Introduction
The use of barbecues or grilling has become increasingly popular in recent years in Korea. High-temperature conditions and the use of charcoal during grilling substantially improve flavor. However, undesirable substances, such as polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines, are produced during charcoal-grilling (Wang et al., 2019). PAHs are a large group of persistent organic compounds containing two or more fused aromatic rings (Gong et al., 2018), which are considered potentially carcinogenic in humans because of their genotoxic properties (Mejborn et al., 2019). Although the mechanisms through which PAHs form in meats during grilling are not understood fully, some studies have reported three mechanisms of PAH formation in grilled meats, as follows: (i) the pyrolysis of organic compounds or fats in meat creates a layer on the meat containing PAHs; (ii) the incomplete combustion of coals produces smoke, which attaches to the surface of meat; and (iii) melting fats drip over the burning charcoal, causing the formation of PAHs that return to the meat via smoke (García-Lomillo et al., 2017; Viegas et al., 2014; Wang et al., 2019). Additionally, various factors affect the formation of PAHs in meat, including cooking temperature, time, and fat content (Sahin et al., 2020). In particular, high doneness and fat contents in meat can increase the PAH contents in grilled meats (Kim et al., 2021). Based on concerns regarding the types of PAHs generated in grilled meat, the United States Environmental Protection Agency listed 16 PAHs with carcinogenic and mutagenic effects, namely, naphthalene, acenaphthene, acenaphthylene, fluorene, anthracene, phenanthrene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, benzo[g,h,i]perylene, and indeno[1,2,3-cd]pyrene (Gong et al., 2018). Although these PAHs are present in charcoal-grilled meat at low levels, frequent consumption of charcoal-grilled meats over a long period of time can be harmful to human health. Therefore, it is important to control the formation of these 16 PAHs in grilled meats.
Various studies have evaluated methods to inhibit PAH formation in meats during cooking (Farhadian et al., 2012; Gibis, 2007; Park et al., 2017). Cooking at lower temperatures and for shorter times can inhibit PAH formation in meat (Farhadian et al., 2012). Additionally, preventing melted fat from meats from dripping onto the heat source reduces benzo[a]pyrene contents in grilled pork belly (Park et al., 2017). In addition, marinating with ingredients with antioxidant properties can reduce PAHs formation during the cooking process (Gibis, 2007). Marinating, a traditional cooking technique, is performed to improve the flavor and tenderness of meat in order to satisfy consumer demand (Fasano et al., 2016). Marinating meats with natural antioxidants, such as polyphenols and sulfhydryl compounds in beer, tea, onion, garlic, and lemon, has been reported to reduce the concentrations of PAHs in meats (Viegas et al., 2014; Wang et al., 2019).
Gochujang, Korean fermented red-pepper paste, is traditionally composed of red pepper, grains (e.g., barley, rice, and/or wheat), and soybean Meju with water (Kwon et al., 2015). Recent studies have reported that Gochujang exhibits bioactivities, including anti-atherosclerotic, anti-obesity, and anticholesterol effects (Kim et al., 2019a; Shin et al., 2016; Yang et al., 2018). These bioactivities are thought to be related to the various biological compounds of Gochujang, including polyphenol compounds Meju and capsaicin derivatives in red pepper (Reyes-Escogido et al., 2011; Yang et al., 2018). Koreans enjoy Gochujang as a seasoning for charcoal-grilled pork belly and typically eat pork belly with dipping in Gochujang or cook pork belly on the grill after marinating with Gochujang (Kwon et al., 2015). According to Chung et al. (2009), Gochujang contained high phenolic acids compared to other phenol contents, which showed greater PAH inhibitory effect than other phenolic compounds, such as flavonoids (Wang et al., 2019). So, Gochujang can be used as effective marinades for inhibiting PAH formation in grilled meat. However, the effects of Gochujang marinade on PAHs formation during charcoal-grilling have not been reported.
