Introduction
Myofibril protein (MP) is one of the major components in the muscles of various animals, and it plays a substantial role in the physicochemical and textural properties of emulsion systems (Choi et al., 2011). MP is a salt-soluble protein that must be extracted from muscle using sodium chloride or sodium phosphates when manufacturing meat products (Kim et al., 2021a). The degree of MP dissolution affects the textural and emulsifying properties of meat products (Zayas, 2012). When MP gelation, myosin contributes mostly to gel formation due to their high hydrophobic interaction and it could dissolve in high ionic strength (Zayas, 2012). For sufficient elution of MP, a large amount of sodium chloride and sodium phosphates are required (Kim et al., 2017; Yong et al., 2020). Sodium chloride and phosphates were used to prepare a gel matrix of myofibrillar protein for a desirable texture and flavor (Lee et al., 2010; Li et al., 2021; Sun et al., 2011). However, high sodium levels cause chronic diseases; thus, a proper control of its degree of ingestion can prevent complications and promote human health (Desmond, 2006; Kim et al., 2021a). Thus, various researchers have developed novel technologies to increase the functional properties of myofibrils to enhance the clearance of sodium phosphates (Yong et al., 2021). In this study, we induced changes in the functional properties of MP that reacted with isolated saccharides (graft reactions) by covalent binding with sugars (Xu et al., 2020).
The grafting reaction with protein isolated-saccharide is an important chemical reaction that can improve techno-functional properties of proteins without additional chemicals (de Oliveira et al., 2016). Functional properties of protein could be improved with conjugate with polysaccharides and this conjugation between amino groups and carbonyl group could be occurred by Maillard reaction (Guan et al., 2006; Xu et al., 2020; Xu et al., 2021). In addition, excellent antioxidant, antimicrobial, gelling, and emulsifying properties observed in the protein-saccharide grafts are beneficial polymers with high thermal stability in food systems (Chevalier et al., 2001; Guan et al., 2006; Xu et al., 2020; Xu et al., 2021). According to Li et al. (2013), soybean protein-glucose graft reactions enhance foaming properties and emulsifying capacity. However, studies on MP-saccharide graft reactions have not been conducted. In this study, we employed different types of saccharides: reducing and non-reducing sugars.
Therefore, a proper selection of saccharides is vital for modifying the protein structure to improve the gelling properties of MPs. Thus, we conducted this study to investigate the effect of various reducing and non-reducing sugars on the protein functionality of MP-saccharide graft reactions.
Materials and Methods
Pork ham (Musculus semitendinosus, Musculus semimembranosus, Musculus biceps femoris) was obtained from a local market and saccharides (dextrose, fructose, sucrose, palatinose, erythitol, lactose, trehalose, sorbitol, and xylitol) were purchased from ES Food (Gunpo, Korea). All chemicals used in the experiments were obtained from Sigma-Aldrich (St. Louis, MO, USA). MP was extracted using the method of Jia et al. (2018). Pork ham was homogenized in four volumes (w/v) with 0.1 M potassium chloride in 0.01 M phosphate buffer (pH 7.4), and the homogenized solution was centrifuged at 2,000×g for 15 min. The residue was washed with four volumes of 0.1 M potassium chloride in 0.01 M phosphate buffer (pH 7.4) at 2,000×g for 15 min, and the residue was washed using Milli-Q water to remove the salt. The washed MP was lyophilized and stored at −20°C.
MP and various saccharides graft reactions were conducted using the method of Wong et al. (2011). One part of the MP powder and one part of the saccharides were fully hydrated and lyophilized. The dried samples were incubated at 60°C for 5 d at 75% humidity. The remaining saccharides were removed by dialysis after dissolving in distilled Milli-Q water and then freeze-dryed.
The grafting degree of MP isolate-saccharides graft reaction was estimated using the method of Vigo et al. (1992) with minor modification. The sample solution (200 μL) and ophthaldialdehyde reagent (4 mL) were incubated at 35°C for 2 min, and the absorbance was measured at 340 nm using a UV/VIS spectrophotometer (Spectra Max Plus 384, Molecular Devices, San Jose, CA, USA).
The browning intensity of the MP isolate-saccharide graft reaction was determined using the method of Li et al. (2019). The absorbance was measured at 420 nm to detect the browning index by dissolving SDS solution (0.1%) in the sample.
