Introduction
The composition of goat milk protein is similar to that of breast milk and its casein fraction is mostly comprised of β-casein, followed by αs-casein (αs1- and αs2-casein) and κ-casein (Bernacka, 2011). Milk protein primarily contains two types of β-casein: A1 and A2 (Jianqun et al., 2016). Interestingly, β-casein in goat milk exists mainly as the A2 type (Cieślińska et al., 2012) and it does not produce β-casomorphin-7 (BCM-7), generated during the process of milk digestion, which may be related with various disorders, such as gastrointestinal disturbances (Ho et al., 2014). It is known that αs1-casein forms hard curds in the stomach, which might cause digestion problems in infants, but its concentration in goat milk is markedly lower than that in other milks (Bernacka, 2011; Yangilar, 2013).
Although goat milk contains β-lactoglobulin (β-lg), which is the primary cause of allergic reactions to this milk (Bossios et al., 2011; Cui et al., 2015), Jandal (1996) announced that goat milk has hypoallergenic properties.
Along with the nutritional benefits of goat milk protein, goat milk has more medium-chain triglycerides and smaller fat globules than cow milk, resulting in better digestibility (Haenlein, 2004; Park, 1994). These properties of goat milk can be exploited in functional foods for people with metabolic disorders as well as infants and the elderly (Alférez et al., 2001).
Research interest in goat milk is steadily increasing, with primary focus on the high digestibility and good hypoallergenic properties of goat milk in comparison to other types of milk (Espejo-Carpio et al., 2016; Tomotake et al., 2009). Therefore, the aim of this study was to prepare an A2 β-casein fraction from goat milk in which β-lg and αs-casein were selectively reduced by pH adjustment and calcium chloride precipitation. We investigated the optimal condition to isolate the A2 β-casein fraction from goat milk and evaluated its nutritional and physicochemical properties. The release of histamine and tumor necrosis factor alpha (TNF-α) from HMC-1 human mast cells exposed to A2 β-casein fraction was measured to evaluate its hypoallergenic properties.
Materials and Methods
Goat milk was obtained from Edam Co. Ltd. (Daejeon, Korea) and skimmed at 5,000 × g for 20 min (Labogene 1736R, Lynge, Denmark). After goat whole casein (GWC) was collected by pH adjustment to pH 4.4 using 1 M HCl to remove whey protein, it was dissolved in distilled water and adjusted up to pH 7.0. The optimal condition to selectively reduce the αs-casein content was investigated using the calcium chloride precipitation method: the GWC suspension was treated with calcium chloride at various concentrations (0.025 to 0.1 M) and for different incubation periods (15 to 60 min) at 25°C. The casein suspension was centrifuged at 10,000 × g for 30 min to collect the supernatant, which contains the A2 β-casein fraction and was freeze-dried using a freeze dryer (Ilshin, Korea). The purity of the A2 β-casein fraction were confirmed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and reverse-phase high-performance liquid chromatography (RP-HPLC) analysis.
The A2 β-casein fraction was resolved on a 12.5% acrylamide gel at 20 mA for 1 h using a Mini-Protean® Tetra System and PowerPacTM HV (Bio-Rad, USA), according to the method of Laemmli (1970). The gel was stained for 2 h with a coomassie blue solution (0.3 M coomassie blue G-250, 40% methanol, and 7% glacial acetic acid). Bands were analyzed using the Molecular Imager® GelDocTM XR plus Imaging system and the Image LabTM software 5.1 (Bio-Rad, USA).
RP-HPLC was performed as described by Bobe et al. (1998), with slight modification to the method. Acetonitrile and water were HPLC-grade, and all other reagents were analytical grade. Bovine whole casein (BWC) was purchased from Sigma-Aldrich (USA). Sample buffer (0.1 M Bis-Tris-HCl, 6 M guanidine hydrochloride, 19.5 mM dithiothreitol, and 5.37 mM sodium citrate, pH 6.8) was mixed with the A2 β-casein fraction or BWC, and the mixture was incubated for 1 h at 25°C and then centrifuged at 12,000 × g for 10 min using a Micro High Speed Refrigerated Centrifuge VS-15000CNF (Vision Scientific, Korea). The supernatant was filtered using a polyvinylidene difluoride syringe filter (pore size 0.22 µm; Woongki Science, Korea) and injected (20 µL) into the HPLC system (Waters, USA) comprised of a Binary HPLC Pump 1525 (Waters, USA), a sample injector, and an absorbance detector. A silica-based C18 RP-HPLC column (250 mm length × 4.6 mm i.d., 5.0 µm; Waters, USA) was used for protein separation with solvents A and B at a flow rate of 1 mL/min. Solvent A and B were composed of 10% and 90% acetonitrile with 0.1% trifluoroacetic acid in HPLC-grade water, respectively. The absorbance was measured at 220 nm using a Photodiode Array Detector 2996 (Waters, USA). The solvent gradient program started at 27% of solvent B and was retained for 5 min after sample injection, followed by increasing proportions of solvent B at 0.5%/min (for 10 min), 0.33%/min (for 3 min), 0.5%/min (for 11 min), 0.25%/min (for 2 min), 0%/min (for 3 min), 0.5%/min (for 2 min), 0.56%/min (for 9 min), and then, the proportion of solvent B was increased to 100%. Before the next sample was injected, the column was maintained under the initial condition for 10 min.