Therefore, in this study, we evaluated the effects of Gochujang marinade on inhibition of 16 PAHs in charcoal-grilled pork belly cooked to different levels of doneness.
Materials and Methods
Gochujang (Sunchang Gochujang; Chungjungone, Seoul, Korea) used in this study was composed of brown rice (20.4%), red pepper powder (3%), and red pepper seasoning (red pepper powder [8.3%], sea salt, garlic, and onion) with soybeans, alcohol, yeast powder, starch syrup, brown glutinous rice flour, sea salt, and isomaltooligosaccharide. Folin-Ciocalteu phenol reagent was purchased from Sigma-Aldrich (St. Louis, MO, USA). All solvents and chemicals used for PAH analysis were high-performance liquid chromatography grade. PAH standards (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[a,h]anthracene, and benzo[g,h,i]perylene) and internal standard (ISTD) solution mix (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) were purchased from Sigma-Aldrich. All the other chemicals were of analytical grade.
The phenol content was measured using Folin-Ciocalteu colorimetric assays, as described by Singleton et al. (1999). The Gochujang was diluted in methanol, and the diluted sample (0.5 mL) was mixed with distilled water (5 mL) and Folin-Ciocalteu phenol reagent (0.5 mL). The mixed solution was incubated for 3 min at 25°C. Subsequently, 1 mL of 1 N Na2CO3 was added, and the solution was incubated for 90 min at 25°C in the dark. The absorbance of the reactant was measured at 760 nm using a spectrophotometer (Molecular Devices, San Jose, CA, USA). The standard curve was established using gallic acid, and the results were expressed as mg gallic acid equivalent (GAE)/g.
ORAC assays were performed according to the method of Kim et al. (2019b). First, 25 μL diluted sample was mixed with 150 μL of 80 nM fluorescein and incubated for 15 min at 37°C. After incubation, to generate peroxyl radicals, 25 μL of 150 mM 2,2’-azobis (2-amidinopropane) hydrochloride was added. The change in the absorbance of the reactant was recorded every minute at excitation (480 nm) and emission (520 nm) wavelengths, at 37°C for 60 min using a spectrophotometer (Molecular Devices). Trolox (Sigma-Aldrich) was used to generate a standard curve, and the results were expressed as mmol trolox equivalent (TE)/g.
The ABTS radical-scavenging activity was evaluated as described by Kim et al. (2019b). To generate the ABTS+ radical, a 14 mM ABTS+ and a 4.9 mM potassium persulfate solution were mixed (1:1, v/v), and the resulting solution was then reacted at 23±1°C for 12 h in the dark. The stock solution was diluted to reach an absorbance of 0.700±0.002 at 735 nm and 30°C. Next, 50 μL sample was reacted with the ABTS+ radical solution (950 μL) for 30 min at 30°C in the dark. The absorbance of the solution was determined at 735 nm. The results were expressed as mmol TE/g.
FRAP assays were carried out according to the method of Kim et al. (2019b). The FRAP working solution was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-S-triazine in 40 mM HCl, and 20 mM FeCl3·6H2O solution at a ratio of 10:1:1 (v/v/v), respectively. Each sample (25 μL) was reacted with the FRAP working solution (175 μL) for 30 min at 37°C in the dark. The absorbance of the reaction solution was determined at 590 nm. The results were expressed as mmol TE/g.
The DPPH radical scavenging activity was determined following the method of Kim and Jang (2021). Each sample (100 μL) was reacted with 0.2 mM DPPH solution (100 μL) in a 96-well microplate. The mixtures were incubated at 25°C for 30 min in the dark, and absorbance was measured at 517 nm. The results were expressed as mmol TE/g.