SDS-PAGE was performed to confirm the MP-isolated saccharide graft reaction (Laemmli, 1970). The MP-isolated saccharide graft reaction samples (10 μg) were mixed with sample buffer and heated at 100°C (5 min). The cooled samples were separated on a 12% Mini-PROTEAN® TGX TM precast gel (Bio-Rad Laboratories, Hercules, CA, USA). Protein bands were stained with Coomassie Brilliant Blue R 250, and glycoproteins were stained. The molecular weights of the bands in the gel were determined using the Precision Plus Protein TM dual-color standards (Bio-Rad Laboratories).
The protein solubility of the MP isolated-saccharide graft reaction samples was estimated using the BCA protein assay under various pH and ionic strength conditions. The pH of the sample was adjusted from 9 to 3 using 1 N NaOH and 1 N HCl, and the ionic strength was regulated by adjusting the NaCl concentration (0–0.5 M). The protein concentration was regulated at 5 mg/mL, and dissolved samples were centrifuged at 6,000×g for 30 min. After obtaining the supernatant, the protein concentration was estimated using the PierceTM BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) to determine protein solubility according to user guide.
The color of the MP-isolated saccharide graft reaction samples was determined using a colorimeter (CR-410, Konica Minolta, Tokyo, Japan). The International Commission on Illumination (CIE) L* (lightness), a* (redness), and b* (yellowness) systems was used to illustrate the color values (D50 illuminant, 2° observer). Colorimeter was calibrated by white plate (Y=87.1, x=0.3166, y=0.3338) and sample height was over 2 cm. The color difference (△E) among the MP isolated saccharide graft reaction samples was calculated according to Kim et al. (2021b).
Four grams of grafted sample and non-grafted sample were homogenized 10 mL of Milli-Q water or soybean oil in 50 mL of conical tubes at 8,000 rpm for 1 min. After centrifuged at 6,000×g for 30 min, supernatant volume was measured to calculate water or oil binding capacity. The retained water or oil per grams of sample were calculated (Beuchat, 1977).
The emulsifying capacity and emulsion stability of the MP-isolated saccharide graft reaction samples were determined according to the protocol of Kim et al. (2021b). The graft reaction samples were dissolved in 0.58 M saline solution (2 mg/mL). Three portions of the protein solution and one portion of soybean oil were homogenized at 10,000 rpm for 1 min. After mixing 50 μL of the emulsion and 0.1% SDS solution (5 mL), the absorbance of the samples was determined at 500 nm at 0 (A0) and 10 min (A10). Emulsifying capacity was demonstrated as A0, and emulsion stability was revealed after comparing the absorbance at 10 min.
All experiments were repeated thrice, and the measurement variation was determined using the SD. One-way analysis of variance (ANOVA) was performed to determine significant differences between treatments using Duncan’s multiple range test (p<0.05) in the SPSS software (Statistical Package for Social Sciences: SPSS ver.20 .0, IBM, Chicago, IL, USA). The saccharide type was considered a fixed term and replicates were considered random terms.
Results and Discussion
Fig. 1 illustrates the grafting degree and browning index of the MP isolate-saccharide graft reaction with various saccharides. According to Li et al. (2019), the degree of grafting determines the active amino content, thereby confirming the extent of the Maillard reaction. Xu et al. (2020) demonstrated that the grafting degree as an indicator of the degree of Maillard reaction. The grafting degree of the MP isolate-saccharide graft reaction with saccharides was higher than that of the control (p<0.05), except for xylitol. The grafting degree of the MP isolate-saccharide graft reaction was significantly higher in the reducing sugar-treated groups (lactose, glucose, fructose, and palatinose). Xiao et al. (2021) reported that the Maillard reaction is the covalent binding between the amino and carbonyl groups (aldehydes, ketones). Reducing sugars have free aldehydes or ketones, and non-reducing sugars do not contain such free groups. The Maillard reaction occurs more readily in reducing sugars than in non-reducing sugars (Yu et al., 2018). Zhang et al. (2015) observed that the macromolecular substances produced undergo aggregation and sedimentation as the Maillard reaction progresses.
