Introduction
Milk is made of milk fats, proteins, carbohydrates, water-soluble vitamins, minerals, and water. The whey protein and a casein exists as protein components. Casein is major component of milk proteins and it is widely studied because it has high availability and nutritional importance. It is about 3% in milk and it is about 80% of all protein in milk. Also, casein protein consisted of beta-casein fragments and molecular weight is about 75,000–375,000 kDa (Chio et al., 2011).
In recent years, calcium intake has increased, but its utilization in the body is low, leading to various deficiency diseases. Lack of calcium is the cause of various diseases such as poor bone growth, fracture, osteoporosis, hypercholesterolosis, arteriosclerosis, hypertension, and hyperlipidemia (Raisz and Smith, 1989; Zhao et al., 2005). In order to increase calcium intake, the use of calcium in the body should be higher than that of eating large amounts (Greger, 1988).
It has been reported that casein itself affects calcium absorption (Bronner, 1987), affecting immune system, so, it affects final immune response and cellular function (Coste and Tome, 1991; Kayser and Meisel, 1996).
Casein protein is characterized with a type of amino acid constituents. The use of casein hydrolysates that retain the bioactive amino acid domain or sequence may be a better alternative to prevent rancidity in foods without affecting food quality (Díaz and Decker, 2004). Also, the enzymatic hydrolysis of casein may act in a body as regulatory components with a hormone activity which can modulate specific physiological functions (Meisel and FitzGerald, 2003).
In vitro and in vivo enzymatic proteolysis of casein proteins produce bioactive peptides (Jelen and Lutz, 1998), which have specific biological functions throughout their ability to affect cellular function (Meisel, 1997). Because casein phosphopeptide (CPP) have a potential effects to deliver calcium as a functional ingredient (Kitts and Yuan, 1992). It is important to examine their immunological activity further. In response to this, 32 articles examining the role of hydrolyzed infant formulas in the prevention of allergies have been investigated (Hays, 2006).
Through this, if casein is hydrolyzed using proteolytic enzyme to develop a specific peptide similar with CPP, superior calcium absorption and anti-inflammatory effects will be expected.
In addition, researchers at the Department of Pediatric Gastrointestinal and Nutrition at Johns Hopkins of Medicine reported that infants fed with totally hydrolyzed casein and infants had a reduced incidence of atopic disease during the five years of life. Also, casein hydrolysates have been reported that it has a biological function on cellular function (Meisel, 1997), and it affects immune system cells (Coste and Tome, 1991; Kayser and Meisel, 1996).
Analysis of bioactive peptides has been made in several studies. These peptide sequences, encrypted within proteins, are liberated in vivo during gastrointestinal digestion or in vitro by fermentation with proteolytic starter cultures or using proteases. BAP generally comprises 2–20 amino acid residues (Poosapati et al., 2018).
In this regard, the objective of this study is development of a specific peptide which have a similar effect with CPP and researchinga of peptide which has excellent physiological properties and promotes calcium absorption.
Materials and Methods
Commercial casein (95% protein) obtained from Meggle food Ingredients (Wasserburg, Germany).
A 10% casein solution prepared by mixing 10% casein and 90% 0.1 M potassium phosphate buffer (pH 7.4). After a sterilization for 10 min at 90°C, a pH is adjusted to 7.0 which is optimum active pH of the enzyme with 1 N NaOH (Sigma, St. Louise, MO, USA).
Commercial enzymes used with Alcalase® 2.4 L FG (Protease from Bacillus licheniformis, 5 U/g), Neutrase® 0.8 L (Protease from Bacillus amyloliquefaciens, 0.8 U/g), Protamex® (Protease from Bacillus sp., 1.5 U/g) and Flavourzyme® (Protease from Aspergillus oryzae, 500 U/g). All commercial enzymes purchased from Novozymes™ (Bagsvaerd, Denmark).
The enzymes added at a ratio of 1:200 (w/v) based on the substrate, and shaken at 120 rpm in a shaking water bath at 50°C. The samples taken at 30 min and inactivate the enzymes at 90°C for 10 min. It preceded to 240 min. After a cooling at 20°C, the supernatant collected by centrifugation for 20 min under conditions of 4,000×g and 4°C. The supernatant was filtered with PVDF 0.22 Syringe membrane filter (Futecs, Daejeon, Korea). Samples stored at –20°C and used for each experiment.