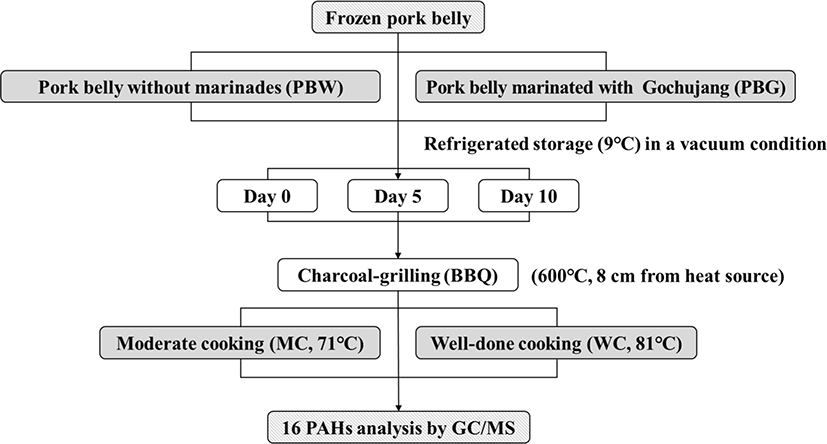
The preparation of PBW and PBG is detailed in Fig. 1. Frozen pork belly (LYD) and Gochujang were purchased from a local supermarket in Korea. The pork belly was cut into cubes measuring 10 cm (length)×5 cm (width)×0.4 cm (thickness). The pork belly was marinated under the following conditions: 71.94% pork belly, 21.58% Gochujang, and 6.48% water, according to previous sensory evaluation and PAHs analysis (data not shown). PBW and PBG were placed into a polyester bag to ripen under vacuum conditions for 24 h at 5°C. The ripened PBW and PBG were stored under vacuum conditions at 9±2°C for 10 days and then used for analysis on days 0, 5, and 10.
Approximately 1 kg charcoal was placed into a garden-type grill (55 cm width, 34 cm length, and 14 cm height; Allcook, Korea). After ignition, grilling was performed over the charcoal at 600°C and at a distance of 8 cm from the heat source. According to internal temperature, doneness of grilled pork belly divided into two; the total grilling times were 3.5 min for moderate cooking (internal temperature: 71°C; MC) and 5 min for well-done cooking (internal temperature: 81°C; WC). The internal temperature used in this study fitted on safe temperature for cooking pork meat by USDA guideline (Jang et al., 2019). No oil was applied to the meat surface, and the meat was turned once during cooking.
PAH analysis was carried out as described by Kim et al. (2021). Pork belly samples (2.5 g) were weighed in conical tubes, and 5 mL ethyl acetate/acetonitrile (20:80, v/v) with ISTD mix (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12, 400 ng/mL) was added. The samples were ultrasonicated for 20 min, and after centrifugation (3,000×g, 7 min), the supernatants were transferred to new conical tubes. Next, 5 mL ethyl acetate/acetonitrile (20:80, v/v) was added to the remaining pellets, and samples were ultrasonicated for 20 min. The combined supernatants were concentrated to 2 mL under vacuum conditions using a rotary evaporator (Scilab Korea, Seoul, Korea), and 0.5 mL distilled water was added. The extracts (2 mL concentrate+0.5 mL distilled water, total=2.5 mL) were cleaned up by passing through a Captiva EMR-Lipid cartridge (Agilent Technologies, Santa Clara, CA, USA). Subsequently, 0.625 mL ethyl acetate/ acetonitrile/water (16:64:20, v/v/v) was eluted through the Captiva EMR-Lipid cartridge. After elution, 1.875 mL eluent was transferred to a new conical tube, mixed with 2.625 mL distilled water and 1.2 mL isooctane, and shaken vigorously. After centrifugation for 7 min at 3,000×g, PAH contents in the supernatant were analyzed by gas chromatography/mass spectrometry (Agilent 8890 GC with an Agilent 5977B GC/MSD; Agilent Technologies).
