0.21 | 1.55±0.35 | 1.55±0.07 | 1.55±0.21 | 1.65±0.49 | 2.30±0.14 | 22 |
| Phe+Tyr | 27.75±0.49c | 23.45±0.35d | 28.70±0.99c | 27.45±1.91c | 35.65±1.06a | 31.70±0.85b | 38 |
| Thr | 9.00±0.21b | 7.40±0.14c | 9.80±0.28a | 8.90±0.00b | 9.65±0.21a | 9.20±0.14b | 23 |
| Val | 8.75±0.21b | 7.50±0.14c | 9.70±0.28a | 8.75±0.07b | 9.50±0.28ab | 9.60±0.57a | 39 |
| Sum of EAA | 96.45±1.06c | 81.95±0.35d | 102.35±1.63b | 95.55±0.07c | 107.75±0.92a | 103.00±3.25b | 271 |
| Ala | 12.85±0.07b | 10.50±0.14e | 13.20±0.00a | 12.00±0.14d | 12.40±0.00c | 12.35±0.21c | |
| Arg | 9.75±0.21a | 7.10±0.99c | 9.30±0.57ab | 8.00±0.71bc | 9.40±0.00ab | 8.55±0.35abc | |
| Asp | 16.50±0.14ab | 12.90±0.00d | 17.30±0.14a | 15.30±0.42c | 17.20±0.57ab | 16.35±0.49b | |
| Glu | 29.10±0.14c | 23.35±0.21d | 31.80±0.28a | 28.25±0.64c | 31.75±0.21a | 30.30±0.57b | |
| Pro | 22.10±0.57a | 15.10±0.57c | 21.20±1.41ab | 13.70±0.85c | 19.00±1.13b | 18.35±1.77b | |
| Gly | 11.85±0.07c | 9.65±0.21e | 12.40±0.00a | 11.30±0.00d | 12.20±0.00ab | 12.10±0.14bc | |
| Ser | 12.60±0.28b | 10.55±0.35c | 13.55±0.21a | 12.35±0.35b | 13.65±0.21a | 12.55±0.07b | |
| Sum of total AA | 114.75±0.21b | 89.15±0.64e | 118.75±1.48a | 100.90±3.11d | 115.60±1.70b | 110.55±1.20c | |
| EAAI | 15.16±0.29b | 13.60±0.21c | 16.05±0.07a | 15.24±0.08b | 16.20±0.51a | 16.45±0.50a | |
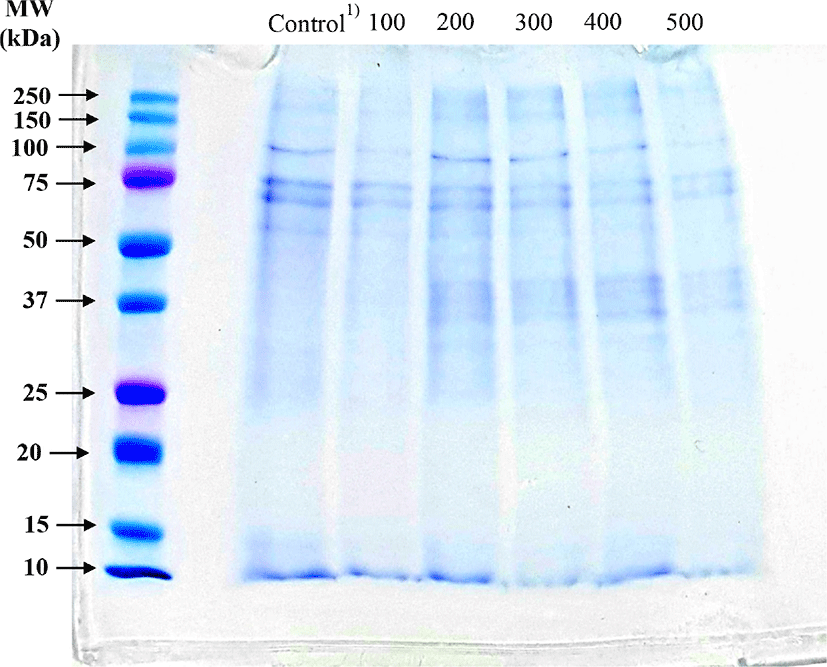
The effect of the high pressure treatment on P. brevitarsis seulensis protein composition is shown in Fig. 1. The observed bands at approximately 75 kDa tended to become faint after high pressure treatment; whereas the bands at approximately 37 kDa became thicker after pressure treatment. In other words, it is possible that the high molecular weight (75 kDa) proteins became low molecular (37 kDa) proteins after treatment with high hydrostatic pressure. Nalinanon et al. (2011) reported that the protein function was observed by protein characteristics with distribution of molecular weight. According to Kim et al. (2020a), the edible insect proteins with molecular weights over 75 kDa in edible insects are at the ground or defatted state. It was reported that the P. brevitarsis seulensis proteins were most plentiful in the range of 10 to 25 kDa and at 35 kDa; however, the protein extracted from P. brevitarsis seulensis appeared only at approximately 35 kDa. Yi et al. (2013) noted that the absence of proteins larger than 75 kDa may have a negative influence on technical functionality of the protein, and skeletal muscle of edible insect composed of the protein size over 95 kDa. These results suggest that edible insect protein subjected to high pressure treatment is reduced in molecular weight.