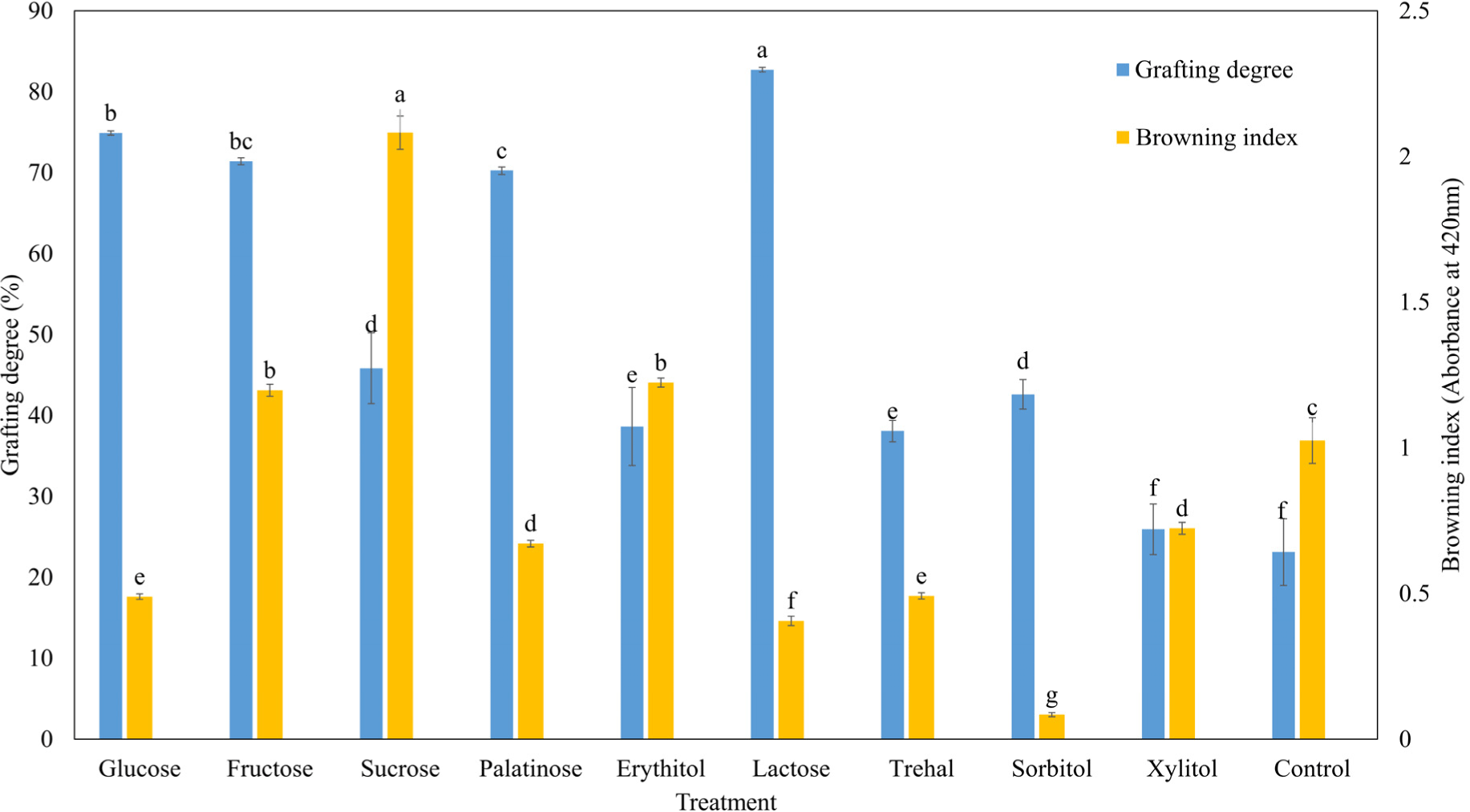
A significant difference in the browning intensity of the MP isolate-saccharide graft reactions with various saccharides was also observed (Fig. 1). The browning intensity of the MP isolate-saccharide graft reaction with fructose, sucrose, and erythitol was higher than that of the control (p<0.05). In general, the browning intensity confirms the progress of the Maillard reaction, and it increases with increasing temperature and time (Nooshkam et al., 2019). Zeng et al. (2017) demonstrated that the Maillard reaction is a complex network of chemical reactions between reducing sugars and amino groups of proteins. Naranjo et al. (1998) showed that the browning intensity is affected by the reducing sugar type, and fructose showed more browning intensity than glucose because fructose has more open chains than glucose. He et al. (2021) reported that high-grafting and low-browning reactions are important for protein-saccharide reactions.
In this study, the glucose, palatinose, and lactose treatments showed the highest grafting degree and lowest browning intensity. Although fructose had the highest grafting degree and browning intensity, it is not a suitable reducing sugar for grafting MP when focusing on the grafting degree and browning intensity.