The 16 PAHs were separated using a DB-EUPAH capillary column (20 m×0.18 mm id, 0.14 μm thickness; Agilent Technologies). Pure helium (99.999%) was used as the carrier gas at a constant flow rate of 1.2 mL/min. The samples (1 μL) were injected in the spitless mode with an injector temperature of 300°C. The temperature of the mass selective detector was 310°C, and the source temperature was 290°C. The oven temperature was initially held at 70°C for 1 min, ramped to 190°C at a rate of 30°C/min, ramped to 290°C at a rate of 10°C/min and held for 5 min, and then finally ramped to 320°C at a rate of 30°C/min and held for 1 min. The mass spectrometer was operated in electron ionization mode (70 eV), and quantitative data acquisition was performed in selective ion monitoring mode. Representative chromatograms of the 16 PAH standards are shown in Fig. 2. All PAHs were quantified using the relative response factors related to ISTD by nine-point calibration curves (9–2,400 ng/mL). The squared correlation coefficients of determination (R2) of the calibration curves were found to be over 0.99. The limit of detection of 16 PAHs was the range of 0.01–0.09 μg/kg. The limit of quantification of 16 PAHs was the range of 0.03–0.28 μg/kg. The average relative recovery of the 16 PAHs was 80.9%–119.5% for pork belly. Moreover, the relative SD was 0.57%–4.62%.
All analyses were expressed as means and SEMs. Statistical analysis was performed with SAS software v.9.4 (SAS Institute, Cary, NC, USA) using one-way analysis of variance (ANOVA). The interaction between doneness and marinating with regard to PAH formation in charcoal-grilled pork belly was evaluated using two-way ANOVA by SAS program. Significant differences in means were determined using Tukey’s tests and results with p-values less than 0.05 were considered significant.
Results and Discussion
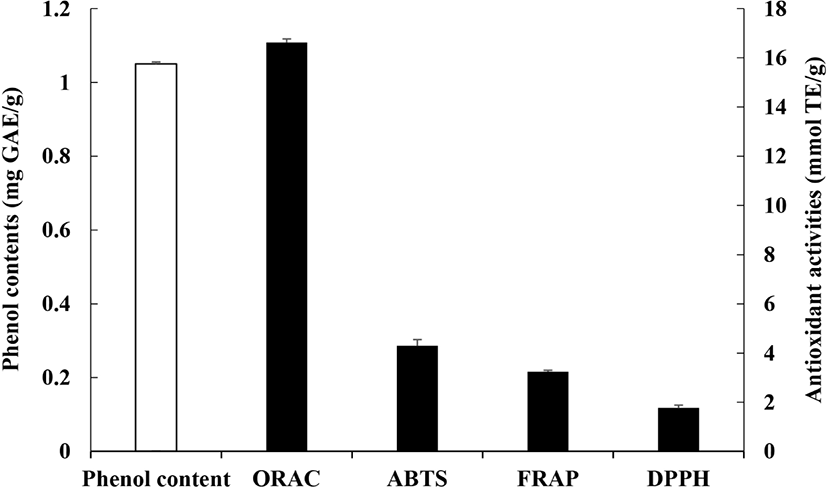
The phenol contents and antioxidant activity of the Gochujang used in this study are shown in Fig. 3. Gochujang had a phenol content of 1.05 mg GAE/g. Red pepper, one of the main components of Gochujang, contains 1.73 mg GAE/g phenols (Marinova et al., 2005). Thus, Gochujang had phenol contents similar to those of broccoli (1.01 mg GAE/g) and apples (1.04 mg GAE/g) (Marinova et al., 2005), but lower phenol contents than green tea (86.3 mg GAE/g dry matter) (Khokhar et al., 2002) and strawberries (2.44 mg GAE/g) (Marinova et al., 2005).