The hydrolysis degree of casein hydrolysates measured using the Lowry method (Lowry, 1951). The Lowry solution A and B prepared for the degree of hydrolysis (DH). To prepare solution A, 1% cupric sulfate (Sigma) and 2% Sodium potassium tartrate (Sigma) mixed at a 1:1 ratio (v/v), and 1.0 mL of the mixed solution and 50 mL of 2% Na2CO3 (DaeJung Chemical & Metals, Siheung, Korea) were mixed. Solution B used to prepare a 1 N Folin-Ciocalteau reagent (Sigma).
First, 1.0 mL of casein hydrolysates and the same amount of 20% TCA (Duksan Chemical, Ansan, Korea) mixed and maintained for 1 h to precipitate 20% TCA soluble protein. It centrifuged at 11,000×g for 20 min. The supernatant and dissolved residue removed in 5.0 mL of 0.1 N NaOH (Sigma).
Thereafter, 1.0 mL of a casein hydrolysates and 5.0 mL of solution A mixed and reacted at room temperature for 10 min. After that, 0.5 mL of solution B added and the contents mixed immediately with vortex mixer. After reacting for 30 min, an absorbance measured at 600 nm. An equivalent amount of protein calculated from standard curve prepared using bovine serum albumin (Sigma). A bovine serum albumin measured in the range of 20 to 200 μg/mL.
The amount of TCA soluble protein measured and calculated by the following equation.
In order to obtain peptide fractions from casein hydrolysates, gel filtration throughout a preparative chromatography system performed (Waters, Milford, MA, USA).
Peptides that identified in SDS-PAGE separated on a preparative scale for further study. The preparative pump (Waters) and preparative liquid chromatography W600 (Waters) used with a Dual λ Absorbance detector W2487 (Waters) and W717 Autosampler (Waters).
The casein hydrolysates dissolved in 5 mM sodium phosphate buffer with 0.15 M NaCl (Sigma), pH 7.0. It filtered with a PVDF 0.45 μm sterile syringe membrane filter (Futecs). After that, casein hydrolysates is loaded 3.0 mL throughout a Hiprep 16/60 Sephacryl S-100 HR column (GE Healthcare Life Sciences, Marlborough, MA, USA) and eluted at flow rate of 1.0 mL/min. A detector was set at 280 nm.
All fractions obtained throughout a gel filtration with Hiprep 16/60 Sephacryl S-100 HR column on preparative chromatography system collected and fractions stored at –20°C (Table 1).
In the experiment of promoting calcium absorption, the method of Naito (1986) and Yamamoto et al. (1994) was slightly modified to measure the effect on precipitation in solution after calcium phosphate formation.
The 10 mM calcium chloride (Sigma) and 20 mM sodium phosphate buffer were prepared. After that, 0.5 mL of 10 mM calcium chloride and 0.5 mL of casein hydrolysate fraction samples mixed. And a 1.0 mL of 20 mM sodium phosphate buffer added. The mixed solution incubated at 37°C for 2 h and centrifuged at 2,000×g for 30 min at 25°C.
Calcium solubility measured using calcium colorimetric kit (Gene Tex, Pkwy Irvine, CA, USA) for the whole solution and the supernatant collected after centrifugation.
The whole solution and supernatant samples dispensed into each 10 μL 96 well plate, followed by mixing of chromogenic reagent to 90 μL and calcium assay buffer to 60 μL. After reacting for 5 min in dark room at room temperature, an absorbance measured at 570 nm.
Calcium concentration calculated according to the first equation below, and calcium solubility calculated according to the second equation below. All protein concentration of casein hydrolysate fraction samples were 200 μg/mL.
*Sa = Sample amount from standard curve
*Sv = Sample volume
RAW 264.7 cell is a macrophage cell of mouse. RAW 264.7 cells obtained from the Dankook University (Cheonan, Korea). Cells grown in Dulbecco’s Modified Eagle Medium (DMEM) including 10% heat-inactivated fetal bovine serum (FBS). The incubator temperature adjusted to 37°C and maintained at 5% CO2.