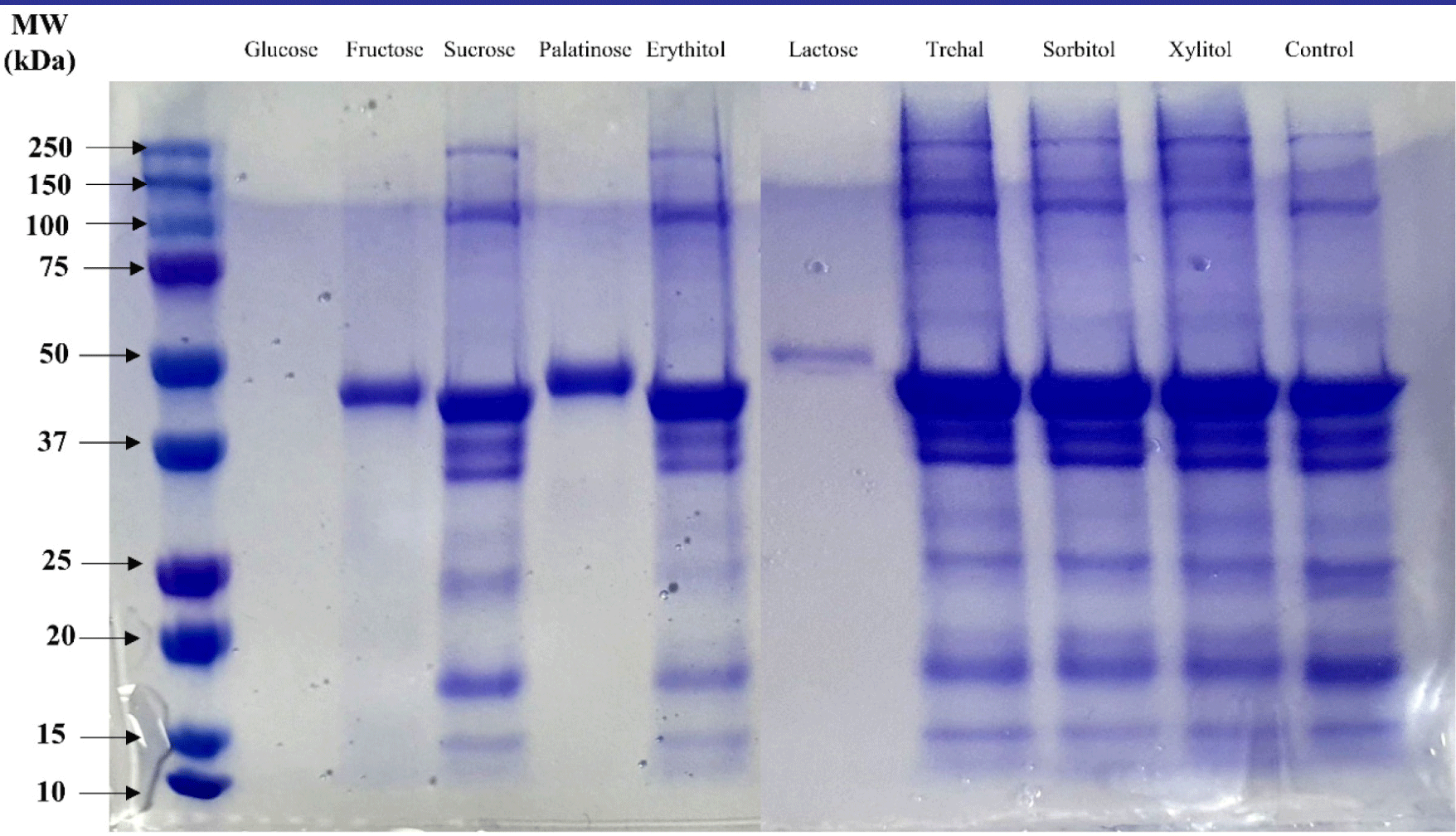
Fig. 2 shows the changes in the molecular-weight distribution of MP grafted with isolate-saccharides. MP that reacted with reducing sugars (glucose, fructose, palatinose, and lactose) had fainter bands than MP that reacted with non-reducing sugars (sucrose, erythitol, trehalose, sorbitol, and xylitol). In the reducing sugar treatments, only protein bands between 37 and 50 kDa appeared and protein bands that appeared in other treatments did not disappear. This result confirmed that myofibrillar protein conjugates with reducing sugars can be produced through the Maillard reaction. Xu et al. (2020) showed that conjugated myofibrillar protein with dextrin is due to covalent bonding between the protein and dextrin, and a single new band appeared after grafting. The carbonyl group of a reducing sugar can react with amino groups, and the Maillard reaction speed can be affected by sugar type (de Oliveira et al., 2016). As shown in Table 1, the highest grafting degree was observed for lactose, followed by dextrose and fructose, and the protein distribution of grafted myofibrillar protein differed by sugar type. These differences in the Maillard reaction speed could affect the occurrence and functionality of proteins in the grafted myofibrils.