The pH and color of the solution of pressure-treated P. brevitarsis seulensis protein are shown in Table 3. The pH of the protein trended to increase as the high pressure levels increased. Chan et al. (2011) reported that the high pressure treatment of muscle proteins resulted in a small increase in pH, possibly due to a decrease in acidic groups in the proteins related to denaturation. A similar trend was observed by Hong et al. (2005), who reported that the pH of pork meat increased with increasing hydrostatic pressure. Hong et al. (2008) reported that the pH of the high pressure treated meat leads to a higher pH, possibly due to greater exposure of acidic groups on the protein surface.
The lightness of the pressure-treated P. brevitarsis seulensis protein solution did not differ significantly (p>0.05) from that of the control. The values for redness and yellowness of the pressure-treated P. brevitarsis seulensis protein solution were lower (p<0.05) than those of the control. Hong et al. (2005) reported that the lightness and redness of pork increased with increasing pressure levels and time of the pressure treatment, and that the yellowness of pork protein did not differ significantly among the high pressure treatments. Marcos et al. (2010) reported that high pressure treatment of sarcoplasmic protein had an independent influence on the color values. In the present study, the color change was observable, but the high pressure treatments did not seem to have a significant effect on the color values.
Foaming capacity and foam stability of the pressure-treated P. brevitarsis seulensis protein are presented in Fig. 2. The forming capacity of the pressure-treated protein solution of P. brevitarsis seulensis was higher (p<0.05) than that of the control, and there was no significant (p>0.05) difference between the high pressure treatment groups except for 100 MPa treatment (Fig. 2A). According to Yi et al. (2013), foaming capacity can be described by protein concentration, protein structure, and ionic strength. Mishyna et al. (2019) found that the protein functionality of protein extract with a lower pH was lower than that of the protein extract with a higher pH. Similarly, in the present study, we report that the pH of the protein solution of P. brevitarsis seulensis was increased by the high pressure treatment.
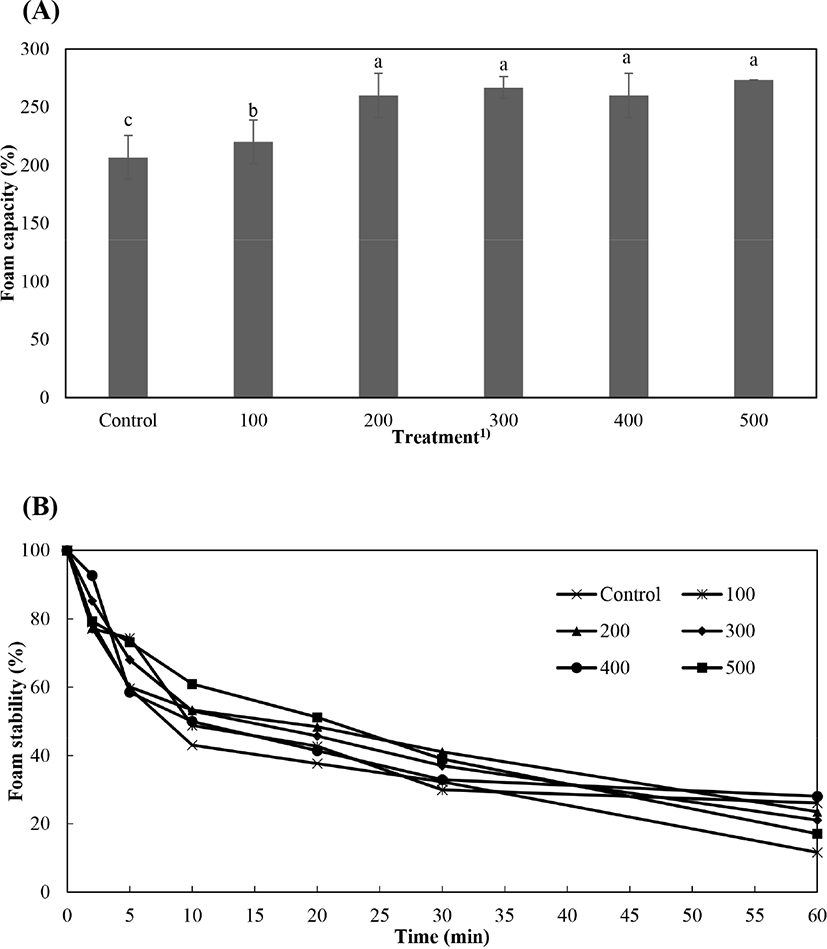
The foam stability of the pressure-treated protein solution of P. brevitarsis seulensis showed acute differences over time (Fig. 2B), tending to decrease with increasing time in the control and in the treatment groups. Kim et al. (2020a) reported that foam stability determines the final quality of food protein. In addition, it was reported that the foam stability of edible insect protein solution showed different trends depending on the species and extraction step (Kim et al., 2020a). According to Zielińska et al. (2018), the foam stability of edible insect protein could be increased depending on surface hydrophobicity, hydrophobic amino acid content and residue location, thiol groups, cations, and anions. Thus, in the present study, we investigated whether high pressure treatment improves the foaming capacity and foam stability of proteins derived from edible insects.