RAW 264.7 cells seeded in 96-well cell culture plates at a density of 5×104 cells/well and incubated for 16–18 h. After removing the medium, RAW 264.7 cells treated with various concentrations of fraction samples for 20–22 h.
After that, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra zolium bromide (Sigma) solution added to each well at a final concentration of 5 mg/mL and incubated (37°C, 5% CO2). After 2–3 h, culture supernatants removed, and 100 μL of dimethyl sulfoxide (Sigma) added to each well to completely and dissolve formazan crystals. The absorbance measured at 540 nm (Fotakis and Timbrell, 2006).
RAW 264.7 cells seeded in 96-well cell culture plates at a density of 5×104 cells/well and incubated for 16–18 h. The amount of nitric oxide (NO) calculated by measuring the amount of nitrite, an oxidized product, in the cell culture supernatants as previously explained. After removing the medium, RAW 264.7 cells treated with various concentrations of fraction samples in medium for 2–3 h.
After that, lipopolysaccharide (LPS) with final concentration of 100 ng/mL treated and stimulated at the same volume in medium for 20–22 h. A Griess reagent added to the supernatant in a ratio of 1:1 (v/v). The absorbance at 540 nm measured in a microplate reader after 15 min at room temperature (Lim, 2010).
RAW 264.7 cells seeded in 96-well plates at a concentration of 5×104 cells/mL for 24 h, preprocessed cell free extracts were prepared. After that, the supernatant taken and the cytokine content measured by a Mouse IL-1α, IL-6 and Mouse TNF-α ELISA kit (Komabiotech, Seoul, Korea) using an enzyme-linked immunosorbent assay (ELISA).
The 100 μL of fraction samples added to each well coated with specific antibodies against cytokines (IL-1α, IL-6, and TNF-α), reacted at room temperature for 2 h. And the supernatant removed and washed five times with washing buffer. Thereafter, detection antibody added to react with antibody. Also, streptavidin-horseradish peroxidase (HRP) conjugated with avidin added and reacted at room temperature for 30 min. After that, it washed for 4 times. A 100 μL of Tetramethylbenzidine (TMB) added to each well as a substrate. After that, it incubates at the room temperature as a proper color development. A stop solution added to each well and absorbance measured at 450 nm (Phelan et al., 2009).
Amino acid profiling preceded according to Waters amino acid analysis AccQ-Tag manual. A waters AccQ-Tag·Fluor reagent kit used for a derivatization of the sample and standard. An amino acid standard (Sigma) used as a standard. The range of sample amount is 0.02–0.08 μg (20–1,000 pmol). Others system condition shown in Table 2 which showed in below.
Results
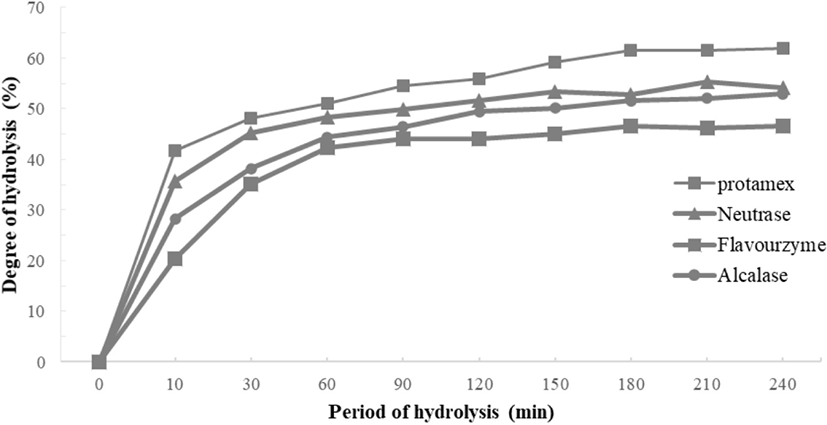
The degree of casein hydrolysis defined as the percentage of total number of peptide bonds in a protein that has been cleaved during hydrolysis (Adler-Nissen, 1986). The DH of the four enzymes tended to increase into a similar pattern. There was no significant difference from 150 min and after increasing from 120 min to 150 min. Among them, the highest DH of the casein hydrolyzed by Protamex® measured, and the DH of the casein hydrolyzed by Neutrase® and Flavourzyme® are similar (Fig. 1).