Protein solubility is a major indicator of protein function. As higher protein solubility has higher functionality, estimation of protein solubility of myofibrillar proteins is important (Zayas, 2012). Fig. 3 shows the effect of various saccharides on the protein solubility of conjugated myofibrillar proteins. In this study, myofibrillar protein conjugated with glucose had higher protein solubility at all pH ranges (Fig. 3A). The general myofibrillar protein has an isoelectric point (pI) around pH 5, and protein solubility decreases around its pI (Zayas, 2012). In this study, there were no changes in the pI, except for the palatinose treatment. The occurrence of a pale, soft, and exudative meat (PSE) can disturb the manufacture of high-quality meat products. Because PSE can occur when the pH of meat is around the pI (pH 5), the pH of meat should be controlled (Barbut et al., 2008). Therefore, the use of palatinose to modify the MP structure improves the quality of meat products susceptible to PSE. When comparing the reducing sugar and non-reducing sugar groups, higher protein solubility was observed in the reducing sugar treatments, except for the lactose treatment. When the pH was changed, the changes in protein solubility increased more in the reducing sugar groups (Zayas, 2012). These results are explained by the increased hydrophilicity of the grafted saccharide and changes in the flexibility of the grafted protein (Mu et al., 2010). The decreased solubility of lactose is due to the highly conjugated myofibrillar protein. According to Li et al. (2019), soybean protein functionality increased with increasing Maillard reaction time (up to 5 h). However, protein functionality decreased when the Maillard reaction time was too long (over 6 h). Fig. 3 demonstrates changes in protein solubility due to ionic strength. Glucose treatment had the highest solubility at 0 M NaCl, and its solubility decreased slightly at 0.25 M NaCl concentration. The protein solubility of fructose and palatinose also increased compared to that in non-grafted treatment (control). This could be due to the increase in hydrophilic residues by reducing sugars (Xu et al., 2020). MP is a salt-soluble protein that has high solubility in saline solution and addition of salt is necessary to manufacture good-quality meat products. However, because of the high risk of sodium intake in chronic diseases, sodium intake or additional amount has to be reduced. In this respect, increased solubility of conjugated myofibrillar proteins at low sodium concentrations help to manufacture low-sodium meat products that are of good quality.
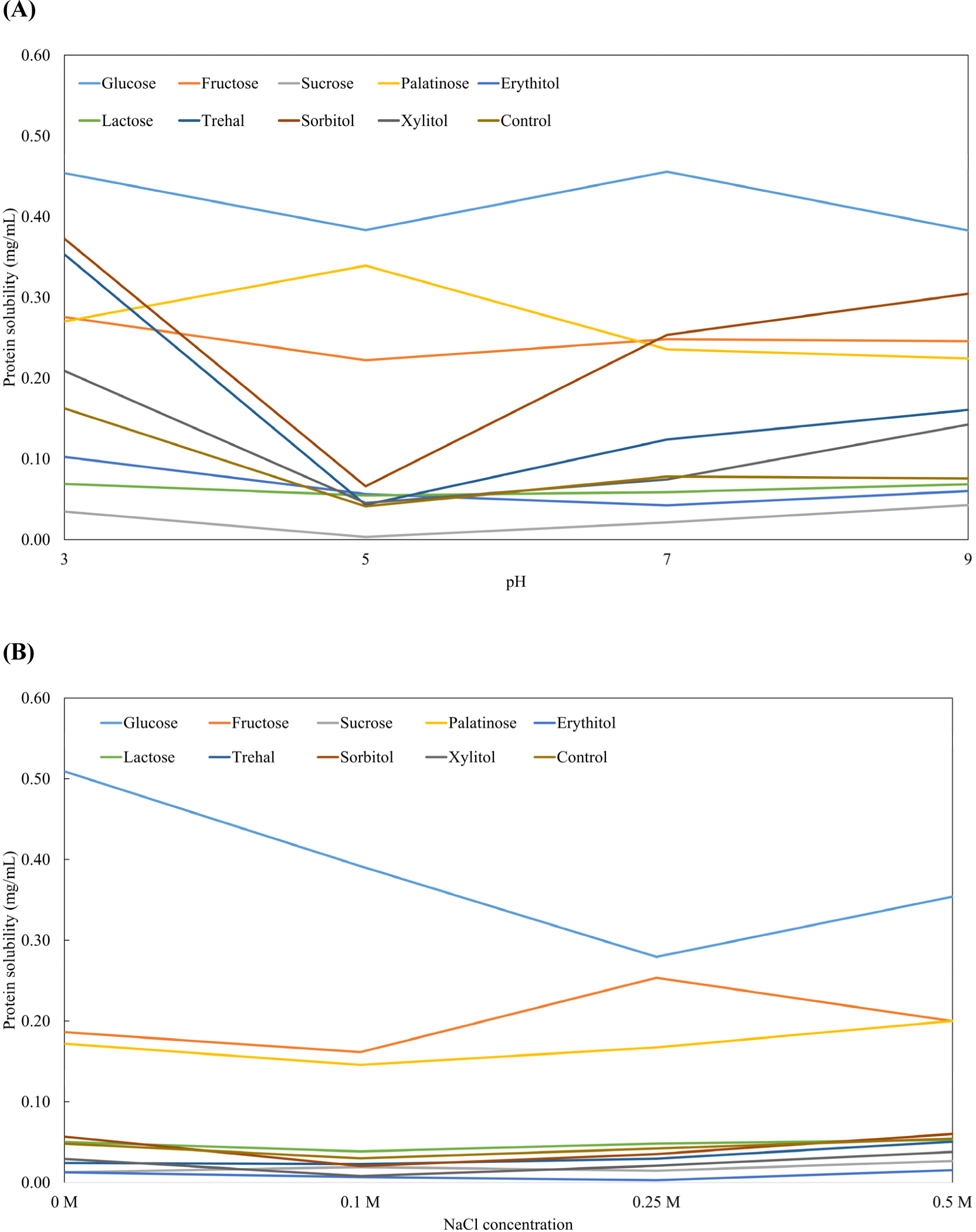
