Introduction
Eggs are valuable livestock products because of their high-quality protein and various nutrients; therefore, they are widely consumed in many countries (Kassis et al., 2010). However, eggs are perishable when not properly handled and stored. Strategies such as addition of antioxidants can maintain egg quality and minimize the oxidation of egg products; however, synthetic antioxidants are potentially toxic. Thus, nowadays, synthetic antioxidants are replaced with natural antioxidants extracted from natural compounds accompanying side effects (Harlina et al., 2015). Many studies have reported the application of plant extracts such as galangal (Harlina et al., 2019), clove (Harlina et al., 2018), and green tea extracts (Ganasen and Benjakul, 2011) to eggs as natural antioxidants.
Lotus (Nelumbo nucifera), an aquatic plant that grows in water and is widely cultivated in Asia (Kim and Park, 2008), is relatively inexpensive and has been verified as safe. Rhizomes, seeds, flowers and leaves in lotus plant have long been used as food or herbal medicine (Mukherjee et al., 2009). In particular, lotus leaves contain abundant phenolic compounds, ascorbic acid, carotenoids, and tocopherols (Huang et al., 2010). Park et al. (2007) reported the free radical scavenging activity of phenolic compounds in lotus leaves and showed that lotus leaves exhibit a potential antioxidant ability for the inhibition of lipid and protein oxidation. Therefore, lotus leaves have been used as a natural antioxidant in foods. For example, Choi et al. (2011) showed that chicken patties treated with lotus leaves had lower 2-thiobarbituric acid (TBA) and volatile basic nitrogen (VBN) contents than the control group. Additionally, Choe et al. (2011) reported that supplemented cooked ground pork with lotus leaf powder reduced the TBA reactive substances (2-thiobarbituric acid reactive substance, TBARS) and peroxide contents and conjugated diene concentration. However, despite these advantages, lotus leaves have rarely been applied to egg products.
Ultrasonication has been conducted for a wide range of food technology processes such as freezing, cutting, drying, tempering, bleaching, sterilization, and extraction (Chemat et al., 2011). Kang et al. (2016) suggested that the application of ultrasonication may produce a faster sodium penetration into baked eggs, simultaneously improves some textural traits as well as flavor of the products. And Sert et al. (2011) reported that ultrasonic treatment was used to improve the sensory properties of eggshells. Jing et al. (2020) reported that the antioxidant activity of egg white protein could be improved by the addition of tea polyphenols using an ultrasound-assisted method.
The purpose of this study was to investigate the effects of lotus leaf hot water extracts on the quality and stability of eggs during storage by using ultrasonication.
Materials and Methods
Eggs that weighed 60–68 g were purchased from a market (Seoul, Korea). Eggs were obtained from ISA Brown laying hens (56 wk of age). And lotus leaves were obtained from the Seon-Wonsa temple (Incheon, Korea). Before soaking the eggs, the eggshells were sterilized with 70% alcohol to remove bacteria, germs, and contaminants on the surface. And the treatment groups are marked with a pencil. To determine the effect of ultrasonication in lotus leaf extracts on egg quality, the eggs were placed in a 40 kHz frequency ultrasonicator (JAC-5020, KODO Technical Research, Hwaseong-Si, Korea) filled with lotus leaf hot water extracts (Table 1) and processed for 30 min. After ultrasonication, the processed eggs were dried and placed on an egg rack with the blunt side of the egg facing up. The eggs were stored at 30°C for 15 d, and measurements were performed at 0, 5, 10, and 15 d.
Twenty eggs were randomly selected to determine the overall quality. The egg weight, Haugh unit (HU), egg grade, albumen height, eggshell thickness, and eggshell breaking strength were measured using a Digital egg tester (DET-6000, NABEL, Kyoto, Japan).
The weight loss was calculated according to a previous report by Wardy et al. (2011). Ten eggs per treatment group were measured with a digital electronic balance. All eggs were measured over the course of 15 d at 5 d intervals. The percentage weight loss was determined as follows:
The egg of all treatment groups (CON, T1, T2, and T3) was broken to separate the shell, and the yolks were separated using an egg separator. The separated yolks were used for TBARS analysis. The TBARS contents were measured using the method reported by Jung et al. (2011). Five grams of egg yolk were added to 15 mL of distilled water and homogenized (HG-15A, DAIHAN Scientific, Wonju, Korea) at 1,130×g for 1 min. One milliliter of the homogenized sample was reacted with 50 μL of butyl hydroxytoluene (7.2% in 100% ethanol) and 2 mL of trichloroacetic acid/TBA reagent (20 mM TBA in 15% trichloroacetic acid). The mixture was heated in a 90°C water bath for 30 min, cooled in ice. And centrifuged (VS-550, VISION SCIENTIFIC, Daejeon, Korea) at 2,090×g for 15 min. The supernatant was filtered using Whatman filter paper No. 1, and the absorbance was measured at 532 nm with spectrophotometer (Optizen 212UV, Mecasys, Daejeon, Korea). The standard curve was measured with malondialdehyde (MDA) prepared by the acidification of 1,1,3,3-tetraethoxypropane. The TBARS contents were evaluated by the standard curve and is expressed as milligrams of MDA per 1 kg of yolk (mg MDA/kg yolk).