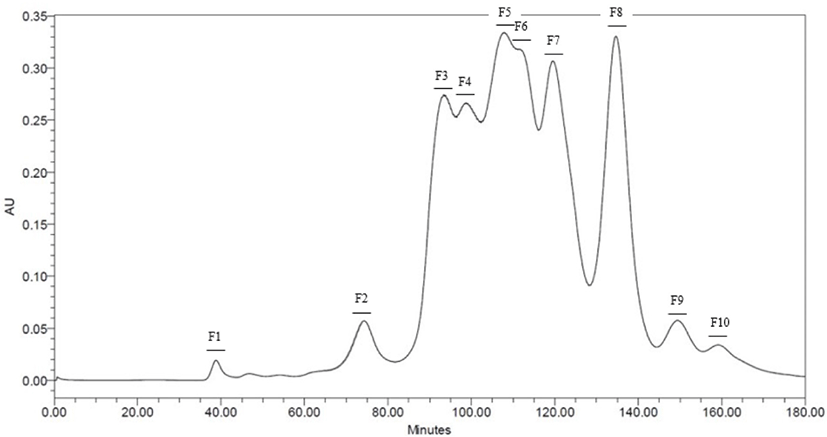
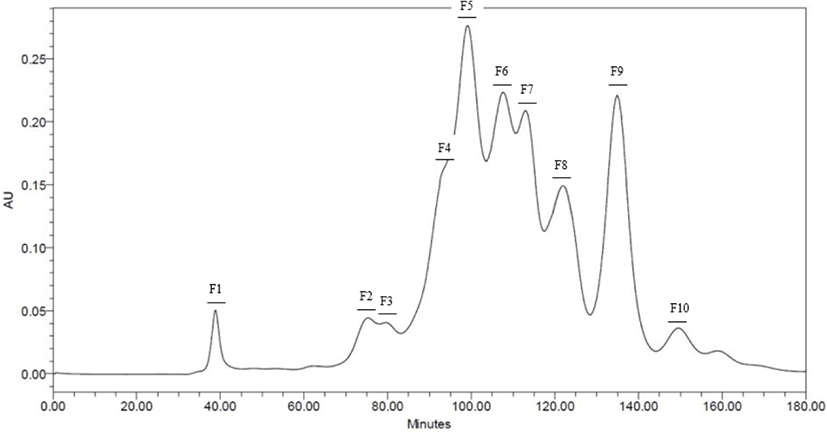
Neutrase® and Protamex® selected throughout the results of DH. Peptide fractions of casein hydrolysates isolated using Hiprep 16/60 Sephacryl S-100 HR column in preparative liquid chromatography system. Each fraction separated by molecular weight. 10 peptide fractions obtained totally. Each fraction compared to the original casein hydrolysates via SDS-PAGE for a molecular weight (Data are not shown) (Figs. 2 and 3).
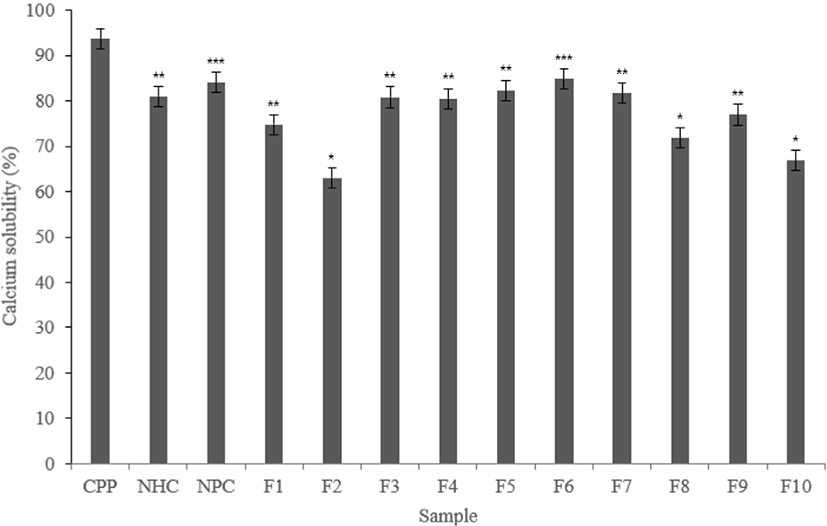
CPP contains phosphoserine and solubilizes calcium, which can promote calcium solubilization ability. In this study, calcium solubility measured to determine whether each fraction promote calcium solubilization ability similar with CPP.