One milligram of A2 β-casein fraction was labeled with phenyl isothiocyanate by the Pico-tag method to determine the general amino acid composition. The labeled sample was mixed in 400 µL of buffer and 10 µL of the mixture was analyzed by RP-HPLC. A Pico-tag column (300 mm length × 3.9 mm, 4.0 µm; Waters, USA) was used with solvents A and B at a flow rate of 1 mL/min. Solvent A consisted of 140 mM sodium acetate with 6% acetonitrile and solvent B comprised 60% acetonitrile in HPLC-grade water. The absorbance was measured at 254 nm using a 2487 UV detector (Waters, USA). The initial concentration of solvent B of 14% was maintained for 9 min after sample injection, followed by increasing percentages of solvent B at 0.5%/s (for 0.2 min), 3.13%/min (for 8.3 min), and 4.5%/s (for 0.2 min), and finally, the proportion of solvent B was decreased to 0%.
The allergenic properties of A2 β-casein fraction were investigated using the method described by Miyazaki et al. (2005), with a slight modification. The HMC-1 cells were supplied by the Pathophysiology Laboratory, Sahmyook University (Korea). The HMC-1 cells were grown in Dulbecco’s modified Eagle’s medium (Cellgro, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) and 1% penicillin/streptomycin (Gibco, USA) at 37°C with 5% CO2 in a humidified atmosphere. The HMC-1 cells (1×105 cells/well) were cultured in a 24-well plate overnight, followed by addition of 100 μL of reagents or samples: the control (without protein), 10 μg/mL of compound 48/80 (C48/80; Sigma-Aldrich, USA) as a positive control, and 100 μg/mL of the A2 β-casein fraction, goat whey protein (GWP), ovalbumin (Sigma-Aldrich, USA), or soy protein (Esfood, Korea), respectively. After the cells were incubated for 24 h, the culture supernatant was collected to measure secreted histamine and TNF-α using the histamine ELISA kit (IBL International, Germany) and human TNF-α ELISA Ready-SET-Go® Kit (eBioscience, USA), respectively, according to the manufacturers’ protocols. The absorbance was read at 450 nm using a microplate reader (Molecular Devices, USA).