VBN was analyzed to determine the extent of albumen deterioration. Five grams of each sample were mixed with 15 mL of distilled water and homogenized at 10,000 rpm for 1 min. Distilled water was added to adjust the mixture to 50 mL, the mixture was filtered with Whatman filter paper No. 4, and 1 mL of the filtrate was placed in the outer chamber of a Conway unit. After placed filtrate, 1 mL of 0.01 N boric acid and 100 μL of Conway reagent were placed in the inner chamber of the unit. After the reaction, 1 mL of potassium carbonate was added to the other side of the outer chamber of the unit. The unit was then sealed and slowly agitated in the horizontal direction to mix the reagents in the outer chamber. The unit was incubated at 37°C for 2 h, after which the liquid of the inner chamber was titrated with 0.02 N sulfuric acid. The VBN contents were determined as follows:
Where, A1 is the volume of sulfuric acid consumed for the sample titration (mL), A0 is the volume of sulfuric acid consumed for the blank titration (mL), and F is the standardized index of 0.02 N sulfuric acid; 28.014 is the amount required to consume 1 mL of 0.02 N sulfuric acid.
Total phenolic contents (TPC) was determined using the Folin-Ciocalteu method, as reported previously, with some modifications (Wei et al., 2011). A total of 20 μL of albumen sample was added to 20 μL of 1 N Folin-Ciocalteu reagent and stirred for 3 min at room temperature. After the reaction, 60 μL of 1 N Na2CO3 was added, and the mixture was incubated in the dark for 90 min. After incubation, 100 μL of distilled water was added. Next, the absorbance of the solution was measured at 725 nm. The results are expressed as milligrams of gallic acid equivalent (GAE) per 1 mL of sample (mg GAE/mL sample).
Total flavonoid contents (TFC) was measured using Dowd’s method as described by Adefegha et al. (2018). One hundred microliters of albumen were mixed with the same amount of 2% (w/v) aluminum chloride and incubated for 10 min at 25°C. Then, the absorbance was measured at 415 nm. Distilled water was used as the blank control, and TFC was calculated based on a standard curve for quercetin. The results are expressed as milligrams of quercetin equivalent (QE) per 1 mL of sample (mg QE/mL sample).
After blending the albumen and 95% ethanol at a ratio of 1:10 (w/v), the mixture was extracted at 60°C in a water bath (SB-1200, EYELA, Shanghai, China) with continuous shaking at a speed of 170 r/min for 2 h. After extraction, the mixture was centrifuged at 2,090×g for 10 min, and the supernatant was used for DPPH radical scavenging activity analysis (Harlina et al., 2019). The DPPH radical scavenging activity was analyzed by slight modification of the method reported by Blois (1958). One hundred microliters of the sample was combined with 100 μL of 0.2 mM DPPH reagent and kept in the dark for 30 min. The absorbance of the reactant was then measured at 517 nm with a spectrophotometer (Multiskan GO, Thermo Fisher Scientific, MA, USA). Radical scavenging activity was expressed as percentage according to the following equation:
Where, A1 is the absorbance of samples, and A0 is the absorbance of control (distilled water).
Results and Discussion
The changes in egg quality and weight loss during storage at 30°C are shown in Table 2. Egg weight and albumen height of all groups significantly decreased after 15 d storage (p<0.05). HU of control, T1, and T2 significantly decreased during storage of 15 d (p<0.05). HU of T3 were observed tend to decrease during storage periods. The HU indicated that CON and T1 exhibited a quality change from grade AA to A after 15 d, whereas T2 and T3 maintained their AA grade. The yolk color for all groups deepened significantly with increasing storage period (p<0.05). No significant differences were observed in eggshell thickness and eggshell breaking strength among the groups during storage. Weight loss of all groups increased significantly with longer storage periods, and the weight loss of T3 was significantly lower than that of CON for entire storage times (p<0.05).
Egg weight typically decreases with time because of the decreased moisture content of the albumen. This decrease occurs because carbon dioxide escapes through the holes in the shell and evaporates as the albumen moisture increases (Robinson, 1987). During storage, the enzymes present in the albumen hydrolyze the amino acid chains and, by destroying the protein structure, release the water that was bound to the large protein molecules, which leads to fluidization and loss of viscosity of the dense albumen. This leads to decreases egg quality and grade.