The ORAC activity of Gochujang was 16.62 mmol TE/g, and the ABTS and DPPH radical scavenging activities were 4.30 and 1.77 mmol TE/g, respectively. Additionally, the FRAP activity of Gochujang was 3.24 mmol TE/g. During Gochujang production, various metabolites, such as polyphenols and amino acid, are produced by microbial fermentation; these components have high nutritional value and are important contributors to antioxidant activity in Gochujang (Lee et al., 2016; Yang et al., 2018).
The contents of 16 PAHs in charcoal-grilled PBW and PBG on days 0, 5, and 10 are shown in Tables 1–3. Several factors affect PAH formation in charcoal-grilled meats, including the amount of fat in the meat, the closeness to the heat source, and the cooking time (Chung et al., 2011). In particular, pyrolysis of proteins, fats, and carbohydrates is accelerated by cooking at high temperature, leading to the generation of PAHs (Kılıç Büyükkurt et al., 2020). In this study, WC increased the PAH contents in PBW and PBG compared with MC (p<0.0001). These results were similar to those of Wang et al. (2019) and Kim et al. (2021), who showed that increased doneness accelerated PAH formation in meats. Phenanthrene was the most abundant compound, with contents ranging from 273.60 to 503.02 μg/kg. Among the four major PAHs detected in this study, benzo[a]pyrene was the most abundant compound, with contents ranging from 16.24 to 63.39 μg/kg. In another study, benzo[a]pyrene was detected in charcoal-grilled pork belly at 8.04 μg/kg (Park et al., 2017), which was lower than the amount reported in this study. Notably, the amount of PAH in charcoal-grilled meat increases when the meat is cooked closer to the charcoal; Park et al. (2017) cooked their pork belly at 15 cm from the charcoal, whereas we cooked our pork belly samples at 8 cm from the charcoal. The total PAH contents in PBW-MC and PBW-WC were 1,507.22 and 2,009.45 μg/kg, respectively. However, PBG reduced the total PAH formation, showing contents of 982.45 and 1,918.90 μg/kg in PBG-MC and PBG-WC, respectively, compared with PBW (p<0.05). Additionally, the contents of the four most abundant PAHs were reduced by PBG (39.86 and 121.26 μg/kg in PBG-MC and PBG-WC, respectively), compared with PBW (p<0.05). Moreover, the contents of 14 PAHs (excluding benzo[b]fluoranthene, benzo[k]fluoranthene, and indono[1,2,3-cd]pyrene) in charcoal-grilled pork belly were affected by treatment (p<0.001, p<0.0001) and doneness (p<0.0001), and treatment and doneness interacted with each other to affect PAH contents (p<0.05, p<0.0001).
On day 5, similar to the results on day 0, 14 PAHs were detected in charcoal-grilled PBW and PBG; chrysene and dibenzo[a,h]anthracene were not detected (Table 2). According to the doneness of charcoal-grilling, WC increased the PAH contents (except the content of naphthalene) in PBW and PBG compared with MC (p<0.0001). Naphthalene contents in charcoal-grilled pork belly were not significantly affected by doneness. Phenanthrene was the most abundant compound, with contents ranging from 319.93 to 751.04 μg/kg. Among the four major PAHs, benzo[a]pyrene was the most abundant, showing contents ranging from 30.62 to 114.84 μg/kg. The total PAH contents for all 16 PAHs in PBW-MC and PBW-WC were 1,919.37 and 3,013.16 μg/kg, respectively; this was increased compared with that from day 0. However, PBG inhibited the formation of total 16PAHs, showing reduced contents of 1,189.63 and 1,402.64 μg/kg for PBG-MC and PBG-WC, respectively, compared with PBW (p<0.05). The contents of the four most abundant PAHs were also reduced in PBG to 70.24 and 100.56 μg/kg for PBG-MC and PBG-WC, respectively, compared with PBW (p<0.05). The contents of 14 PAHs (except naphthalene) in charcoal-grilled pork belly were affected by treatment (p<0.0001) and doneness (p<0.05, p<0.0001). An interaction effect was also observed between treatment and doneness for PAH contents (p<0.01, p<0.0001).