Protein solubility was measured by the method of Bera and Mukherjee (1989), with slight modification to the method. After 1 g of A2 β-casein fraction or BWC was suspended in 100 mL of distilled water, the pH of the suspensions was adjusted to pH 2, 4, 6, 8, or 10 using 1 M HCl or 1 M NaOH. The suspensions were stirred for 1 h at 25°C and then centrifuged at 12,000 × g for 20 min. The protein content in the supernatants was determined by the Quick StartTM Bradford 1×Dye Reagent (Bio-Rad, USA) and the protein solubility was calculated as follows:
protein solubility (%) = (protein content of supernatant at designated pH/total protein content of sample) × 100
Results
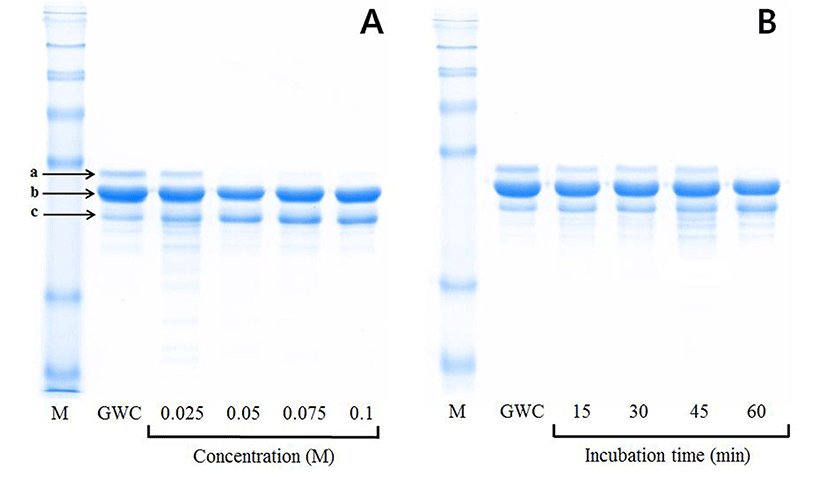
The optimal conditions to selectively decrease the β-lg and αs-casein contents in the final product, the A2 β-casein fraction, were examined. Firstly, whey protein including β-lg was completely removed from goat skim milk using pH adjustment to pH 4.4, followed by calcium chloride precipitation. As shown in Fig. 1, the amount of αs-casein among three major goat caseins (αs-casein, β-casein, and κ-casein) was selectively reduced in the A2 β-casein fraction, depending on increasing calcium chloride concentration and incubation time. Degradation of αs-casein showed no significant difference when calcium chloride was added at a concentration higher than 0.05 M and the mixture was incubated for longer than 1 h at 25°C. The A2 β-casein fraction was the most effectively prepared by treatment with 0.05 M calcium chloride for 1 h at 25°C.
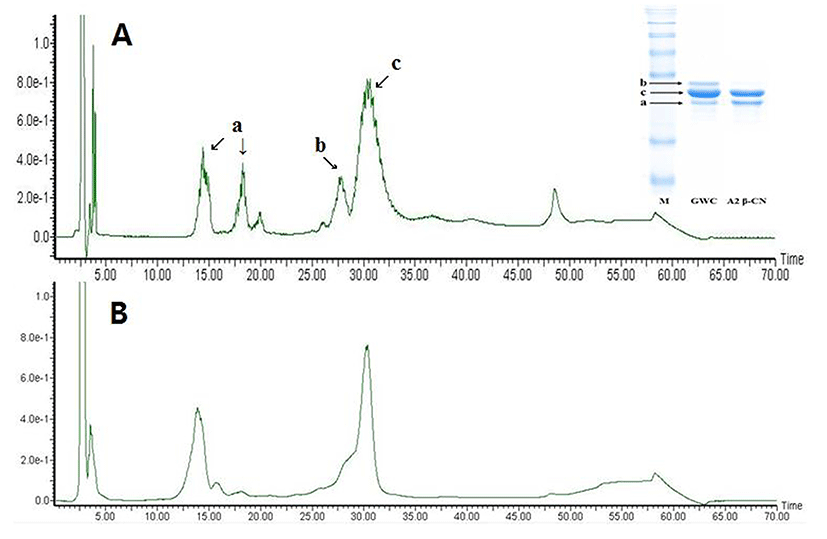
Selective degradation of αs-casein in the A2 β-casein fraction was confirmed by SDS-PAGE and RP-HPLC (Fig. 2). The αs-casein was effectively reduced in the A2 β-casein fraction compared to GWC. The proportions of αs-casein and β-casein in A2 β-casein fraction changed from 19.5% to 1.9% and from 52.9% to 63.8%, respectively.