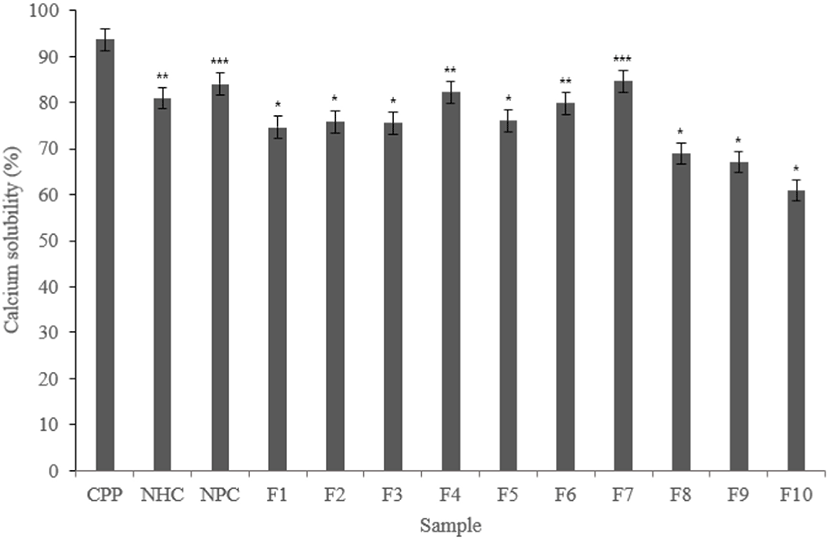
According to experimental results, CPP showed high calcium solubility with 93.67%. Phosphoserine contained in CPP solubilizes calcium, resulting in high calcium solubility.
Both Neutrase® and Protamex® hydrolysate fractions showed lower calcium solubility than CPP. Also, F4 and F7 in the Neutrase® fractions showed lower than CPP but about 80% calcium solubility. The F6 have a similar level with 79.84%. The Protamex® fractions showed lower calcium solubility than CPP, but F3, F4, F5, F6, and F7 showed calcium solubility of about 80% or more.
This may show the potential of calcium solubilization ability. Based on the results of the above experiments, fractions that showed good activity selected (Figs. 4 and 5).
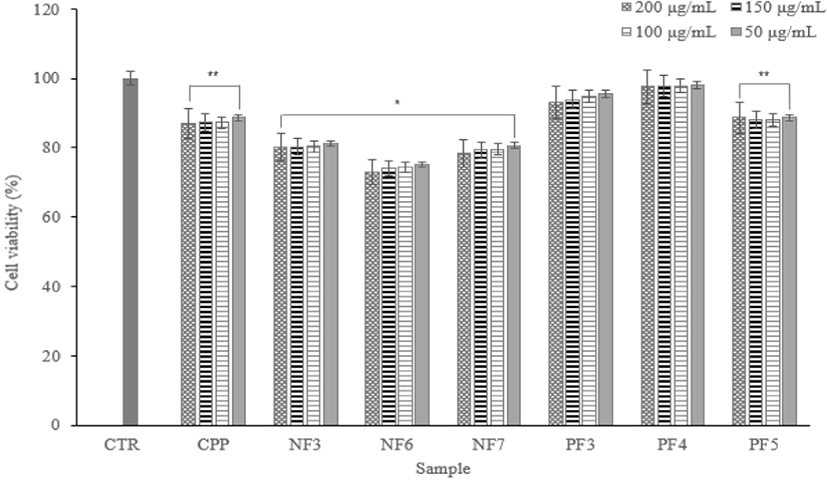
Cytotoxicity test via the MTT assay is used in vitro toxicology experiment widely. According to experimental results, despite the increase in concentration, there were no significant differences by concentration. Except for a NF6, there were no significance on concentration differences and cell death. In addition, it showed cell viability of 80% or more.