On day 10, similar to the results from days 0 and 5, 14 PAHs were detected in charcoal-grilled PBW and PBG; chrysene and dibenzo[a,h]anthracene were not detected (Table 3). WC still increased the contents of all PAHs in PBW and PBG compared with MC (p<0.01, p<0.0001). Phenanthrene was the most abundant compound, with contents ranging from 227.88 to 782.97 μg/kg. Among the four major PAHs, benzo[a]pyrene was the most abundant compound, with contents ranging from 20.49 to 134.75 μg/kg. The contents of the total 16 PAHs in PBW-MC and PBW-WC were 2,254.98 and 3,225.90 μg/kg, respectively, indicating increased contents compared with days 0 and 5. However, PBG inhibited the formation of the total 16 PAHs to 883.07 and 11,540.15 μg/kg for PBG-MC and PBG-WC, respectively, compared with PBW (p<0.05). Additionally, the contents of the four most abundant PAHs were reduced in PBG, showing contents of 49.76 and 107.89 μg/kg for PBG-MC and PBG-WC, respectively, compared with PBW (p<0.05). For 14 types of PAHs, the contents in charcoal-grilled pork belly were affected by treatment (p<0.0001) and doneness (p<0.01, p<0.0001). The interaction effect between treatment and doneness was observed for some of the PAHs and all four of the major PAHs (p<0.001, p<0.0001), but not for fluorene, phenanthrene, and the total 16 PAHs.
Few studies have evaluated the effects of storage on PAH formation in grilled meats. In this study, we found that PAH contents in pork belly were increased as the storage time increased except naphthalene and fluorene, which these contents showed an increase on day 5 and decrease on day 10. Similar to our results, Zhao et al. (2018) reported that the concentration of PAHs in oil increased with storage time and that increases in oxidation and radical formation during storage could be attributed to increased PAH concentrations. Although the reason for the increase in PAH contents during storage and after charcoal-grilling is not fully understood, our findings suggested that increased oxidation in pork belly during storage could contribute to production of high PAH contents in pork belly on days 5 and 10 when charcoal-grilling.
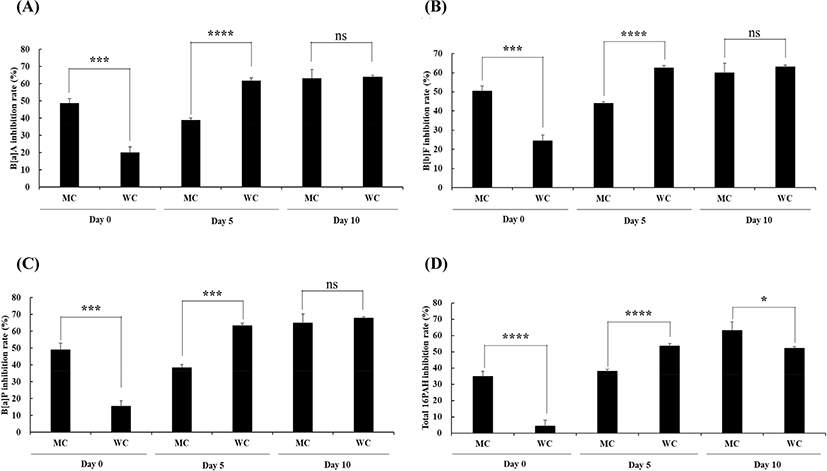
The Gochujang marinade showed inhibitory effects on PAH formation in charcoal-grilled pork belly during storage (Tables 1–3). The inhibition rates of the three major PAHs (benzo[a]anthracene, B[a]A; benzo[b]fluoranthene, B[b]F; benzo[a]pyrene, B[a]P) and the 16 total PAHs were calculated in PBG and PBW (Fig. 4). On day 0, the inhibition rates of MC on each of the three major PAHs and on the total 16 PAHs were higher than those of WC (34.82%–50.47% versus 4.51%–24.30%; p<0.05). The highest and lowest PAH inhibitory effects were observed for B[b]F and the total 16 PAHs, respectively. This may be because of the high PAH contents in charcoal-grilled pork belly subjected to WC, results in apparent weakening of the inhibitory effects of Gochujang. However, the inhibition rates of WC on the three major PAHs and the total 16 PAHs on day 5 were higher than those of MC (p<0.05). Finally, on day 10, there were no significant differences in inhibition rates between MC and WC for the three major PAHs. Additionally, the inhibition rates of the three major PAHs and the 16 total PAHs increased up to 52.26%–67.80% on day 10. These results indicated that the inhibitory activity of Gochujang on PAH contents in charcoal-grilled pork belly was increased as the storage time increased. Similar to our results, García-Lomillo et al. (2017) reported that red wine pomace seasoning on beef patties for 9 days increased the PAH inhibitory effect compared with that on day 1. This suggests that the inhibitory effects of polyphenols on PAH formation are exerted during storage (García-Lomillo et al., 2017). Therefore, storage of pork belly marinated Gochujang for 10 days could increase the PAH inhibitory effect when charcoal-grilling.