In this study, T3 showed the highest weight, HU, grade, albumen height and lowest weight loss during storage. This is a result of the high content of lotus leaf extracts of T3, and it is because moisture retention is improved as the free sugar component of the lotus leaf (Park et al., 2014). Thus, a relatively small amount of water loss might occur in the lotus leaf hot water extracts treatment group, thereby maintaining high egg quality and low weight loss. This is consistent with the findings of a previous study, wherein the quality of duck eggs was maintained during storage because of the treatment with Melinjo (Gnetum gnemon Linn) leaf extract (Mukhlisah et al., 2020).
These results suggest that lotus leaf hot water extract is highly effective in improving the egg quality (HU, egg grade, albumen height (mm), and yolk color) and decreasing weight loss during 15 d of storage.
Fig. 1 shows the changes in the TBARS values of the egg yolks during storage for 15 d. The TBARS values increased significantly in all groups as the storage period increased (p<0.05). The TBARS values of the CON, T1, T2, and T3 egg yolks were 0.03, 0.01, 0.02, and 0.01 mg MDA/kg yolk at 0 d of storage, respectively. The TBARS values of T3 was significantly lower than those of the other groups (p<0.05), and the TBARS value of CON (0.12 mg MDA/kg yolk) was twice that of T3 (0.06 mg MDA/kg yolk) after 15 d of storage.
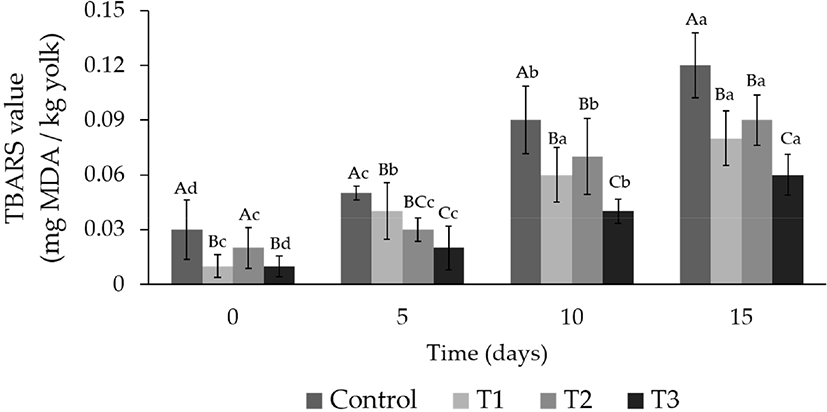
The value of TBARS, the secondary product of lipid oxidation, is expressed as the MDA contents. At high concentrations of MDA compound can adversely affect the flavor and aroma of food items, making them inedible (Osawa et al., 2005).
The active compounds of lotus leaves can terminate free-radical reactions and scavenge reactive oxygen species (Harlina et al., 2018; Park et al., 2007). It was observed that the TBARS value significantly decreased during all storage periods because of the antioxidant action of the active compounds contained in the lotus leaf hot water extract.
The changes in the VBN values of the albumens during storage are shown in Fig. 2. The VBN values of all groups increased significantly with time (p<0.05). The range of initial VBN value was from 0.75 to 1.06 mg%, and there were no significant differences among groups (p<0.05). However, the VBN value of CON (7.84 mg%) increased significantly (p<0.05) after 10 d of storage and was the highest (11.58 mg%) after 15 d of storage. During 5, 10, and 15 d of storage, the VBN values of T3 were significantly lower, ranging from 0.75 to 5.10 mg%, than those of the other groups (p<0.05).
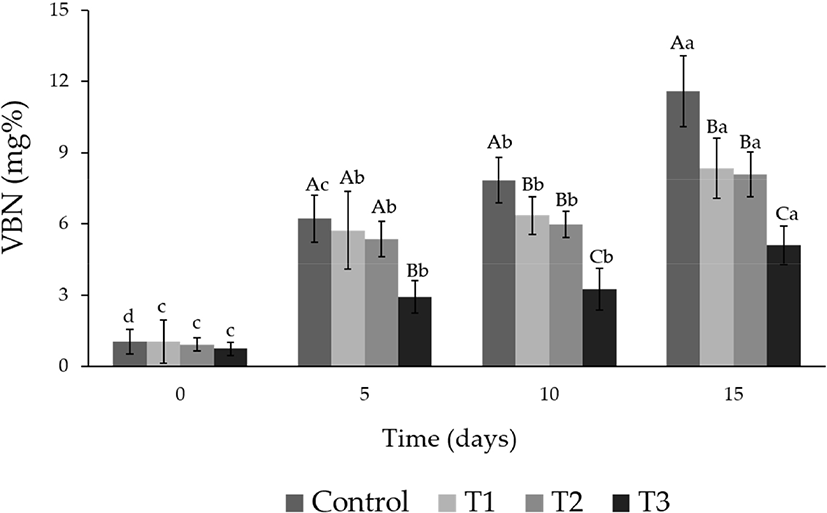
VBN in protein foods is a substance produced by bacterial reduction of protein decomposed into low molecular weight substances such as albumose, peptone, peptide, and amino acid (Crespo et al., 1978). The increase in VBN contents was due to bacterial growth and enzyme action, so it is used as an indicator of the degree of protein deterioration. In our study, the group treated with lotus leaf extract found lower VBN values than the other groups. This is the result of suppressing the growth of microorganisms due to the antimicrobial activity (Li and Xu, 2008) and antioxidant effect (Choi et al., 2011) of polyphenol compounds contained in lotus leaves. Thus, we observed that phenolic compounds of lotus leaf extracts prevent the breakdown of albumens. This suggests that the antibacterial action of lotus leaf hot water extract is related to the reduction of VBN values of albumens.