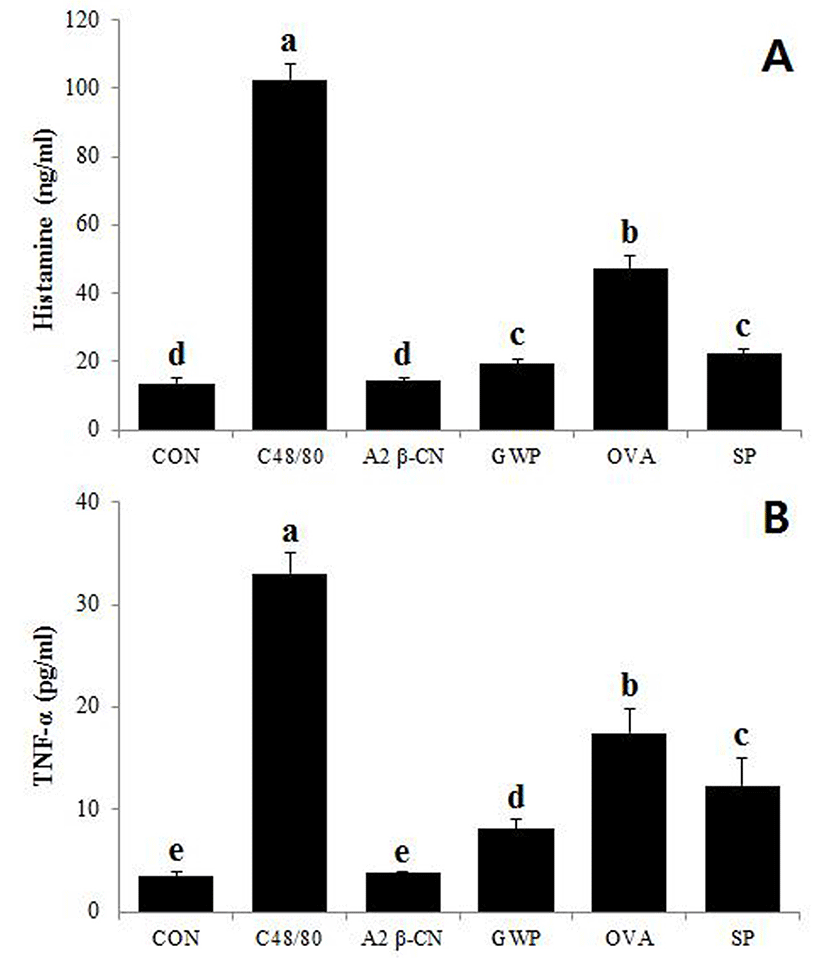
The HMC-1 cells were cultured with the A2 β-casein fraction or other protein samples (GWP, ovalbumin, soy protein) to measure the released contents of histamine and TNF-α. The C48/80 was included as a positive control. As shown in Fig. 3, addition of C48/80, GWP, ovalbumin, or soy protein significantly (p<0.01) induced the release of histamine or TNF-α in comparison to the control, while addition of the A2 β-casein fraction did not stimulate their release.
The percentages of essential and non-essential amino acids in the A2 β-casein fraction were 52.79% and 47.21%, respectively (Table 1). The A2 β-casein fraction was rich in leucine (11.91%) and valine (9.83%) among essential amino acids, and in proline (17.71%) and glutamine (9.83%) among non-essential amino acids.
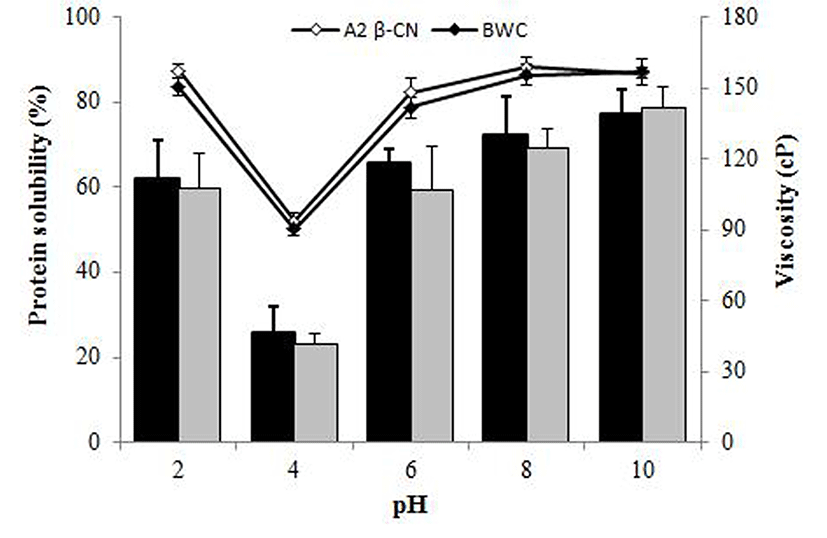
The protein solubility and viscosity of A2 β-casein fraction and BWC were determined in the pH range of 2 to 10 (Fig. 4). No significant differences were observed at any pH. The protein solubility of the A2 β-casein fraction and BWC was 61.9% and 59.7%, respectively, at pH 2 and decreased to 25.6% and 23.1%, respectively, at pH 4. Their solubility gradually increased up to 77.3% and 78.6% above pH 4, respectively. The viscosity of the A2 β-casein fraction and BWC was the lowest at pH 4.0 and increased from 93.7 cP to 158.7 cP and 90.3 cP to 157.0 cP, respectively, at alkaline pH.