Previous studies have reported that marinades, such as onions, garlic, beer, tea, and vinegar, can reduce the PAH contents of grilled meats (Cordeiro et al., 2020; Viegas et al., 2014; Wang et al., 2019). These ingredients contain abundant amounts of phenolic compounds and sulfhydryl compounds, resulting in high antioxidant activity. In particular, antioxidants can inhibit PAH formation in meats by blocking free radical generation in pyrolysis and eliminating the free radicals (Cordeiro et al., 2020; Viegas et al., 2014; Wang et al., 2019). Gochujang is fermented by Aspergillus oryzae, and during fermentation, metabolites such as furan, phenolics, and heptelidic acid derivatives can be produced, exerting various biological effects (Lee et al., 2016). Moreover, during the fermentation, total polyphenols and flavonoids in Gochujang increase, thereby enhancing its antioxidant activity (Yang et al., 2018). Therefore, the high phenolic content and antioxidant activity of Gochujang can inhibit PAH formation in charcoal-grilled pork belly.
The PAH formation during grilling is the result of pyrolysis of nutrients on the surface of meats caused by exposure to high-temperature flame, and incomplete combustion of coal results in adherence of smoke particles to surface of the meat. Therefore, blocking the surface using a marinade can reduce the PAH contents in grilled meats (Kılıç Büyükkurt et al., 2020). Because Gochujang is a paste with high viscosity, it can easily cover the surface of pork belly, which may help block the surface of pork belly from the flame and smoke.
Conclusion
The phenol contents and antioxidant activities of Gochujang may affect the reduction of PAH contents in charcoal-grilled pork belly. Cooking at a high internal temperature of 81°C (well-done cooking) increased the formation of 16 PAHs in charcoal-grilled pork belly compared with moderate cooking at 71°C. Interactions were observed between doneness and marinating with regard to PAH contents in charcoal-grilled pork belly. Moreover, the inhibitory effects of Gochujang marinade on the total 16 PAHs in charcoal-grilled pork belly increased up to 63.06% after storage for 10 days and cooking at moderate temperature. Importantly, the amount of carcinogenic benzo[a]pyrene in charcoal-grilled PBG was 67.80% on day 10 after well-done cooking. These results indicated that Gochujang marinade of pork belly could inhibit PAH formation after charcoal-grilling and that the inhibitory effects of this marinade increased during the 10 days of storage. These findings provided a preliminary data for the inhibitory effects of Gochujang on PAH formation, while the specific mechanism for PAH inhibitory effect of Gochujang marinade on charcoal-grilled pork belly should be investigated in the future. It was suggested that marinating pork belly with Gochujang may be an effective processing method to reduce the intake of PAHs when consuming charcoal-grilling meat.