Therefore, it confirmed that there are no cytotoxicity of fraction samples on macrophage cells (Fig. 6).
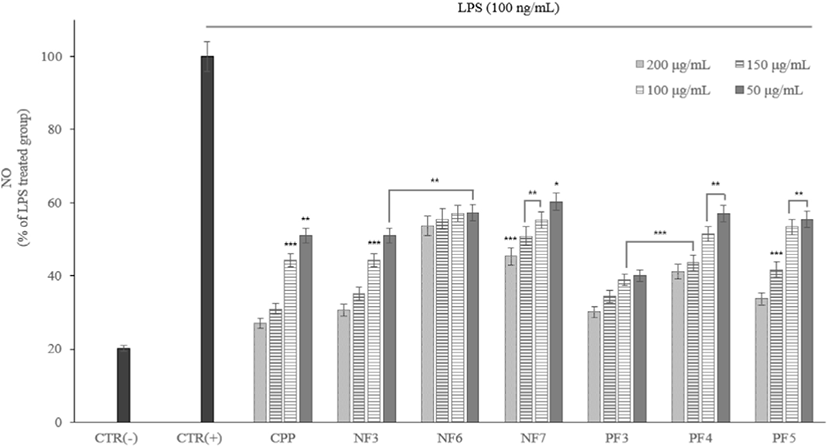
The NO effect an important role in a blood coagulation, blood pressure regulation and immune function against cancer cells. However, it oxidized like reactive oxygen species (ROS) and converted into active NO. It produces oxidants that cause cytotoxicity. The NO produced by cells exposed to inflammatory mediators increased in tissue damage or various inflammatory diseases.
According to the results of the study, as the concentration of fraction samples increased, NO production inhibited. The MTT experiments showed that inhibition of NO production not caused by cytotoxicity (Fig. 7).
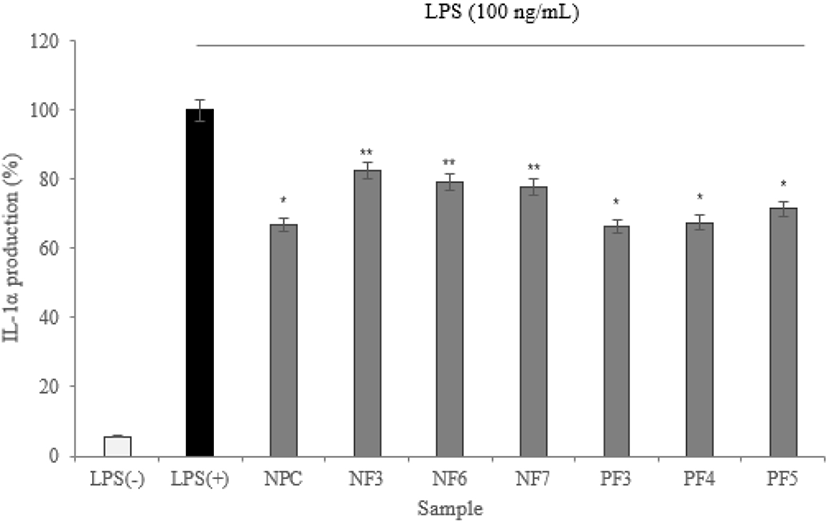
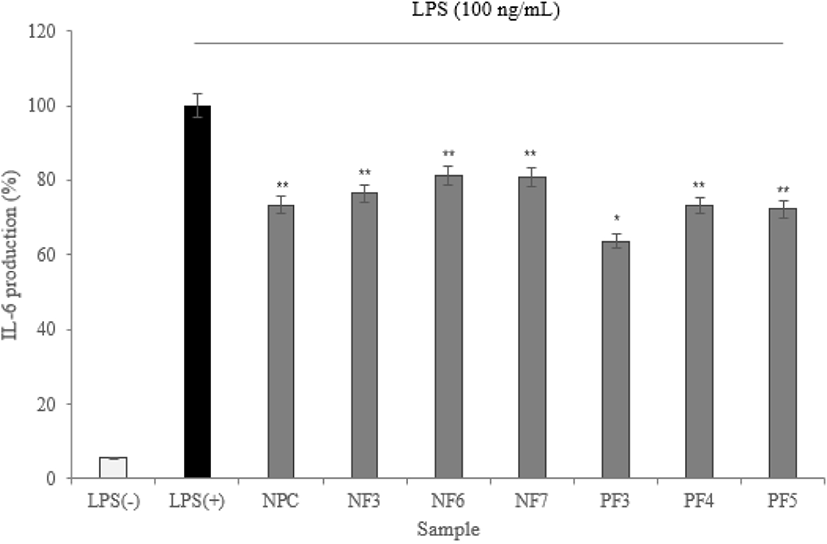
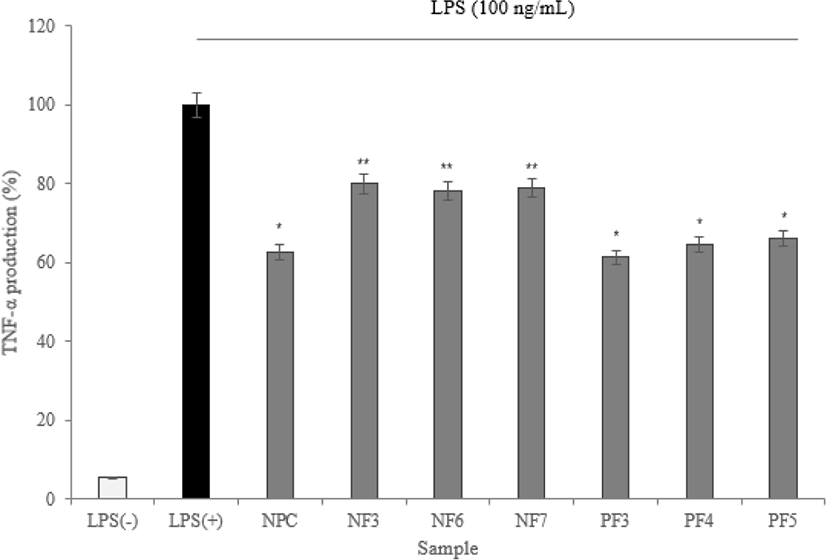
To maintain immune balance, they must be direct or indirect interactions of immune cells. The cytokines can induce proliferation, differentiation, changes in function and activity of various immune cells. A disease is mostly associated with inflammation, and inflammatory cells secrete inflammatory cytokines that induce inflammation (Barland et al., 2004). In this study, the expression levels of IL-1α, IL-6, and TNF-α measured.
According to the results of cytokine measuring using ELISA, three cytokine (IL-1α, IL-6, and TNF-α) production was significantly lower than LPS(+) group (* p<0.05). Based on results, the possibility of anti-inflammatory activity found in the hydrolysate fractions. In addition, the low expression level of TNF-α from macrophage effect by samples prevents the activation of proinflammatory cytokines which is produced by the expression of TNF-α. It reduces the inflammatory response (Figs. 8–10).
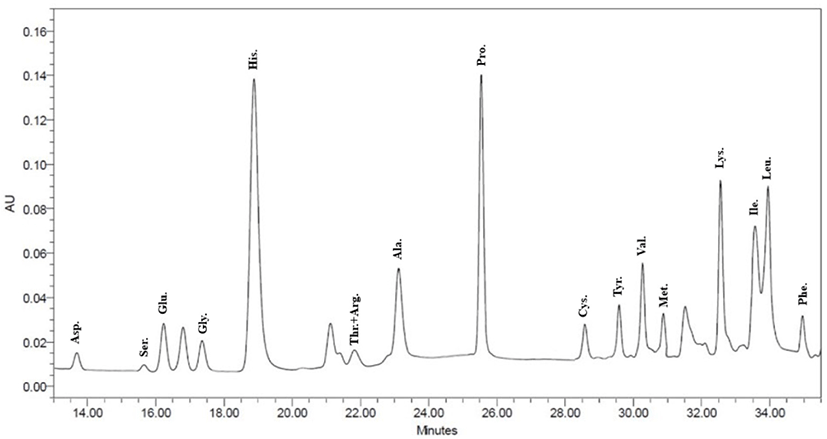
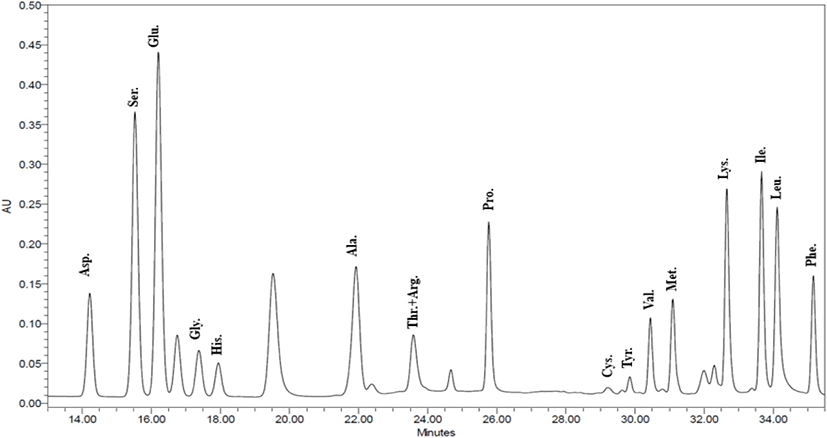