Discussion
In general, β-casein exists as A1 or A2 type in milk, and both types differ in amino acid sequence. The A1 type contains histidine at position 67 of the β-casein amino acid sequence, whereas A2 type has a proline at this position (Farrell et al., 2004). A few studies have reported that BCM-7 (f60-66 of β-casein: Tyr-Pro-Phe-Pro-Gly-Pro-Ile) which is generated by proteolytic digestion of A1 type β-casein, might cause various disorders, such as gastrointestinal problems (Ho et al., 2014). However, goat β-casein is mainly composed of A2 type, and thus, the A2 β-casein fraction prepared in this study may be considered as a safe food material.
Milk αs-casein is comprised of αs1- and αs2-casein. The goat milk contains mainly αs2-casein and a small amount of αs1-casein (Park et al., 2007). During the digestion process, αs1-casein may interfere with digestion by being curdled in the stomach (Tomotake et al., 2006). In contrast, αs2-casein hardly forms solid curds. Espejo-Carpio et al. (2016) reported that goat milk has hypoallergenic properties despite the fact that it contains β-lg which may cause allergic reactions. For these reasons, goat milk is believed to be more digestible and has lower allergenicity than other types of milk.
In this study, to maximize the utilization of goat milk protein, we investigated optimal conditions to selectively reduce the amounts of both αs-casein and β-lg in goat milk protein. After casein was separated from whey to remove β-lg by pH adjustment, calcium chloride precipitation method was used to selectively reduce αs-casein in GWC. The degradation of αs-casein in GWC was the most effective when the GWC suspension was treated with 0.05 M calcium chloride at 25°C for 1 h, as verified by SDS-PAGE and RP-HPLC analyses.
Previously, Murphy and Fox (1991) isolated a β-caseinenriched fraction from milk by adjusting temperature and twice ultrafiltration, which might be time-consuming and costly for commercial application. However, the procedure developed in the current study is more efficient than other methods.
Allergic reaction to the A2 β-casein fraction was investigated by measuring the release of histamine and TNF-α from HMC-1 cells. In general, allergens activate Th2 lymphocytes through Langerhans cells, resulting in production of immunoglobulin E (IgE) (Novak and Bieber, 2005). The IgE binds to high-affinity IgE receptor on mast cells, causing the release of histamine and TNF-α (Je et al., 2013). While the secretion of both inflammatory indicators significantly increased when the cells were treated with C48/80, GWP, ovalbumin, or soy protein, the A2 β-casein fraction did not stimulate their release in comparison to the control. Allergic reaction induced by GWP was supposed to be due to the existence of β-lg. Cordle (2004) and Zhang et al. (2015) reported that ovalbumin and soy protein also induce the release of histamine and TNF-α.
The A2 β-casein fraction contains a substantial amount of essential amino acids (52.79%). In particular, it is abundant in leucine (11.91%), valine (9.83%), and isoleucine (6.48%). These amino acids are known as branched-chain amino acids, which improve not only insulin resistance and hypoalbuminemia (Kawaguchi et al., 2009; Muto et al., 2005) but also protein synthesis and recovery of patients (Wang et al., 2003).
The physiochemical properties, including protein solubility and viscosity, of the A2 β-casein fraction were investigated in comparison with those of BWC, which is widely used as a protein material in various foods. The solubility of A2 β-casein fraction and BWC was the lowest at pH 4, the near isoelectric point of casein, and it gradually increased both below and above pH 4, similar to the results reported by Gani et al. (2015). The viscosity of both samples showed a similar tendency and was considered to be affected by the net charge of casein depending on the pH. Moreover, emulsifying activity index and emulsifying stability index of each sample changed according to variation in protein solubility (data not shown), similar to the results reported by Raikos et al. (2014), and Lee et al. (2006).
Conclusions
An A2 β-casein fraction with effectively reduced αs-casein and β-lg contents was prepared from goat milk by adjusting the pH and treating GWC suspension with 0.05 M calcium chloride for 1 h at 25°C; this was confirmed by SDS-PAGE and RP-HPLC. The A2 β-casein fraction did not stimulate histamine and TNF-α release from HMC-1 cells, and it has abundant essential amino acids, including branched-chain amino acids (leucine, valine, and isoleucine), which can improve nutrition and health. Moreover, the A2 β-casein fraction can be considered as a substitute for BWC (widely used as a protein source for various foods) because the physicochemical properties of the A2 β-casein fraction were proven to be similar to those of BWC. These results indicate that the A2 β-casein fraction not only has improved digestibility and hypoallergenic properties, but also may have potential as a functional food material.