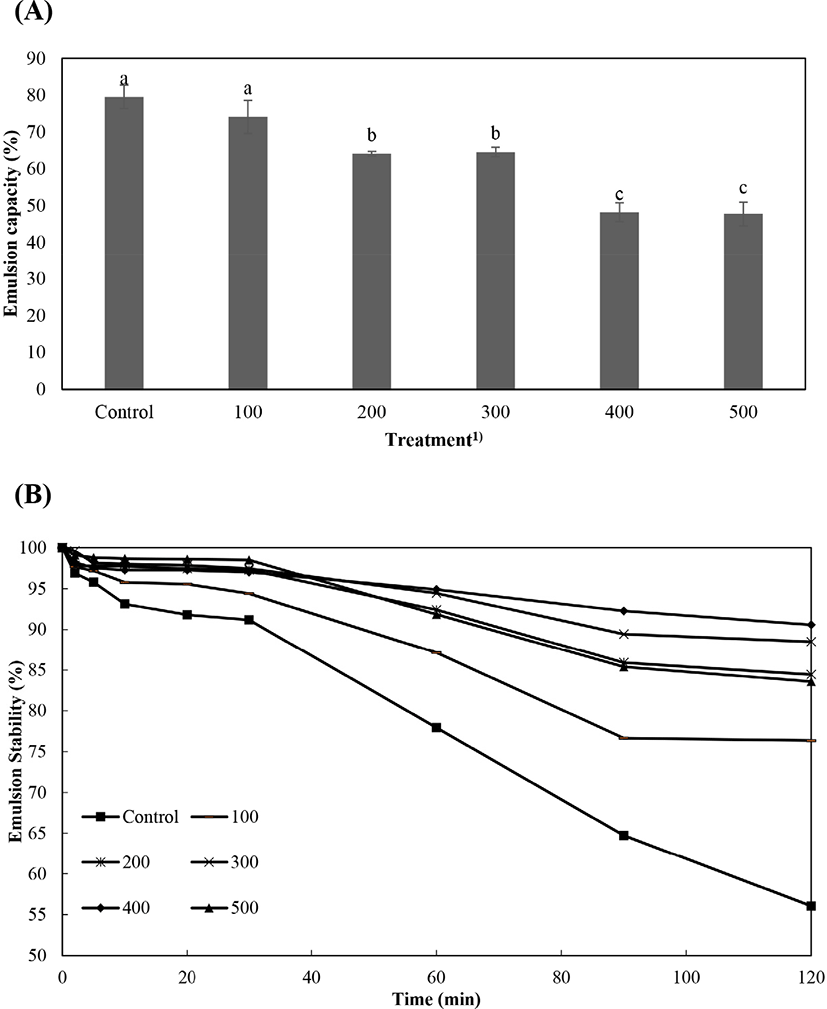
In Fig. 3, we present the representative emulsion capacity and emulsion stability of the pressure-treated protein derived from P. brevitarsis seulensis. The control group and the 100 MPa treatment group had the highest (p<0.05) emulsion stability, and the emulsion stability tended to decrease with increasing hydrostatic pressure (Fig. 3A). In similar studies, it has been reported that pressure denaturation of animal proteins resulted in destabilizing interactions in emulsions that decreased the emulsion capacity of the protein (Villamonte et al., 2016; O'Sullivan et al., 2016). In addition, Mishyna et al. (2019) reported that the emulsion capacity of edible insect protein can be affected by the solubility, concentration, and hydrophobicity of the protein. In the present study, the emulsion stability of the pressure-treated protein solution varied over time, tending to decrease with increasing time in all treatment groups (Fig. 3B). Villamonte et al. (2016) reported that the emulsion stability improved when the proteins were treated at 200 MPa hydrostatic pressure, due to escaped droplets in the aggregated droplets network at oil droplet concentration. Mishyna et al. (2019) reported that the lower molecular weight of edible insect proteins might affect their emulsion stability. In the present study, we found that both the emulsion capacity and emulsion stability of the protein solution of P. brevitarsis seulensis can be improved with hydrostatic pressure treatment up to 200 MPa.
Conclusion
With this study, we have demonstrated the technical functional properties of a protein solution of P. brevitarsis seulensis treated with high hydrostatic pressure. In general, edible insect proteins have poor technical functional properties compared to other animal proteins, and thus their utilization as a food material is currently insufficient. We applied high pressure to a protein solution from P. brevitarsis seulensis in order to demonstrate how the technical functional properties of edible insect protein can be improved. We confirmed the improvement of technical functional properties of P. brevitarsis seulensis proteins extracted under high pressure (200 MPa). In conclusion, we propose that high pressure treatment can improve the technical functional properties of proteins derived from edible insects, thereby increasing the utilization of edible insects as a protein resource.