Based on the above experiment results, NF3 and PF3 selected. According to results of confirming amino acid sequence of the selected fractions throughout the AccQ-Tag system, 17 species of amino acids and several species of unknown amino acids identified (Figs. 11 and 12; Table 3). Both fractions had the highest content of phenylalanine and the lowest content of threonine and arginine.
Discussion
In this study, proteolytic enzymes used to focus on the function of specific peptides derived from the hydrolysis of a casein.
According to results, the DH of the four enzymes tended to increase in a similar pattern. There was no significant difference after increasing from 120 min to 150 min. DH of Protamex® found to increase most appropriately. All enzymatic hydrolysates maintained from 30 to 40 min and gradually increased after 50-60 min. This is similar to the previous report (Wang et al., 2013). In this study, the casein contained 95% of protein, and the retention time of hydrolysis was about 10 min.
In this study, fractions separated by molecular weight throughout the Hiprep 16/60 Sephacryl S-100 HR column. In another study, FPLC used either size exclusion or ion exchange chromatography (Kaminarides and Anifantakis, 1993). Chromatographic methods for peptide separation are very diverse, but the purpose of this study was to obtain peptides similar to CPP, so fractions separated using the column separated by molecular weight.
In the case of calcium solubility experiments, no fractions with calcium solubilization higher than CPP found. However, it has a similar level of calcium solubilization, which has confirmed the potential to the CPP. The calcium is solubilized by gastric acid, and most of it is absorbed in the small intestine. If excess phosphate ions present in the small intestine before absorption, calcium reacts to form of calcium phosphate and it is released into the body. Therefore, there is a need to have material capable of preventing such as a precipitation reaction, amino acids and peptides have been reported to have such properties from before (Naito et al., 1972).