The changes in TPC and TFC of the albumens are shown in Table 3. TPC significantly decreased in all groups (p<0.05) as storage time increased. At 0 d, the TPC of CON, T1, T2, and T3 were 1.46, 1.85, 1.61, and 2.25 mg GAE/mL, which decreased to 1.25, 1.58, 1.48, and 1.73 mg GAE/mL, respectively, after 15 d of storage. The TPC of T3 was significantly higher than those of the other groups for entire times (p<0.05). Similarly, TFC significantly decreased in all groups as the storage period increased (p<0.05), and the TFC of T3 (0.48 mg QE/mL) was significantly higher than that of CON (0.26 mg QE/mL) after 15 d of storage (p<0.05).
Oh et al. (2013) reported that the TPC of lotus leaf hot water extract was 20.17±0.37 mg GAE/g tea. Also, it has been reported that abundant phenolic compounds, including kaempferol, quercetin, and isoquercetin (Choe et al., 2011; Park et al., 2014), have been extracted from lotus leaves. Phenolic compounds, a class of chemical components containing one or more acidic hydroxyl residues, are some of the most effective antioxidant ingredients that contribute to the antioxidant activity of natural foods (Velioglu et al., 1998).
Flavonoids, one type of phenolic compound, have attracted extensive attention because of their strong antioxidant activity, as well as their ability to reduce the formation of free radicals and scavenge free radicals (Zhu et al., 2015). Phenolic compounds and flavonoids are known to exhibit antioxidant effects through activities such as regenerating α-tocopherol, scavenging free radicals, and chelating metal ions (Rice-Evans et al., 1996).
It could be suggested that enhanced TPC and TFC of albumen groups treated lotus leaf extracts may result from the phenolic compounds, which play an essential role as antioxidant.
Therefore, the results suggest that the TPC and TFC of the eggs were improved by the antioxidant activity of the lotus leaf hot water extract.
The DPPH radical scavenging activities of the albumens are shown in Table 4. The initial DPPH radical scavenging activities were 3.25%, 15.09%, 3.87%, and 7.33% for CON, T1, T2, and T3, respectively. The DPPH radical scavenging activity of T3 was significantly higher than those of the other groups during storage (p<0.05). This is consistent with a previous study that confirmed that the components of the plant extract are absorbed by the egg and have a positive effect on the antioxidant activity (Harlina et al., 2018).
DPPH radical scavenging activities are commonly calculated by measuring the reduction in free radicals by electrons transferred from antioxidants. Their aromatic features and conjugated structures with numerous different hydroxyl groups make phenolic compounds effective electron or hydrogen atom donors for scavenging free radicals and reactive oxygen species (Zhang and Tsao, 2016). In general, a greater number of hydroxyl groups in a phenolic structure was thought to yield superior antioxidant activity. In this study, the involvement of a large amount of phenolic compounds in lotus leaf extracts indicated that a large number of phenolic hydroxyl groups were introduced into albumen.
Therefore, it is suggested that the improvement of the DPPH radical scavenging activity might be related to the increased total phenol contents in eggs treated with lotus leaf hot water extracts.
Conclusion
This study was performed to investigate the effects of lotus leaf hot water extracts as a natural ingredient for quality and stability of eggs during storage.
The egg quality, weight loss, stability indicators (TBARS and VBN contents), TPC and TFC contents, and DPPH radical scavenging activity were determined. During storage, T3 showed that highest egg quality (HU, egg grade, albumen height) and low weight loss. Also, T3 had low TBARS and VBN contents and delayed lipid and protein deterioration. The TPC and TFC and DPPH radical scavenging activity of T3 were significantly higher than those of CON (p<0.05).
The results suggest that lotus leaf hot water extract is a highly effective natural ingredient for maintaining the quality and stability of eggs during storage.