Table 1 shows the color of the MP isolate-saccharide graft reaction with the saccharides. The lightness of the MP isolate-saccharide graft reaction with glucose, fructose, palatinose, and lactose was lower than that of the control (p<0.05). The redness of the graft reaction with reducing sugars was higher (p<0.05), followed by that of fructose, palatinose, lactose, and glucose. The yellowness of the graft reaction with saccharides was higher than that of the control (p<0.05), except for the sucrose and erythitol treatments. The color difference of the graft reaction with saccharides was higher, followed by that of glucose, fructose, palatinose, and lactose. The color values and differences showed a similar tendency to the grafting degree of the graft reaction, indicating a higher level in the reducing sugar group (glucose, fructose, palatinose, and lactose). Starowicz and Zieliński (2019) demonstrated that color development was attributed to the Maillard reaction, due to the production of melanoidins (brown-colored high-molecular-weight compounds formed by the condensation of amines and carbonyls). Li et al. (2018) reported that the color difference of the Maillard reaction between proteins and saccharides markedly increased steps of Maillard reaction. Therefore, the degree of Maillard reaction can be deduced based on the change in color, consistent with our study findings.
WBC and OBC are important factors in emulsion-based foods because the meat emulsion system can collapse during heating with denaturation of proteins (Zayas, 2012). The increased hydrophilic residue by saccharides can increase WBC and the cooking yield of the final meat products (de Oliveira et al., 2016). Fig. 4 shows differences in WBC and OBC observed. Palatinose and lactose treatments had the highest values for WBC, fructose, sorbitol, and control treatments (p<0.05). Generally, the WBC count of conjugated myofibrillar proteins with reducing sugars increases (de Oliveira et al., 2016). However, the glucose treatment had the lowest WBC count (p<0.05), and myofibrillar protein conjugated with non-reducing sugars (sucrose, erythitol, sorbitol, and xylitol) had a higher WBC count (p<0.05). This could be due to the changes in the structural characteristics of the glucose treated group. Pore size between proteins is important for immobilizing the moisture content of meat proteins (Zayas, 2012). According to Fig. 2, the molecular weight of the glucose treatment was not observed. The highly cross-linked myofibrillar protein could not capture water molecules, and free water can be immersed after centrifugation (Hemung and Chin, 2015). Therefore, glucose treatment was not appropriate for manufacturing meat products with a high-cooking yield. There was no significant difference in OBC among the saccharide treatments (p>0.05), except for glucose. In conclusion, increasing hydrophilic residues by reducing sugar can increase WBC count, and the use of reducing sugar can enhance the WBC count of meat products.