Except for the NF6, there were no significance on concentration differences and cell death. In addition, it showed with cell viability of 80% or more.
According to results of the study, as the concentration of fraction samples increased, NO production inhibited. The MTT experiments showed that inhibition of NO and NO production was not caused by cytotoxicity. In previous study, there were results that hydrolyzed proteins significantly inhibited NO production in NO assay experiments comparing non-hydrolyzed protein and hydrolyzed protein (Mukhopadhya et al., 2014).
In addition, studies on the inhibitory ability of inflammatory cytokines of casein hydrolysates reported that it have high activities of Alcalase® hydrolysates (Mao et al., 2007).
According to results of confirming amino acid sequence of selected fractions throughout the AccQ-Tag system, 17 species of amino acids and several species of unknown amino acids identified. Both fractions had the highest content of phenylalanine. Except for the αs1-casein, there are common sequence feature and it is defined a N-terminal tyrosine residue. Also, it is absolutely essential for activities. Typically, a second aromaticamino acid residue, such as phenylalanine, is also present in the third or fourth position (Clare and Swaisgood, 2000).
Therefore, these protein fractions must be obtained and produced active peptides from them for use as dietary supplements and milk based nutraceuticals. This study identified the potential of new biologically active peptides derived from milk proteins that affect the food and healthcare industry.