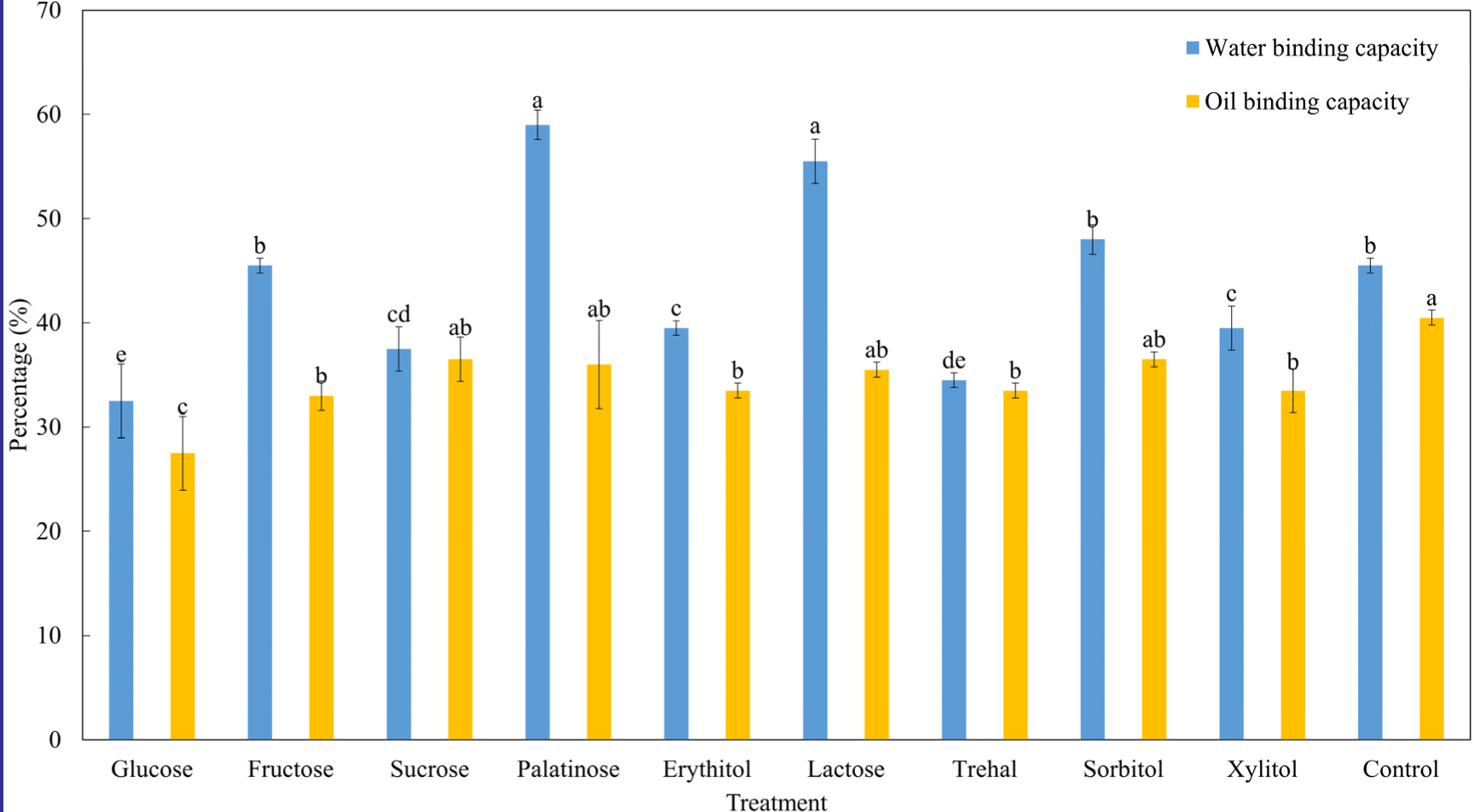
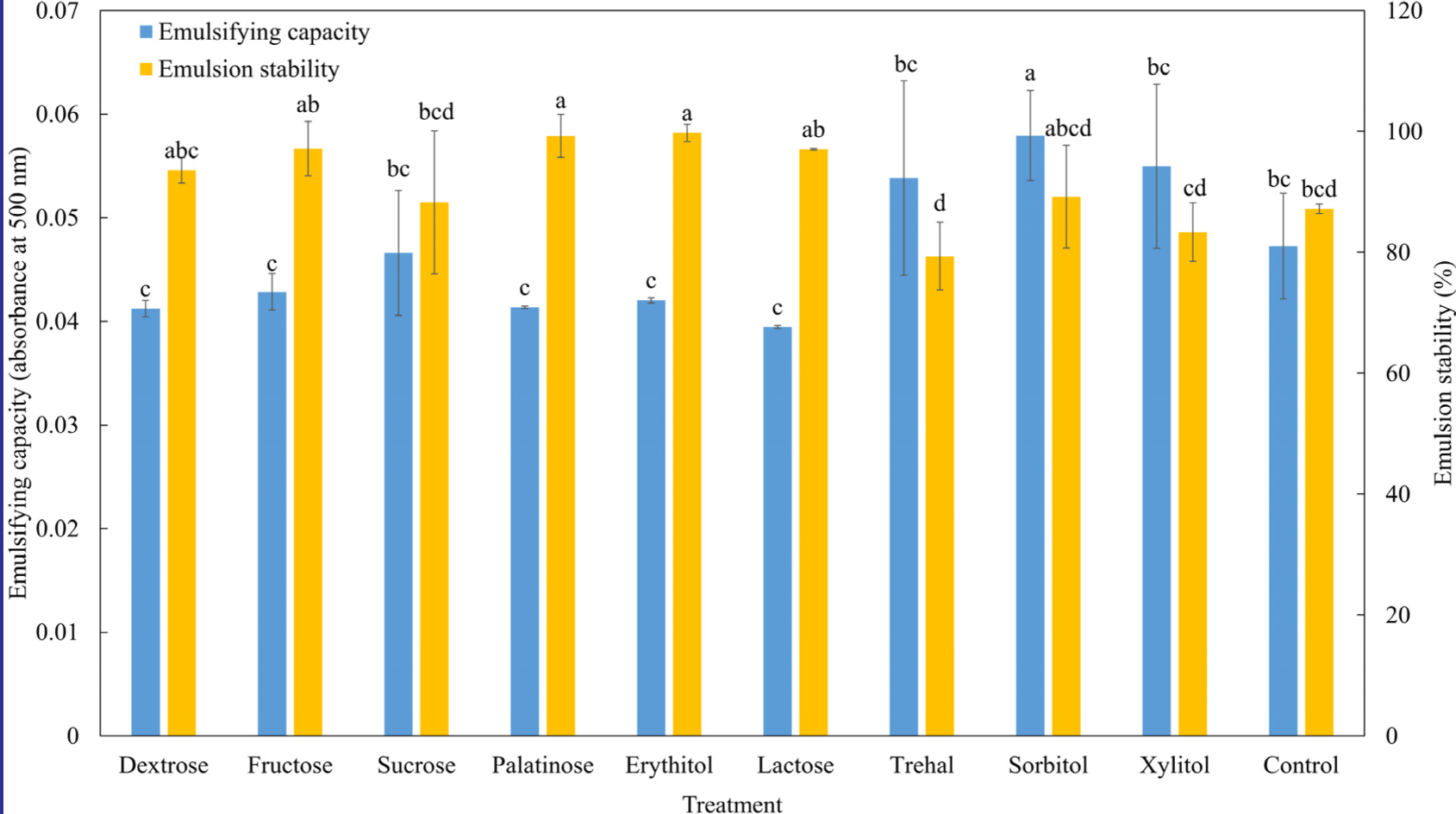
Fig. 5 illustrates the emulsifying properties (emulsification capacity and emulsion stability) of the MP isolate-saccharide graft reaction with saccharides. The emulsification capacity of the MP isolate-saccharide graft reaction with sorbitol was higher than that of the control (p<0.05), while the other MP isolate-saccharide graft reactions with saccharide treatment groups were similar to the control (p<0.05). The emulsion stability of the MP isolate-saccharide graft reaction with palatinose and erythitol was significantly higher than that of the control (p<0.05). Xu et al. (2020) showed that conjugated myofibrillar proteins and polysaccharides improve emulsification capacity and stability. However, the findings of this study was inconsistent with our results regarding the emulsification capacity and stability. Zayas (2012) reported that emulsifying capacity deteriorates as the balance between hydrophobicity and hydrophilicity collapses. Xu et al. (2020) demonstrated that covalent linking of hydrophilic groups can improve absorbability at the oil/water interface. Thus, sorbitol might be a suitable saccharide for improving the emulsifying properties of the MP isolate-saccharide graft reaction.
Conclusion
This study was aimed at estimating the effect of the quality of the MP isolate-saccharide graft reaction with various saccharides. The MP isolate-saccharide graft reaction has a high-grafting degree and low-browning intensity when grafted with reducing sugars. In addition, the graft reaction with reducing sugars improves the protein functionality. However, glucose treatment which had high grafting degree had low WBC. Therefore, excessive grafted protein might be not proper to enhance protein functionality. In this study, palatinose and lactose to improve water holding capacity and sorbitol to improve emulsification capacity. Thus, reducing sugars have a good protein functionality in the MP isolate-saccharides graft reaction. In addition, these enhanced characteristics might improve quality characteristics of low sodium level meat products.













